Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition
Abstract
1. Introduction
2. Results and Discussion
2.1. UPLC-MSE Analyses to Unveil the Chemical Complexity and Diversity of PCs in Rice Bran Extracts
2.2. Focus on the Tentative Identification of Phenolic Compounds by Solvent
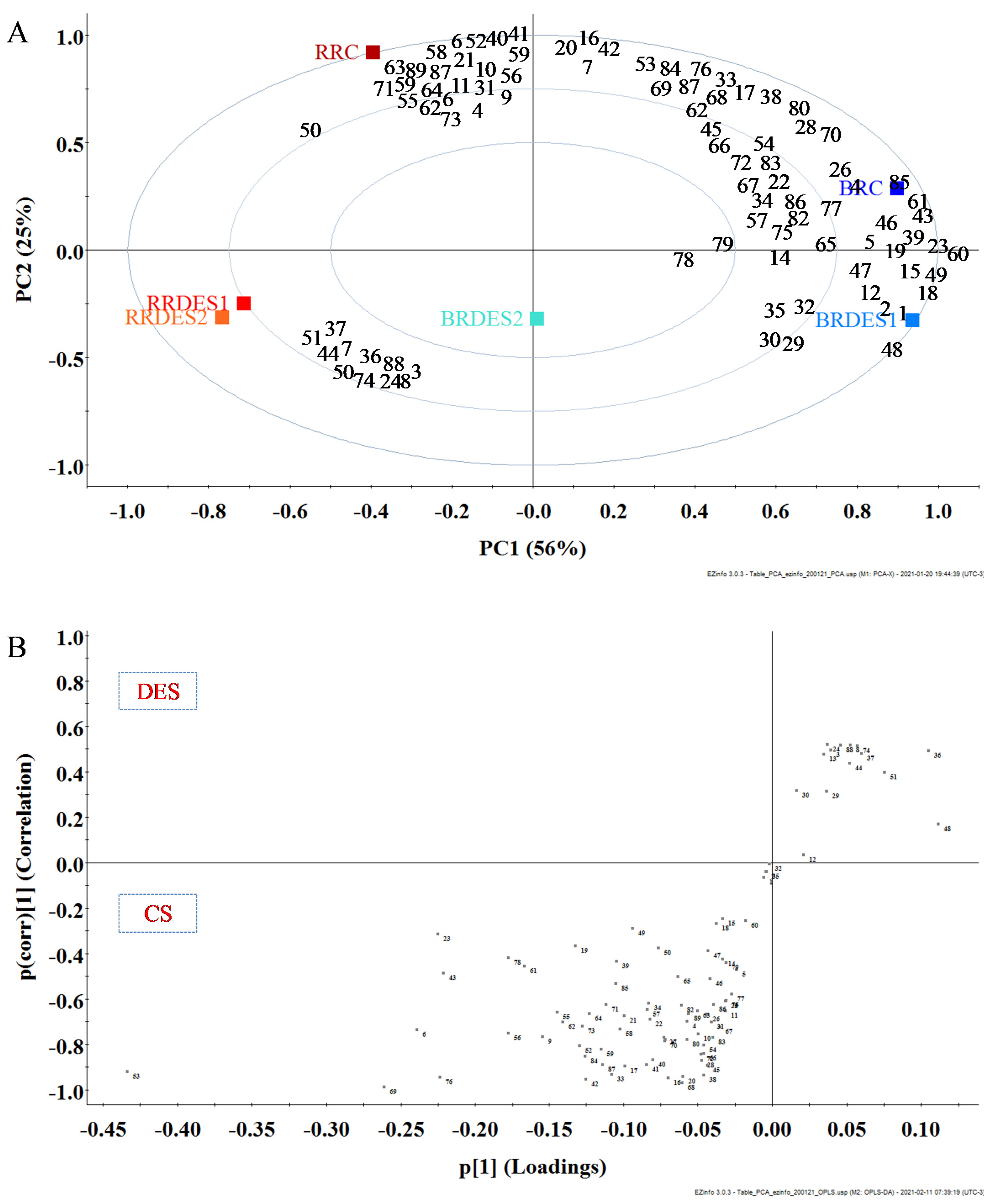
2.3. Multivariate Analysis from the Measured Relative Abundance of Phenolic Compounds
2.4. Further Characterization of Extracts
2.4.1. Quantitative Examination of the Typical Phenolic Acids Found in Cereals
2.4.2. Scavenging Ability of Rice Bran Extracts Assessed by the DPPH Radical Assay
3. Materials and Methods
3.1. Chemicals
3.2. Pigmented Rice Brans
3.3. Preparation of Deep Eutectic Solvents
3.4. Extraction of Phenolic Compounds from Rice Brans
3.5. HPLC-DAD Characterization
3.6. Metabolomic Analysis and Data Processing
3.7. DPPH Assessment of the Reducing Power of Extracts
3.8. Statistics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borresen, E.C.; Ryan, E.P. Chapter 22—Rice Bran: A Food Ingredient with Global Public Health Opportunities. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 301–310. [Google Scholar]
- Li, K.J.; Borresen, E.C.; Jenkins-Puccetti, N.; Luckasen, G.; Ryan, E.P. Navy Bean and Rice Bran Intake Alters the Plasma Metabolome of Children at Risk for Cardiovascular Disease. Front. Nutr. 2018, 4, 71. [Google Scholar] [CrossRef]
- Khir, R.; Pan, Z. Chapter 2—Rice. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–58. [Google Scholar]
- Tong, C.; Bao, J. Rice lipids and rice bran oil. In Rice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–168. [Google Scholar]
- Zarei, I.; Luna, E.; Leach, J.E.; McClung, A.; Vilchez, S.; Koita, O.; Ryan, E.P. Comparative Rice Bran Metabolomics across Diverse Cultivars and Functional Rice Gene–Bran Metabolite Relationships. Metabolites 2018, 8, 63. [Google Scholar] [CrossRef]
- Goodyear, A.; Kumar, A.; Ehrhart, E.; Swanson, K.S.; Grusak, M.A.; Leach, J.E.; Dow, S.W.; McClung, A.; Ryan, E.P. Dietary rice bran supplementation prevents Salmonella colonization differentially across varieties and by priming intestinal immunity. J. Funct. Foods 2015, 18, 653–664. [Google Scholar] [CrossRef]
- Nealon, N.; Worcester, C.; Ryan, E.P. Lactobacillus paracaseimetabolism of rice bran reveals metabolome associated withSalmonellaTyphimurium growth reduction. J. Appl. Microbiol. 2017, 122, 1639–1656. [Google Scholar] [CrossRef]
