Promotion of In Vitro Hair Cell-like Cell Differentiation from Human Embryonic Stem Cells through the Regulation of Notch Signaling
Abstract
:1. Introduction
2. Results
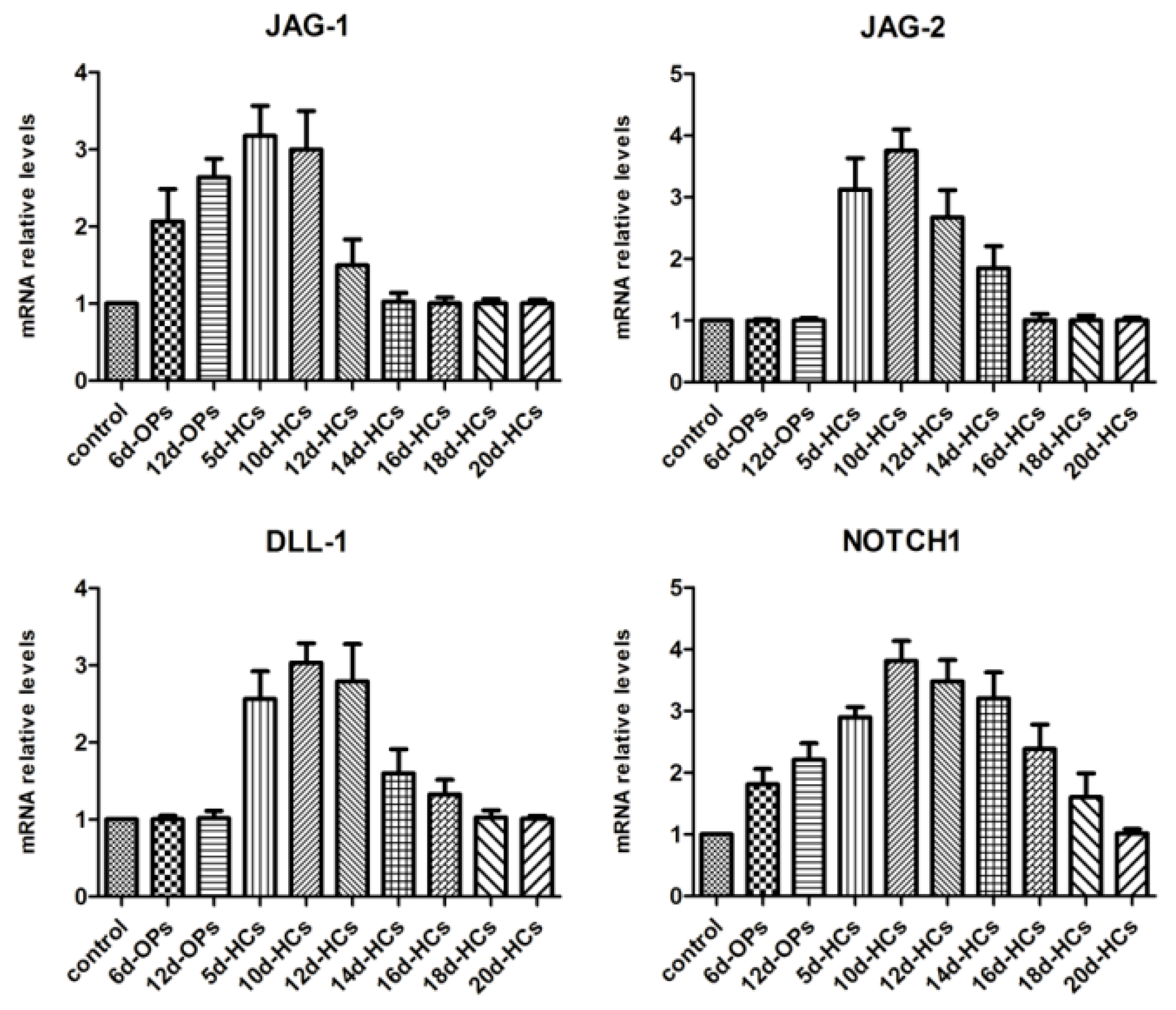
2.1. Temporal Expression Pattern of Notch Ligands and Receptors during hESCs Hair Cell Differentiation
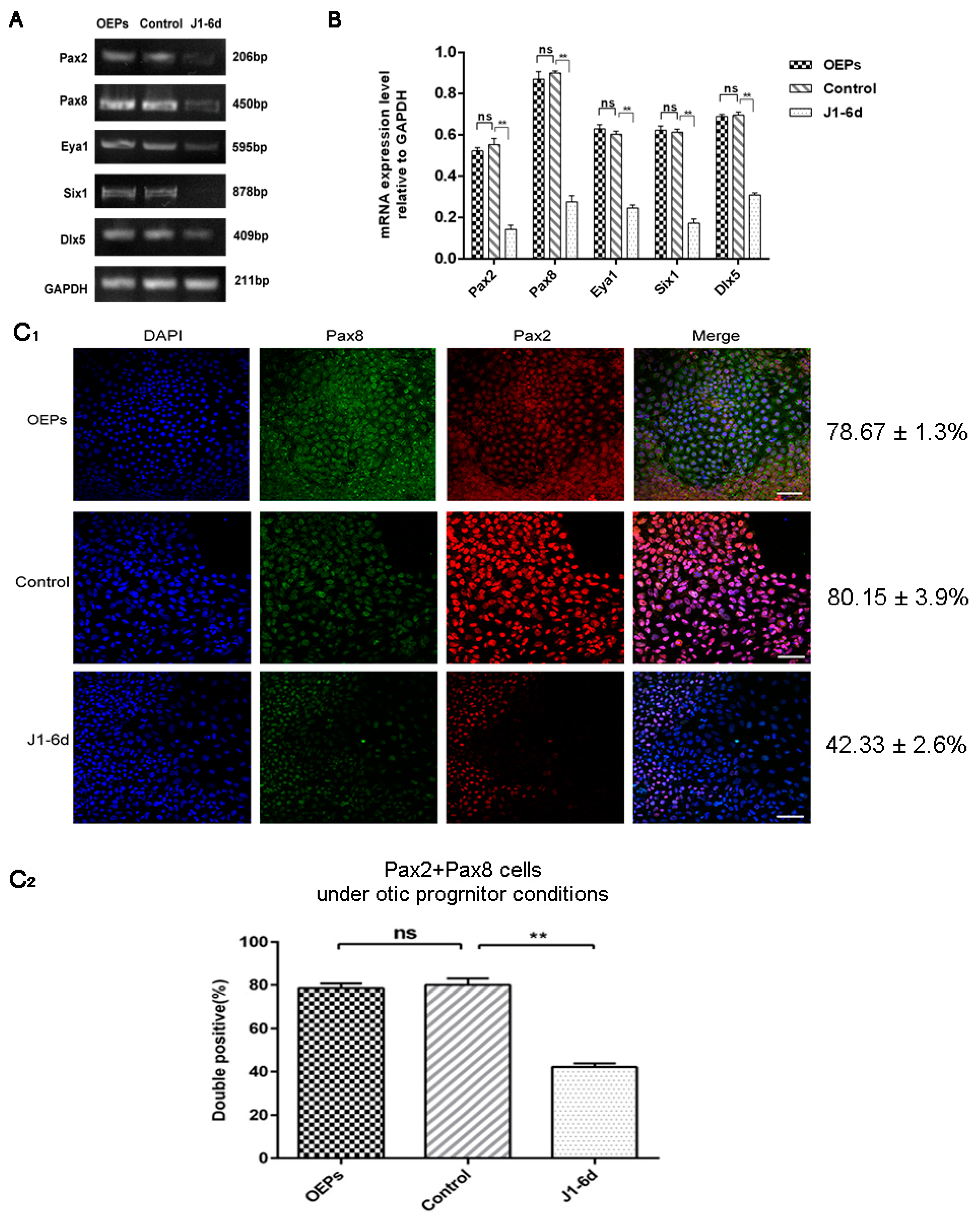
2.2. The Effects of ShRNA Silencing on the Expression of Target Genes
2.3. The Expression of Early Otic-Specific Genes in OPCs Infected with JAG-1-shRNA2
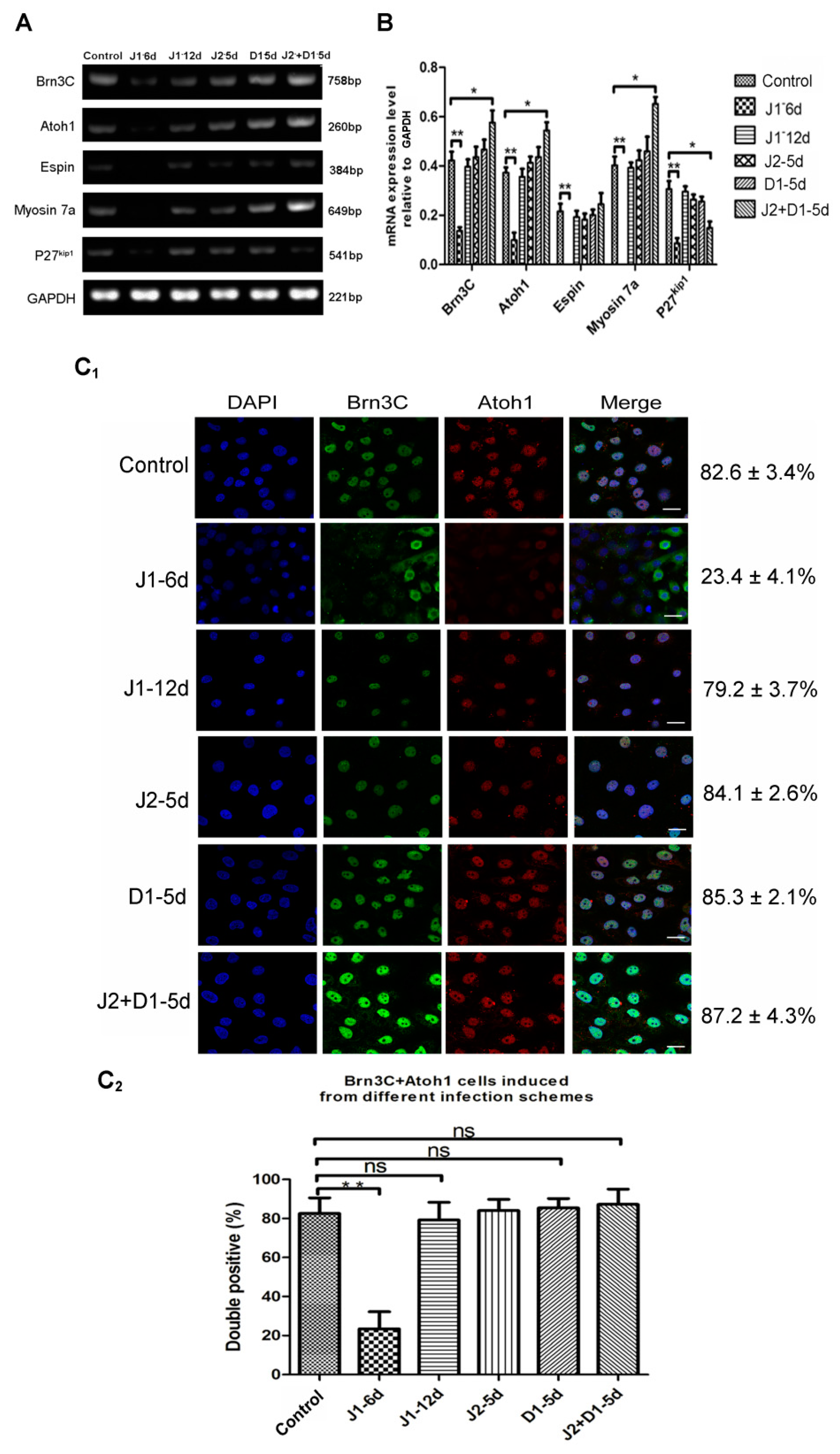
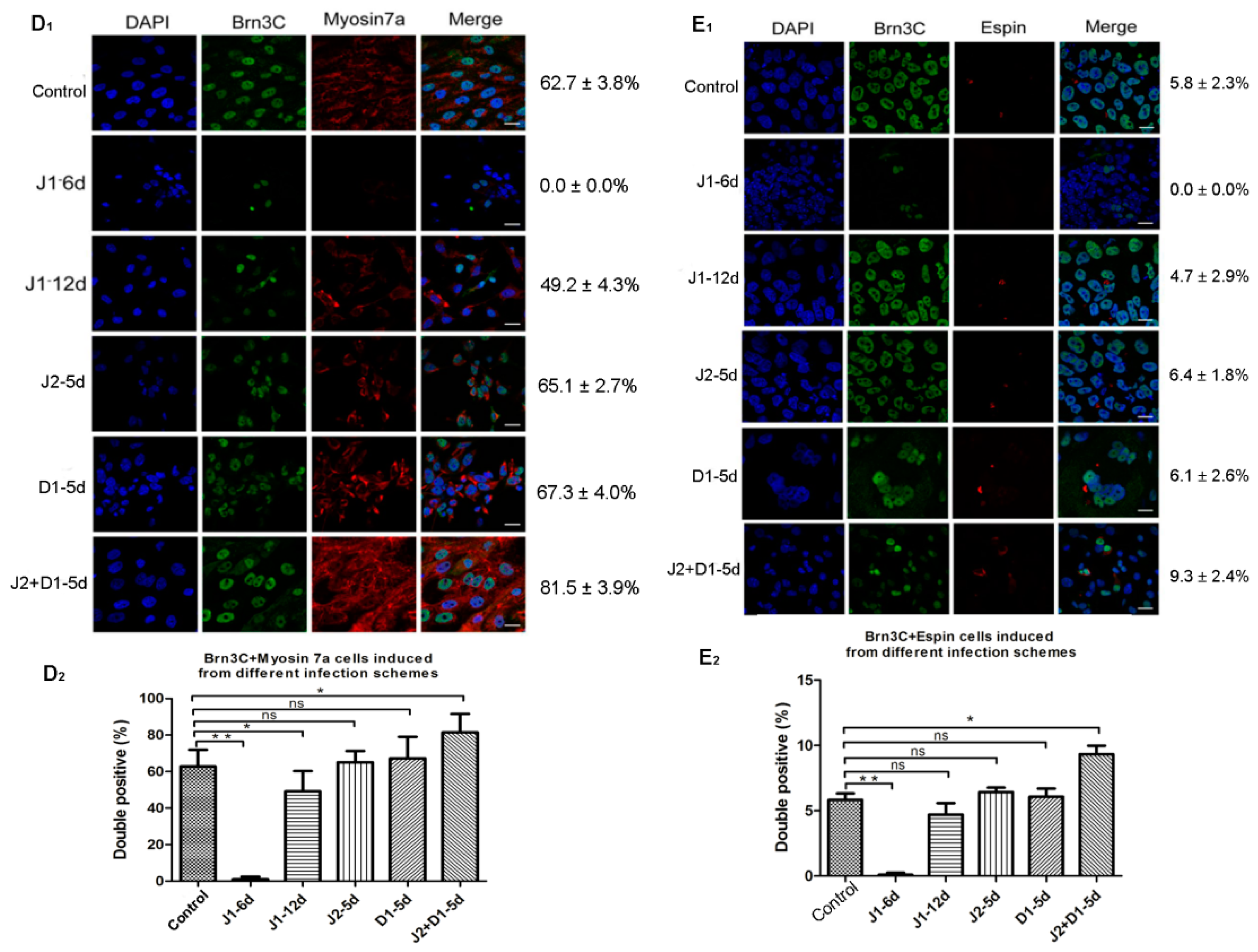
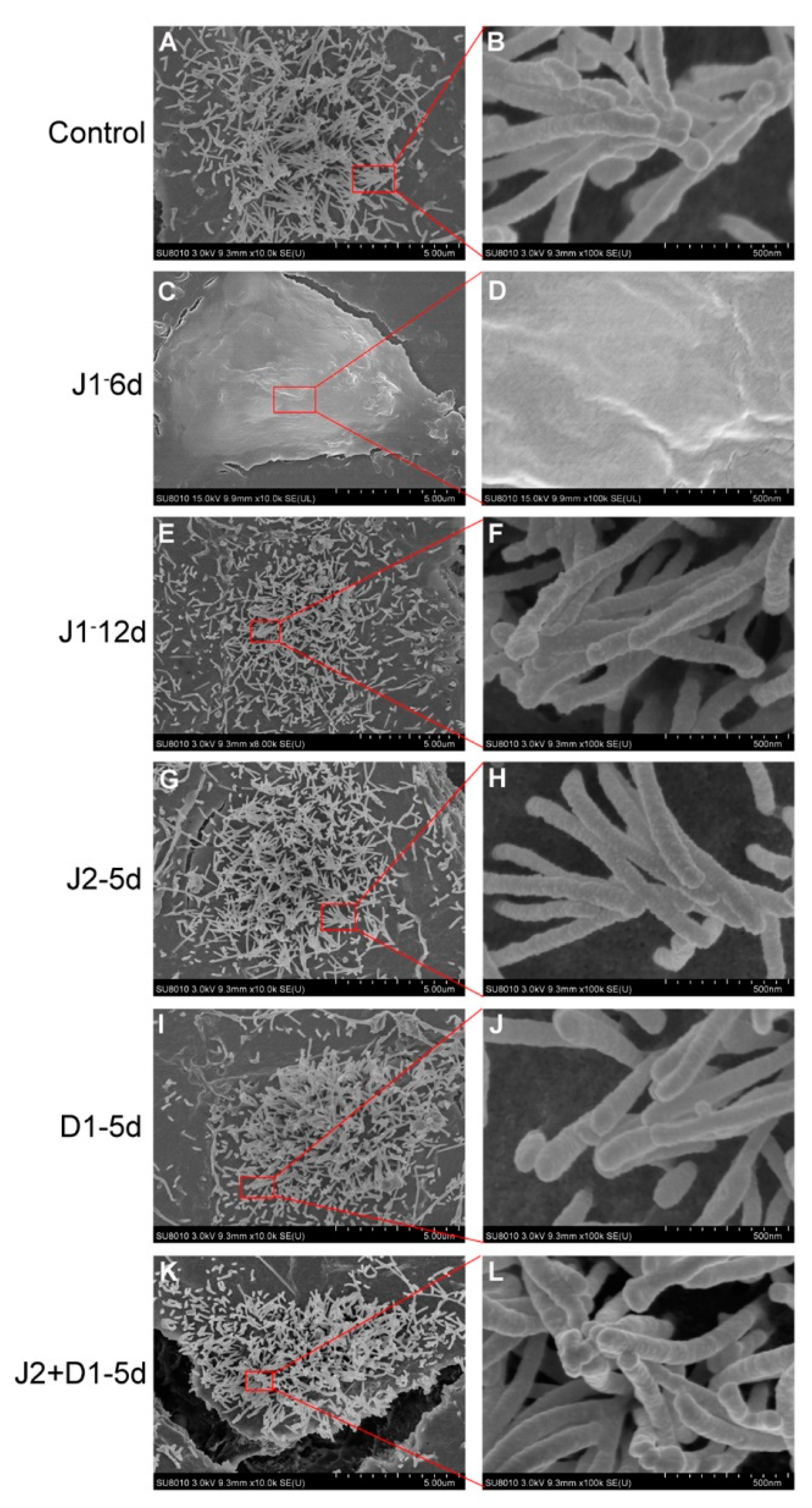
2.4. Analysis of Hair Cell-Specific Markers and Morphological Characteristics
3. Discussion
4. Materials and Methods
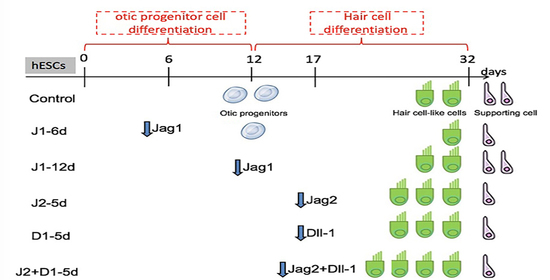
4.1. Two-Step Induction to Generate Hair Cell-like Cells from hESCs
4.2. The Expression Pattern of Genes Specific for Notch Signaling Analyzed during the Different Stages of hESC Differentiation to Hair Cell-like Cells
4.3. Recombinant Lentiviral Vector Construction and Cell Transfection
4.4. Western Blot
4.5. Gene Expression Specific Analysis
4.6. Immunocytochemistry
4.7. SEM Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Canalis, E. Notch and the regulation of osteoclast differentiation and function. Bone 2020, 138, 115474. [Google Scholar] [CrossRef] [PubMed]
- McCarter, A.C.; Wang, Q.; Chiang, M. Notch in Leukemia. Adv. Exp. Med. Biol. 2018, 1066, 355–394. [Google Scholar] [CrossRef]
- Meurette, O.; Patrick, M. Notch Signaling in the Tumor Microenvironment. Cancer Cell. 2018, 34, 536–548. [Google Scholar] [CrossRef] [Green Version]
