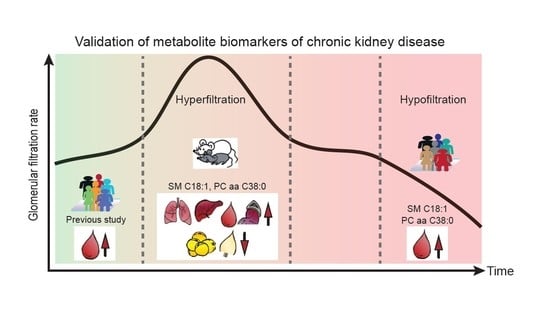
Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient db/db Mouse
Abstract
:1. Introduction
2. Results
2.1. Associations of the Two Metabolites with eGFR and CKD in Hyperglycemic Individuals
2.1.1. Characteristics of the KORA FF4 Study Participants
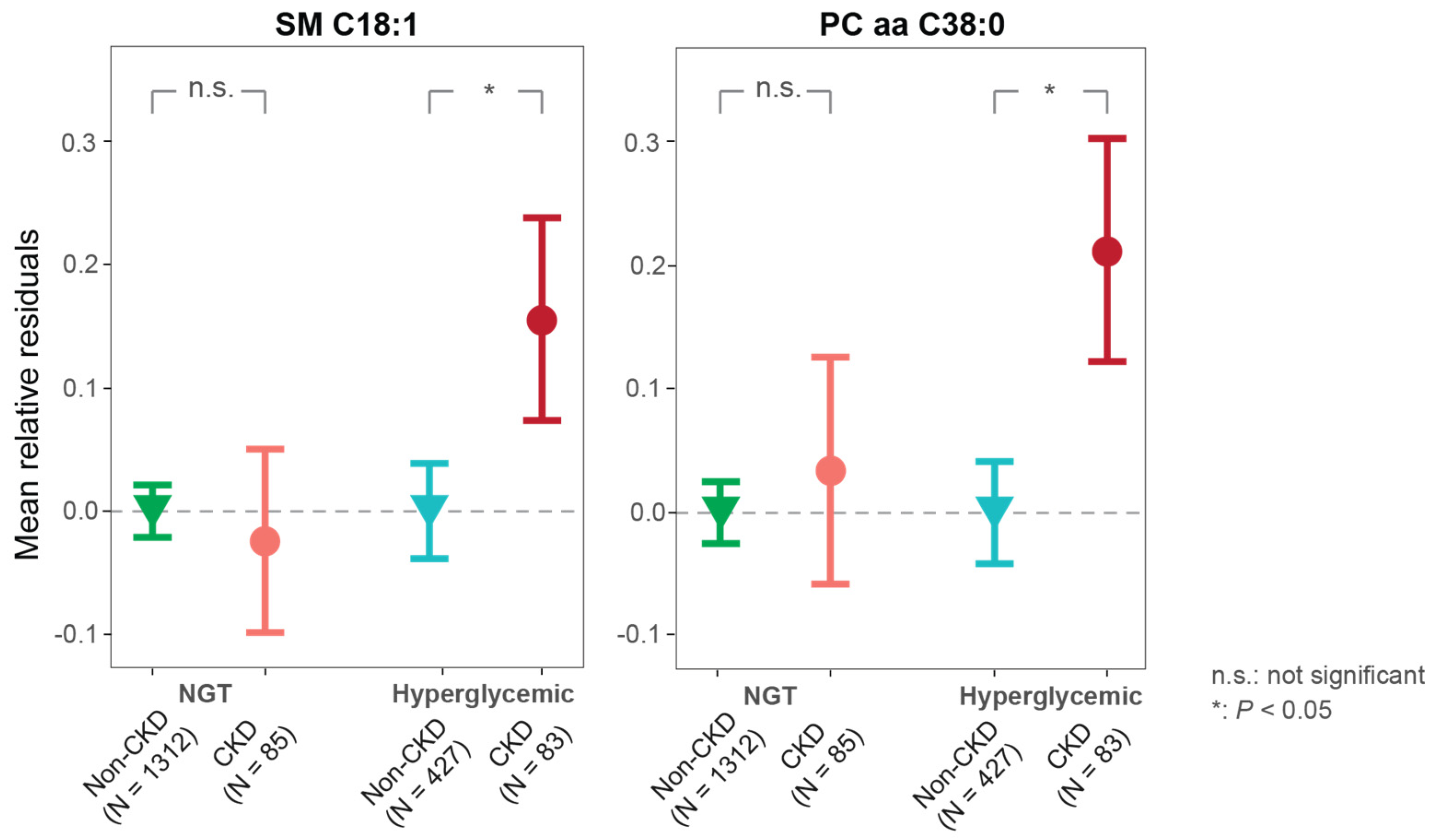