- Zarei, I.; Oppel, R.C.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Modulation of plasma and urine metabolome in colorectal cancer survivors consuming rice bran. Integr. Food Nutr. Metab. 2019, 6. [Google Scholar] [CrossRef]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly)phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic Compounds and Bioactivities of Pigmented Rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Chakuton, K.; Puangpronp, D.; Nakornriab, M. Phytochemical Content and Antioxidant Activity of Colored and Non-colored Thai Rice Cultivars. Asian J. Plant. Sci. 2012, 11, 285–293. [Google Scholar] [CrossRef]
- Petroni, K.; Landoni, M.; Tomay, F.; Calvenzani, V.; Simonelli, C.; Cormegna, M. Proximate Composition, Polyphenol Content and Anti-inflammatory Properties of White and Pigmented Italian Rice Varieties. Univers. J. Agric. Res. 2017, 5, 312–321. [Google Scholar] [CrossRef]
- Hansakul, P.; Junmarkho, K. Thai pigmented rice bran extracts inhibit production of superoxide, nitric oxide radicals and inducible nitric oxide synthase in cellular models. Asian Pac. J. Trop. Biomed. 2019, 9, 291. [Google Scholar] [CrossRef]
- Sunthonkun, P.; Palajai, R.; Somboon, P.; Chua, L.S.; Ungsurangsri, M.; Soontorngun, N. Life-span extension by pigmented rice bran in the model yeast Saccharomyces cerevisiae. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Khammanit, R.; Lomarat, P.; Anantachoke, N.; Sato, V.H.; Ungsurungsie, M.; Mangmool, S. Inhibition of Oxidative Stress through the Induction of Antioxidant Enzymes of Pigmented Rice Bran in HEK-293 Cells. Nat. Prod. Commun. 2017, 12, 1934578X1701200727. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of extraction conditions on phenolic content, anthocyanin content and antioxidant activity of bran extracts from Thai rice cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative Evaluation of Different Extraction Techniques and Solvents for the Assay of Phytochemicals and Antioxidant Activity of Hashemi Rice Bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef]
- Ramon, B.M.R.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Cifuentes, A. Food analysis and Foodomics. J. Chromatogr. A 2009, 1216, 7109. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2011, 31, 49–69. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG). Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical Profiles of Black, Red, Brown, and White Rice from the Camargue Region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Hrazdina, G. Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry 1981, 20, 297–303. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Extraction solvents affecting phytochemicals in food colorant prepared from purple glutinous rice bran. Appl. Biol. Chem. 2017, 60, 181–189. [Google Scholar] [CrossRef]