- Bray, S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Tateya, T.; Sakamoto, S.; Imayoshi, I.; Kageyama, R. In vivo overactivation of the Notch signaling pathway in the developing cochlear epithelium. Hear. Res. 2015, 327, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Groves, A.K. Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudet, N.; Żak, M. Notch Signalling: The Multitask Manager of Inner E-ar Development and Regeneration. Adv. Exp. Med. Biol. 2020, 1218, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Fekete, D.M. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 2016, 143, 4003–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahlou, H.; López-Juárez, A.; Fontbonne, A.; Nivet, E.; Zine, A. Modeling human early otic sensory cell development with induced pluripotent stem cells. PLoS ONE 2018, 13, e0198954. [Google Scholar] [CrossRef] [PubMed]
- Murata, J.; Ikeda, K.; Okano, H. Notch signaling and the developing inner ear. Adv. Exp. Med. Biol. 2012, 727, 161–173. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, S.; Ni, W.; Chen, Y.; Li, W. Unidirectional and stage-dependent roles of Notch1 in Wnt-responsive Lgr5+ cells during mouse inner ear development. Front. Med. 2019, 13, 705–712. [Google Scholar] [CrossRef]
- Lin, V.; Golub, J.S.; Nguyen, T.B.; Hume, C.R.; Oesterle, E.C.; Stone, J.S. In-hibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J. Neurosci. 2011, 31, 15329–15339. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, J.; Gálvez, H.; Neves, J.; Abelló, G.; Giraldez, F. Differential regula-tion of Hes/Hey genes during inner ear development. Dev. Neurobiol. 2015, 75, 703–720. [Google Scholar] [CrossRef]