2.1.2. Inverse Associations of the Two Metabolites with eGFR in Hyperglycemic Individuals
2.1.3. Associations of the Two Metabolites with CKD Are Specific for Hyperglycemia
2.2. Organ-Specific Trends of the Candidate Biomarkers in Diabetic Mice
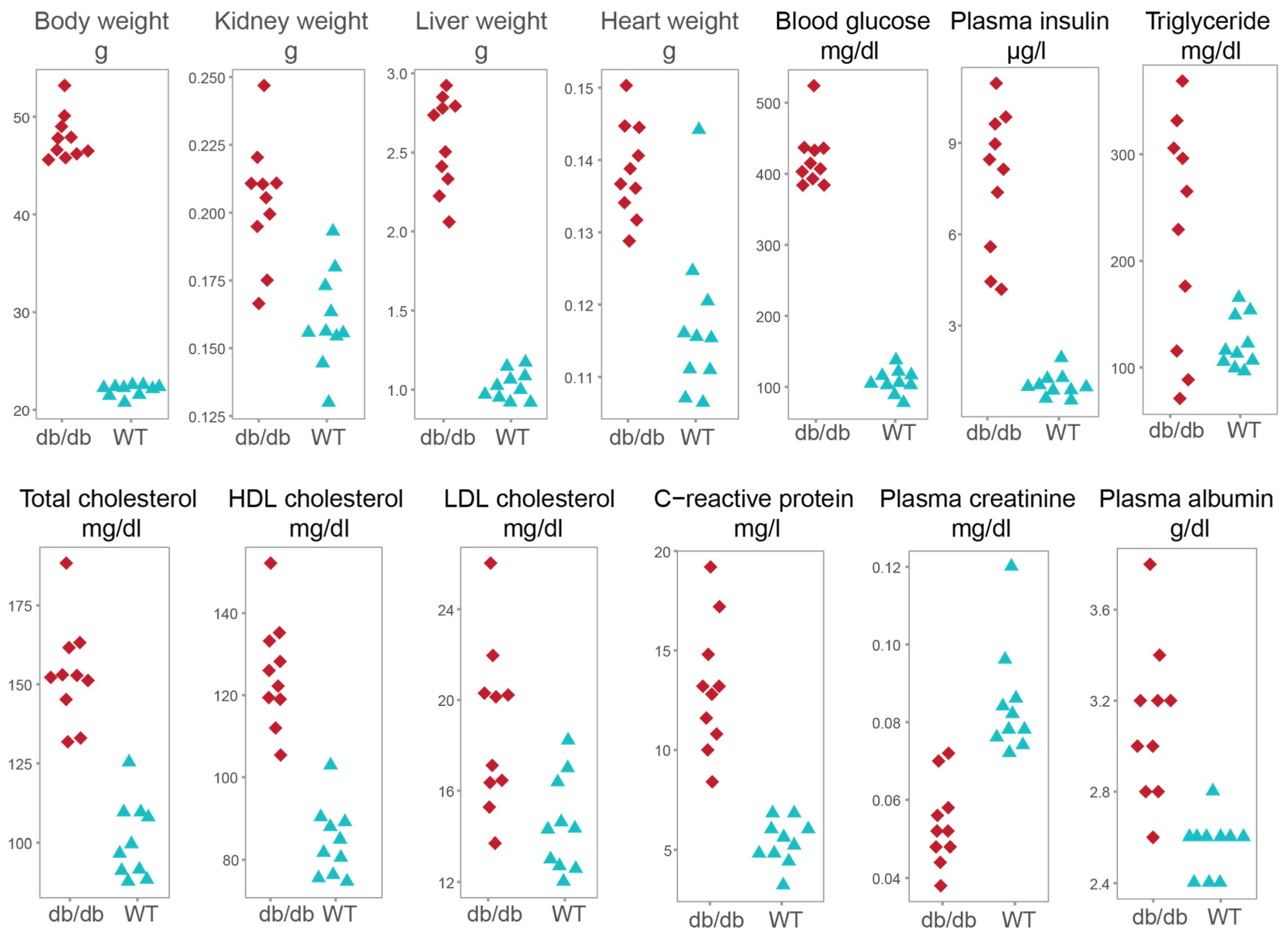
2.2.1. Characteristics of the Mouse Model