- Gakh, A.A.; Anisimova, N.Y.; Kiselevsky, M.V.; Sadovnikov, S.V.; Stankov, I.N.; Yudin, M.V.; Rufanov, K.A.; Krasavin, M.Y.; Sosnov, A.V. Dihydro-resveratrol—A potent dietary polyphenol. Bioorg. Med. Chem. Lett. 2010, 20, 6149–6151. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Tang, B.; Peng, C.-Y.; Darko, K.O.; Tao, T.; Huang, Y.; Su, Q.; Yang, X. Paeonol, a Powerful Natural Product with Broad Biological Spectra by Inhibiting Inflammatory Pathway. Curr. Tradit. Med. 2016, 2, 72–79. [Google Scholar] [CrossRef]
- Park, B.-Y.; Oh, S.-R.; Ahn, K.-S.; Kwon, O.-K.; Lee, H.-K. (–)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharmacol. 2008, 8, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Janvier, M.; Hollande, L.; Jaufurally, A.S.; Pernes, M.; Ménard, R.; Grimaldi, M.; Beaugrand, J.; Balaguer, P.; Ducrot, P.-H.; Allais, F. Syringaresinol: A Renewable and Safer Alternative to Bisphenol A for Epoxy-Amine Resins. ChemSusChem 2017, 10, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Koffas, M.A. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017, 2, 259–266. [Google Scholar] [CrossRef]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- Li, C.-Z.; Jin, H.-H.; Sun, H.-X.; Zhang, Z.-Z.; Zheng, J.-X.; Li, S.-H.; Han, S.-H. Eriodictyol attenuates cisplatin-induced kidney injury by inhibiting oxidative stress and inflammation. Eur. J. Pharmacol. 2016, 772, 124–130. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K.; Hussan, K.P.S. Chapter 5—Structural Evaluation and Toxicological Study of a Bitter Masking Bioactive Flavanone, ‘Eriodictyol’. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–60. [Google Scholar]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef]
- Irakli, M.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Simultaneous determination of phenolic acids and flavonoids in rice using solid-phase extraction and RP-HPLC with photodiode array detection. J. Sep. Sci. 2012, 35, 1603–1611. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Factors influencing antioxidant compounds in rice. Crit. Rev. Food Sci. Nutr. 2016, 57, 893–922. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ -oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Sabir, A.; Rafi, M.; Darusman, L.K. Discrimination of red and white rice bran from Indonesia using HPLC fingerprint analysis combined with chemometrics. Food Chem. 2017, 221, 1717–1722. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; McClung, A.M.; Chen, M.-H. Phytochemicals and Antioxidant Capacities in Rice Brans of Different Color. J. Food Sci. 2010, 76, C117–C126. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Lima, L.R.D.S.; Nascimento, F.R.; Nascimento, T.P.D.; Cameron, L.C.; Ferreira, M.S.L. Metabolomic approach for characterization of phenolic compounds in different wheat genotypes during grain development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]