- Ko, E.-B.; Hwang, K.-A.; Choi, K.-C. Prenatal toxicity of the environmental pollutants on neuronal and cardiac development derived from embryonic stem cells. Reprod. Toxicol. 2019, 90, 15–23. [Google Scholar] [CrossRef]
- Chen, W.; Jongkamonwiwat, N.; Abbas, L.; Eshtan, S.J.; Johnson, S.L.; Kuhn, S.; Milo, M.; Thurlow, J.K.; Andrews, P.W.; Marcotti, W.; et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 2012, 490, 278–282. [Google Scholar] [CrossRef]
- Ronaghi, M.; Nasr, M.; Ealy, M.; Durruthy, D.R.; Waldhaus, J.; Diaz, G.H.; Joubert, L.M.; Oshima, K.; Heller, S. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 2014, 23, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Tang, Z.H.; Chen, J.R.; Shi, H.S.; Chen, J.L.; Wang, C.C.; Zhang, C.; Li, L.; Chen, P.; Wang, J.F. Induction of differentiation of human embryonic stem cells into functional hair-cell-like cells in the absence of stromal cells. Int. J. Biochem. Cell Biol. 2016, 81, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, J.; Hu, N.; Liu, A.; Zhu, H.; Li, L.; Tian, Y.; Chen, X.; Quan, L. Lentivirus-mediated down-regulation of CK2α inhibits proliferation and induces apoptosis of malignant lymphoma and leukemia cells. Biochem. Cell Biol. 2018, 96, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.A. Treating Disease at the RNA Level with Oligonucleotides. N. Engl. J. Med. 2019, 380, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Feng, H.; Zhao, F.; Zhang, L.; Xu, S.; Zhang, C.; Zhao, C.; Qin, G. FANCD2 knockdown with shRNA interference enhances the ionizing radiation sensitivity of nasopharyngeal carcinoma CNE-2 cells. Neoplasma 2021, 68, 40–52. [Google Scholar] [CrossRef]