| Putative Compound | [M – H]− | RT (min) | Molecular Formula | Score | FS | Fragments /Intensity | ME | IS | Black Rice Bran | Red Rice Bran | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | DES1 | DES2 | CS | DES1 | DES2 | ||||||||||
| 1 | Gallic acid * | 169.0131 | 1.26 | C7H6O5 | 37.3 | 0 | Nd | −6.89 | 94.40 | ||||||
| 2 | Dihydroxybenzoic acid isomer I * | 153.0181 | 1.41 | C7H6O4 | 37.1 | 0 | Nd | −8.25 | 94.68 | ||||||
| 3 | 4-Hydroxymandelic acid/Vanillic acid isomer I | 167.0334 | 1.46 | C8H8O4 | 36.1 | 0 | Nd | −9.59 | 90.85 | ||||||
| 4 | Dihydroxybenzoic acid isomer II | 153.0180 | 1.48 | C7H6O4 | 37.1 | 0 | Nd | −8.46 | 94.67 | ||||||
| 5 | Caffeoylquinic acid isomer I | 353.0863 | 1.54 | C16H18O9 | 35.5 | 0 | Nd | −4.19 | 82.23 | ||||||
| 6 | 4-Hydroxymandelic acid/Vanillic acid isomer II | 167.0337 | 1.64 | C8H8O4 | 37.8 | 0 | Nd | −7.85 | 97.57 | ||||||
| 7 | Dihydroxybenzoic acid isomer III | 153.0179 | 1.64 | C7H6O4 | 36.7 | 0 | Nd | −9.25 | 93.70 | ||||||
| 8 | Apigenin 7-O-glucoside | 419.1351 | 1.73 | C21H24O9 | 36.2 | 0 | Nd | 0.77 | 81.93 | ||||||
| 9 | Isorhamnetin/Rhamnetin/Nepetin | 315.0498 | 1.74 | C16H12O7 | 37.7 | 0 | Nd | −3.95 | 93.23 | ||||||
| 10 | Irilone | 297.0392 | 1.75 | C16H10O6 | 35.6 | 0 | Nd | −4.20 | 82.89 | ||||||
| 11 | Dihydro-p-coumaric acid/Methoxyphenylacetic acid | 165.0545 | 1.81 | C9H10O3 | 36.3 | 0 | Nd | −7.41 | 90.07 | ||||||
| 12 | Dihydroxybenzoic acid isomer IV | 153.0180 | 1.85 | C7H6O4 | 38 | 0 | Nd | −8.46 | 99.40 | ||||||
| 13 | Eriodictyol isomer I | 287.0567 | 1.90 | C15H12O6 | 38.6 | 11.7 | 165.0543 (19%) | 2.05 | 83.77 | ||||||
| 14 | Esculetin | 177.0180 | 1.91 | C9H6O4 | 37.4 | 0 | Nd | −7.54 | 95.53 | ||||||
| 15 | Quercetin 3-O-glucoside isomer I | 463.0870 | 1.94 | C21H20O12 | 37.4 | 0 | Nd | −2.69 | 90.16 | ||||||
| 16 | Syringic acid/Gallic acid ethyl ester/3,4-Dihydroxyphenyllactic acid | 197.0444 | 2.09 | C9H10O5 | 36.5 | 0 | Nd | −6.00 | 89.58 | ||||||
| 17 | Homovanillic acid/Dihydrocaffeic acid | 181.0494 | 2.13 | C9H10O4 | 37.6 | 0 | Nd | −6.85 | 95.91 | ||||||
| 18 | Myricetin | 317.0290 | 2.16 | C15H10O8 | 38.9 | 5.13 | 124.0146 (17%), 123.0076 (11%) | −4.04 | 94.33 | ||||||
| 19 | Coumaroylquinic acid isomer I | 337.0916 | 2.27 | C16H18O8 | 43.2 | 20.7 | 119.0488 (100%), 191.0549 (5%), 20111.0434 (2%) | −3.70 | 99.55 | ||||||
| 20 | 4-Hydroxymandelic acid/Vanillic acid isomer III | 167.0335 | 2.29 | C8H8O4 | 36.2 | 0 | Nd | −9.01 | 90.85 | ||||||
| 21 | Cyanidin 3-O-beta-D-sambubioside | 580.1488 | 2.38 | C26H29O15+ | 36.2 | 0.631 | 115.0401 (2%) | 9.41 | 90.56 | ||||||
| 22 | Methylgallic acid | 183.0289 | 2.42 | C8H8O5 | 38.6 | 0 | Nd | −5.28 | 98.86 | ||||||
| 23 | Feruloylquinic acid isomer I | 367.1023 | 2.45 | C17H20O9 | 57.3 | 91 | 134.0359 (100%), 193.0494 (29%), 200.0442 (25%), 117.0333 (14%), 123.0436 (7%), 155.0335 (2%) | −3.18 | 99.18 | ||||||
| 24 | Dihydroresveratrol | 229.0878 | 2.54 | C14H14O3 | 36.2 | 0 | Nd | 3.43 | 85.26 | ||||||
| 25 | Scopoletin | 191.0332 | 2.54 | C10H8O4 | 37 | 0 | Nd | −9.04 | 95.14 | ||||||
| 26 | 4-Hydroxymandelic acid/Vanillic acid isomer IV | 167.0337 | 2.56 | C8H8O4 | 36.5 | 0 | Nd | −7.53 | 90.85 | ||||||
| 27 | 4′-O-Methylepigallocatechin | 319.0809 | 2.58 | C16H16O7 | 35.5 | 0 | Nd | −4.54 | 82.64 | ||||||
| 28 | Feruloyl glucose | 355.1016 | 2.58 | C16H20O9 | 40.7 | 15.9 | 177.0545 (41%) | −5.08 | 93.62 | ||||||
| 29 | Bergapten/Xanthoxin | 215.0335 | 2.60 | C12H8O4 | 44.9 | 36.8 | 191.0333 (100%) | −6.78 | 95.46 | ||||||
| 30 | Psoralen | 185.0233 | 2.60 | C11H6O3 | 36.2 | 0 | Nd | −6.24 | 88.21 | ||||||
| 31 | (+)-Catechin * | 289.0705 | 2.67 | C15H14O6 | 35.7 | 0 | Nd | −4.38 | 83.74 | ||||||
| 32 | Kaempferide | 298.0465 | 2.69 | C16H11O6- | 39.4 | 20.9 | 175.0388 (76%), 134.0360 (27%), 193.0127 (13%), 117.0330 (1%) | −6.01 | 82.88 | ||||||
| 33 | Caffeic acid * | 179.0335 | 2.72 | C9H8O4 | 38 | 0 | Nd | −7.97 | 98.69 | ||||||
| 34 | Trihydroxyisoflavone | 269.0443 | 2.73 | C15H10O5 | 38.6 | 0 | Nd | −4.63 | 98.27 | ||||||
| 35 | Isorhamnetin 3-O-glucoside/Isorhamnetin 3-O-galactoside | 477.1021 | 2.75 | C22H22O12 | 40.8 | 16.2 | 429.0818 (1%), 59.0113 (1%) | −3.68 | 92.19 | ||||||
| 36 | Hydroxymatairesinol isomer I | 373.1303 | 2.77 | C20H22O7 | 43.2 | 28.9 | 205.0494 (100%),223.0601 (62%), 179.0700 (12%), 221.0805 (6%), 181.0491 (4%), 193.0854 (1%), 105.0331 (1%) | 2.67 | 90.02 | ||||||
| 37 | Syringaresinol isomer I | 417.1560 | 2.80 | C22H26O8 | 37.7 | 0 | Nd | 1.17 | 89.93 | ||||||
| 38 | Dicaffeoylquinic acid | 515.1221 | 2.83 | C25H24O12 | 38.4 | 5.72 | 307.0909 (3%) | 4.94 | 92.15 | ||||||
| 39 | Coumaroylquinic acid isomer II | 337.0917 | 2.84 | C16H18O8 | 45.2 | 32.1 | 245.0803 (50%), 119.0486 (15%), 93.0327 (13%), 243.0651 (11%) | −3.56 | 98.01 | ||||||
| 40 | (-)-Epicatechin | 289.0700 | 2.84 | C15H14O6 | 46.9 | 48.8 | 257.0438 (100%), 243.0651 (11%) | −6.23 | 92.53 | ||||||
| 41 | 4-Hydroxymandelic acid/Vanillic acid isomer V | 167.0337 | 2.90 | C8H8O4 | 51.6 | 75.8 | 151.0385 (100%), 123.0437 (14%), 135.0435 (4%), 105.0332 (1%) | −7.79 | 90.85 | ||||||