- Zhao, H.; He, L.; Yin, D.; Song, B. Identification of β-catenin target genes in colorectal cancer by interrogating gene fitness screening data. Oncol. Lett. 2019, 18, 3769–3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, H.; Shuai, B. Exogenous siRNAs against chitin synthase gene suppress the growth of the pathogenic fungus Macrophomina phaseolina. Mycologia 2020, 112, 699–710. [Google Scholar] [CrossRef]
- He, W.; Tu, M.; Du, Y.; Li, J.; Pang, Y.; Dong, Z. Nicotine Promotes AβPP Nonamyloidogenic Processing via RACK1-Dependent Activation of PKC in SH-SY5Y-AβPP695 Cells. J. Alzheimer’s Dis. 2020, 75, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Pei, Y.; Li, C.; Zhu, M. Periodicity and dosage optimization of an RNAi model in eukaryotes cells. BMC Bioinform. 2019, 20, 340. [Google Scholar] [CrossRef]
- Ma, J.; Chen, S.J.; Qin, Y.; Zhang, Y.Y.; Sun, X.F. In vivo and in vitro Experiment of E74-Like Factor 5 Overexpression Inhibiting the Biological Behavior of Colon Cancer Cells. J. Sichuan Univ. (Med. Sci. Ed.). 2021, 52, 430–437. [Google Scholar] [CrossRef]
- Luna-Escalante, J.C.; Formosa-Jordan, P.; Ibañes, M. Redundancy and cooperation in Notch intercellular signaling. Development 2018, 145, dev154807. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Li, W.; Huang, M.; Quan, Y.-Z.; Scheffer, D.; Tian, C.; Tao, Y.; Liu, X.; Hochedlinger, K.; Indzhykulian, A.; et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat. Commun. 2019, 10, 5530. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.B.; West, M.B.; Du, X.; Iwasa, Y.; Raphael, Y.; Kopke, R.D. Gene therapy for hair cell regeneration: Review and new data. Hear. Res. 2020, 394, 107981. [Google Scholar] [CrossRef]
- Hartman, B.H.; Reh, T.A.; Bermingham-McDonogh, O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc. Natl. Acad. Sci. USA 2010, 107, 15792–15797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Koesters, R.; Bouchard, M.; Gridley, T.; Pfannenstiel, S.; Plinkert, P.K.; Zhang, L.; Praetorius, M. Jagged1-mediated Notch signaling regulates mammalian inner ear development independent of lateral inhibition. Acta Oto-Laryngol. 2012, 132, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Abello, G.; Petrovic, J.; Giraldez, F. Patterning and cell fate in the inner ear: A case for Notch in the chicken embryo. Dev. Growth Differ. 2013, 55, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovic, J.; Formosa-Jordan, P.; Luna-Escalante, J.C.; Abelló, G.; Ibañes, M.; Neves, J.; Giraldez, F. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development 2014, 141, 2313–2324. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jia, S.; Liu, H.; Tateya, T.; Guo, W.; Yang, S.; Beisel, K.W.; He, D.Z.Z. Characterization of Hair Cell-Like Cells Converted from Supporting Cells After Notch Inhibition in Cultures of the Organ of Corti From Neonatal Gerbils. Front. Cell. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.M.; Nelson, J.C.; Zhang, H.; Kiernan, A.E.; Groves, A.K. Notch-mediated lateral induction is necessary to maintain vestibular prosensory identity during inner ear development. Dev. Biol. 2020, 462, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Macchiarulo, S.; Morrow, B.E. Tbx1 and Jag1 act in concert to modulate the fate of neurosensory cells of the mouse otic vesicle. Biol. Open 2017, 6, 1472–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiernan, A.E.; Cordes, R.; Kopan, R.; Gossler, A.; Gridley, T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 2005, 132, 4353–4362. [Google Scholar] [CrossRef] [Green Version]