| 42 | 3,4-Dihydroxyphenyllactic acid | 197.0441 | 2.98 | C9H10O5 | 44.2 | 34.6 | 134.0357 (90%) | −7.18 | 94.67 | ||||||
| 43 | Feruloylquinic acid isomer II | 367.1023 | 2.99 | C17H20O9 | 42.7 | 17.5 | 134.0357 (100%), 173.0443 (76%), 191.0546 (31%), 117.0330 (10%), 111.0436 (10%), 155.0332 (7%), 75.0065 (5%) | −3.03 | 99.41 | ||||||
| 44 | Rosmarinic acid | 359.0775 | 3.02 | C18H16O8 | 35.9 | 0 | Nd | 0.85 | 80.69 | ||||||
| 45 | Quercetin 3-O-rutinoside/Kaempferol 3-O-sophoroside/Quercetin 3-O-rhamnosyl-galactoside/Kaempferol 3,7-O-diglucoside isomer I | 609.1446 | 3.21 | C27H30O16 | 38 | 0 | Nd | −2.44 | 92.86 | ||||||
| 46 | Tetrahydroxyisoflavone isomer I | 285.0391 | 3.27 | C15H10O6 | 47.1 | 52.9 | 151.0386 (100%) | −4.70 | 88.27 | ||||||
| 47 | Eriodictyol 7-O-glucoside | 449.1075 | 3.30 | C21H22O11 | 45.7 | 34.3 | 103.0386 (100%), 181.0498 (22%), 122.0359 (16%), 311.0760 (10%), 99.0075 (7%) | −3.24 | 97.82 | ||||||
| 48 | Dihydroxybenzoic acid isomer V | 153.0181 | 3.31 | C7H6O4 | 38 | 0 | Nd | −8.22 | 99.29 | ||||||
| 49 | Quercetin 3-O-rutinoside/Kaempferol 3-O-sophoroside/Quercetin 3-O-rhamnosyl-galactoside/Kaempferol 3,7-O-diglucoside isomer II | 609.1454 | 3.38 | C27H30O16 | 42.7 | 16.9 | 300.0264 (8%) | −1.16 | 98.00 | ||||||
| 50 | Luteolin 7-O-rutinoside/Kaempferol 3-O-rutinoside/Chrysoeriol 7-O-apiosyl-glucoside | 593.1495 | 3.39 | C27H30O15 | 37.1 | 1.66 | 103.0387 (7%), 175.0600 (2%) | −2.81 | 87.33 | ||||||
| 51 | Didymin/Poncirin | 593.1884 | 3.40 | C28H34O14 | 36.7 | 2.47 | 103.0387 (100%), 175.0600 (23%) | 1.41 | 82.63 | ||||||
| 52 | Salvianolic acid D | 237.0395 | 3.44 | C11H10O6 | 38.2 | 0 | Nd | −3.88 | 95.39 | ||||||
| 53 | p-coumaric acid * | 163.0389 | 3.47 | C9H8O3 | 40.8 | 12.3 | 163.0388 (28%), 119.0488 (21%) | −6.97 | 99.39 | ||||||
| 54 | Phloridzin | 435.1277 | 3.48 | C21H24O10 | 37.2 | 7.68 | 103.0387 (100%) | −4.47 | 83.45 | ||||||
| 55 | Schisandrin B | 399.1835 | 3.51 | C23H28O6 | 37.3 | 0 | Nd | 5.39 | 92.86 | ||||||
| 56 | Tectoridin | 461.1080 | 3.54 | C22H22O11 | 41.8 | 16.4 | 341.0654 (4%), 146.0341 (1%) | −2.00 | 95.23 | ||||||
| 57 | Glycitin | 445.1129 | 3.59 | C22H22O10 | 44.3 | 29.7 | 326.0777 (29%), 283.0593 (22%), 379.0769 (16%) | −2.51 | 94.59 | ||||||
| 58 | Isorhamnetin 3-O-rutinoside | 461.1067 | 3.63 | C22H22O11 | 50.6 | 69.8 | 324.0255 (100%), 279.0288 (29%), 99.0070 (7%), 73.0274 (5%) | −4.82 | 88.73 | ||||||
| 59 | Ferulic acid * | 193.0495 | 3.64 | C10H10O4 | 40 | 10.6 | 137.0590 (13%) | −5.68 | 96.05 | ||||||
| 60 | Paeoniflorin | 479.1549 | 3.66 | C23H28O11 | 38.1 | 0 | Nd | −2.09 | 92.84 | ||||||