- Redeker, C.; Schuster, G.K.; Kremmer, E.; Gossler, A. Normal developme-nt in mice over-expressing the intracellular domain of DLL-1 argues against revers-e signaling by DLL-1 in vivo. PLoS ONE 2018, 8, e79050. [Google Scholar] [CrossRef] [Green Version]
- Brooker, R.; Hozumi, K.; Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 2006, 133, 1277–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowik, A.D.; Bermingham-McDonogh, O. Notch signaling in mammalian hair cell regeneration. Trends Dev. Biol. 2013, 7, 73–89. [Google Scholar]
- Basch, M.L.; Brown, R.M.; Jen, H.-I.; Semerci, F.; Depreux, F.; Edlund, R.K.; Zhang, H.; Norton, C.R.; Gridley, T.; Cole, S.E.; et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife 2016, 5, e19921. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.; Parada, C.; Chamizo, M.; Giráldez, F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: A mechanism for sensory organ specification. Development 2011, 138, 735–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Martin, G.V.; Kelley, M.W.; Gridley, T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr. Biol. 2000, 10, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Maass, J.C.; Gu, R.; Basch, M.L.; Waldhaus, J.; Lopez, E.M.; Xia, A.; Oghalai, J.S.; Heller, S.; Groves, A.K. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front. Cell. Neurosci. 2015, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudet, N.; Ariza-McNaughton, L.; Lewis, J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development 2007, 134, 2369–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lentivirus. | Item | V Value | C Value | N Value | D Value | Viral Titer | Mean Titer |
|---|---|---|---|---|---|---|---|
| JAG-1-shRNA2 | 1 | 10 | 37 | 1 × 105 | 1 | 3.70 × 108 | |
| 2 | 1 | 3.98 | 1 × 105 | 1 | 3.98 × 108 | 3.70 × 108 | |
| 3 | 0.1 | 0.34 | 1 × 105 | 1 | 3.40 × 108 | ||
| JAG-2-shRNA4 | 1 | 10 | 32 | 1 × 105 | 1 | 3.20 × 108 | |
| 2 | 1 | 3.52 | 1 × 105 | 1 | 3.52 × 108 | 3.20 × 108 | |
| 3 | 0.1 | 0.29 | 1 × 105 | 1 | 2.90 × 108 | ||
| DLL-1-shRNA3 | 1 | 10 | 27.6 | 1 × 105 | 1 | 2.76 × 108 | |
| 2 | 1 | 3.05 | 1 × 105 | 1 | 3.05 × 108 | 2.74 × 108 | |
| 3 | 0.1 | 0.24 | 1 × 105 | 1 | 2.40 × 108 | ||
| NC-shRNA | 1 | 10 | 42 | 1 × 105 | 1 | 4.20 × 108 | |
| 2 | 1 | 4.46 | 1 × 105 | 1 | 4.46 × 108 | 4.19 × 108 | |
| 3 | 0.1 | 0.39 | 1 × 105 | 1 | 3.90 × 108 |
| Gene | Primer | Sequence | Tm |
|---|---|---|---|
| Pax8 | Sense | 5′- ACC CCC AAG GTG GTG GAG AAG A -3′ | 62 °C |
| Antisense | 5′- CTC GAG GTG GTG CTG GCT GAA G -3′ | ||
| Pax2 | Sense | 5′- GAG CGA GTT CTC CGG CAA C -3′ | 60 °C |
| Antisense | 5′- GTC AGA CGG GGA CGA TGT G -3′ | ||
| Six1 | Sense | 5′- GAC TCC GGT TTT CGC CTT TG -3′ | 57 °C |
| Antisense | 5′- TAG TTT GAG CTC CTG GCG TG -3′ | ||
| Dlx5 | Sense | 5′- TTC CAA GCT CCG TTC CAG AC -3′ | 57 °C |
| Antisense | 5′- GTA ATG CGG CCA GCT GAA AG -3′ | ||
| Eya-1 | Sense | 5′- TCA GAT GCT ATC TGC CGC TG -3′ | 57 °C |
| Antisense | 5′- GTG CCA TTG GGA GTC ATG GA -3′ | ||
| Atoh1 | Sense | 5′- GCC GCC CAG TAT TTG CTA CA -3′ | 57 °C |
| Antisense | 5′- GCT AGC CGT CTC TGC TTC TG -3′ | ||
| Myosin7A | Sense | 5′- CAC ATC TTT GCC ATT GCT GAC -3′ | 55 °C |