| 61 | Tetrahydroxyisoflavone isomer II | 285.0392 | 3.73 | C15H10O6 | 39.8 | 5.06 | 117.0331 (9%), 105.0330 (4%), 132.0206 (3%) | −4.38 | 99.00 | ||||||
| 62 | Violanone | 315.0860 | 3.75 | C17H16O6 | 43.2 | 23.3 | 165.0543 (32%) | −4.45 | 98.07 | ||||||
| 63 | 3,7-Dimethylquercetin/Jaceosidin/Tricin isomer I | 329.0654 | 3.80 | C17H14O7 | 37.4 | 0 | Nd | −3.97 | 91.62 | ||||||
| 64 | Diosmin | 607.1656 | 3.81 | C28H32O15 | 38.6 | 1.83 | 89.0229 (100%) | −2.04 | 93.78 | ||||||
| 65 | Tetrahydroxyisoflavone isomer III | 285.0393 | 3.88 | C15H10O6 | 38.4 | 0.795 | 123.0074 (1%) | −4.03 | 95.99 | ||||||
| 66 | Schisantherin A | 535.2012 | 3.90 | C30H32O9 | 34.9 | 0.218 | 191.0701 (1%) | 7.14 | 82.26 | ||||||
| 67 | Gardenin B | 357.0968 | 3.91 | C19H18O7 | 47.4 | 51.3 | 209.0445 (100%), 315.0861 (45%), 239.0552 (32%), 327.0860 (22%), 345.0952 (17%), 251.0550 (13%), 177.0547 (14%), 181.0491 (12%) | −3.24 | 89.72 | ||||||
| 68 | Hesperidin | 609.1820 | 3.93 | C28H34O15 | 36 | 0.274 | 161.0596 (4%) | −0.78 | 80.56 | ||||||
| 69 | Syringaldehyde | 181.0495 | 4.29 | C9H10O4 | 38.4 | 0 | Nd | −6.18 | 99.23 | ||||||
| 70 | Eriodictyol isomer II | 287.0549 | 4.34 | C15H12O6 | 38.1 | 3.22 | 147.0071 (16%), 119.0123 (12%), 123.0075 (2%) | −4.19 | 92.29 | ||||||
| 71 | 3,7-Dimethylquercetin/Jaceosidin/Tricin isomer II | 329.0655 | 4.38 | C17H14O7 | 38.3 | 5.77 | 122.0355 (3%), 146.0350 (3%) | −3.70 | 90.18 | ||||||
| 72 | Nobiletin | 401.1229 | 4.52 | C21H22O8 | 37.4 | 0 | Nd | −3.14 | 90.76 | ||||||
| 73 | Hydroxymatairesinol isomer II | 373.1281 | 4.62 | C20H22O7 | 38.1 | 2.26 | 146.0343 (10%) | −3.23 | 91.85 | ||||||
| 74 | Syringaresinol isomer II | 417.1560 | 4.74 | C22H26O8 | 36.6 | 0 | Nd | 1.32 | 84.73 | ||||||
| 75 | Isorhamnetin isomer I | 315.0495 | 4.79 | C16H12O7 | 49.4 | 59.6 | 175.3038 (100%), 160.0152 (20%) | −4.75 | 92.69 | ||||||
| 76 | Tetrahydroxyisoflavone isomer V | 285.0393 | 5.01 | C15H10O6 | 42.7 | 21.6 | 133.0280 (100%), 132.0207 (9%), 179.0343 (4%) | −4.21 | 96.95 | ||||||
| 77 | 3,7-Dimethylquercetin/Jaceosidin/Tricin isomer II | 329.0648 | 5.04 | C17H14O7 | 40 | 10.8 | 121.0281 (35%), 139.0387 (4%), 147.0434 (3%), 119.0121 (2%) | −5.78 | 96.01 | ||||||
| 78 | Quercetin | 301.0342 | 5.06 | C15H10O7 | 49.6 | 53.2 | 151.0023 (100%), 121.0181 (35%), 178.9973 (19%) | −3.80 | 99.43 | ||||||
| 79 | Dihydroquercetin * | 303.0499 | 5.16 | C15H12O7 | 38.2 | 0 | Nd | −3.67 | 95.13 | ||||||
| 80 | Trihydroxyisoflavanone isomer II | 271.0595 | 5.38 | C15H12O5 | 37.4 | 0 | Nd | −6.09 | 94.02 | ||||||
| 81 | Isorhamnetin isomer II | 315.0498 | 5.56 | C16H12O7 | 40.1 | 6.44 | 117.0334 (13%) | −3.73 | 98.49 | ||||||