| Antisense | 5′- AGA AGA GAA CCT CAC AGG CAT -3′ | ||
| Espin | Sense | 5′- CAG GCA TGT CCT CAC CCA AT -3′ | 55 °C |
| Antisense | 5′- CGT GGC GGA GTT TGT TCT TG -3′ | ||
| Brn3c | Sense | 5′- TGC AAG AAC CCA AAT TCT CC -3′ | 55 °C |
| Antisense | 5′- GAG CTC TGG CTT GCT GTT CT -3′ | ||
| P27kip1 | Sense | 5′- CTG GAG CGG ATG GAC GCC AGA C -3′ | 62 °C |
| Antisense | 5′- CGT CTG CTC CAC AGT GCC AGC -3′ | ||
| GAPDH | Sense | 5′- GAA GGT CGG AGT CAA CGG -3′ | 58 °C |
| Antisense | 5′- GGA AGA TGG TGA TGG GAT T-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Yang, Y.; Chen, J.; Tang, Z.; Peng, Q.; Wang, J.; Ding, J. Promotion of In Vitro Hair Cell-like Cell Differentiation from Human Embryonic Stem Cells through the Regulation of Notch Signaling. Metabolites 2021, 11, 873. https://doi.org/10.3390/metabo11120873
Chen F, Yang Y, Chen J, Tang Z, Peng Q, Wang J, Ding J. Promotion of In Vitro Hair Cell-like Cell Differentiation from Human Embryonic Stem Cells through the Regulation of Notch Signaling. Metabolites. 2021; 11(12):873. https://doi.org/10.3390/metabo11120873
Chicago/Turabian StyleChen, Fengjiao, Ying Yang, Jianling Chen, Zihua Tang, Qian Peng, Jinfu Wang, and Jie Ding. 2021. "Promotion of In Vitro Hair Cell-like Cell Differentiation from Human Embryonic Stem Cells through the Regulation of Notch Signaling" Metabolites 11, no. 12: 873. https://doi.org/10.3390/metabo11120873
APA StyleChen, F., Yang, Y., Chen, J., Tang, Z., Peng, Q., Wang, J., & Ding, J. (2021). Promotion of In Vitro Hair Cell-like Cell Differentiation from Human Embryonic Stem Cells through the Regulation of Notch Signaling. Metabolites, 11(12), 873. https://doi.org/10.3390/metabo11120873