| 82 | 6-Hydroxyluteolin/Morin | 301.0338 | 5.56 | C15H10O7 | 37.5 | 0 | Nd | −5.33 | 93.63 | ||||||
| 83 | Urolithin A | 227.0337 | 5.56 | C13H8O4 | 39.8 | 19 | 183.0435 (100%), 182.0360 (24%), 167.0486 (7%) | −5.42 | 86.04 | ||||||
| 84 | Trihydroxyisoflavanone isomer III | 271.0598 | 5.64 | C15H12O5 | 40 | 9.09 | 119.0487 (100%) | −4.98 | 96.67 | ||||||
| 85 | Hesperetin/Homoeriodictyol | 301.0704 | 5.73 | C16H14O6 | 42.8 | 21.1 | 193.0492 (23%), 134.0358 (19%), 164.0097 (7%), 149.0591 (6%) | −4.41 | 97.88 | ||||||
| 86 | Dihydroxykaempferol | 317.0290 | 5.75 | C15H10O8 | 42 | 23.2 | 271.0234 (100%), 107.0124 (11%) | −4.10 | 91.65 | ||||||
| 87 | Hispidulin | 299.0545 | 5.80 | C16H12O6 | 37.6 | 0 | Nd | −5.51 | 94.43 | ||||||
| 88 | Paeonol | 165.0545 | 6.71 | C9H10O3 | 36.4 | 0 | Nd | −7.14 | 90.07 | ||||||
| 89 | Rosmanol | 345.1688 | 6.72 | C20H26O5 | 37.4 | 0 | Nd | −5.74 | 93.66 | ||||||
| Number of identifications | # 79 | # 49 | # 58 | # 67 | # 16 | # 43 | |||||||||
 | |||||||||||||||
| Name | # 1 | # 2 | # 3 | Molar Ratio | aW | Water Content | pH |
|---|---|---|---|---|---|---|---|
| DES1 | Choline chloride | 1.2-propanediol | Water | 1:1:1 | 0.51 ± 0.03 | 7.2 ± 0.5 | 5.42 |
| DES2 | Choline chloride | Lactic acid | - | 1:10 | 0.29 ± 0.00 | 6.3 ± 0.25 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.C.B.; Barouh, N.; Durand, E.; Baréa, B.; Robert, M.; Micard, V.; Lullien-Pellerin, V.; Villeneuve, P.; Cameron, L.C.; Ryan, E.P.; et al. Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition. Metabolites 2021, 11, 110. https://doi.org/10.3390/metabo11020110
Santos MCB, Barouh N, Durand E, Baréa B, Robert M, Micard V, Lullien-Pellerin V, Villeneuve P, Cameron LC, Ryan EP, et al. Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition. Metabolites. 2021; 11(2):110. https://doi.org/10.3390/metabo11020110
Chicago/Turabian StyleSantos, Millena Cristina Barros, Nathalie Barouh, Erwann Durand, Bruno Baréa, Mélina Robert, Valérie Micard, Valérie Lullien-Pellerin, Pierre Villeneuve, Luiz Claudio Cameron, Elizabeth P. Ryan, and et al. 2021. "Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition" Metabolites 11, no. 2: 110. https://doi.org/10.3390/metabo11020110
APA StyleSantos, M. C. B., Barouh, N., Durand, E., Baréa, B., Robert, M., Micard, V., Lullien-Pellerin, V., Villeneuve, P., Cameron, L. C., Ryan, E. P., Ferreira, M. S. L., & Bourlieu-Lacanal, C. (2021). Metabolomics of Pigmented Rice Coproducts Applying Conventional or Deep Eutectic Extraction Solvents Reveal a Potential Antioxidant Source for Human Nutrition. Metabolites, 11(2), 110. https://doi.org/10.3390/metabo11020110