2.2.2. Analysis of Creatinine in Eight Murine Tissues
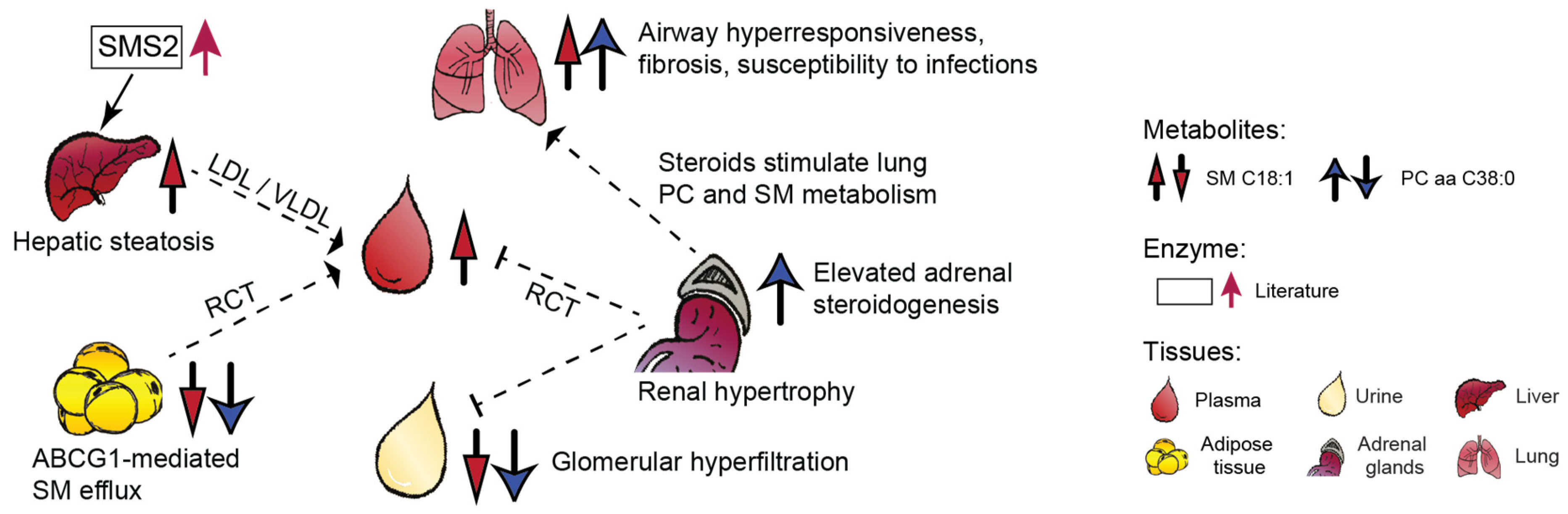
2.2.3. Organ-Specific Trends of the Two Metabolites
3. Discussion
4. Materials and Methods
4.1. Study Participants, Outcome Definition
4.2. Mouse Study
4.3. Metabolite Quantification and Normalization
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alicic, R.Z.; Neumiller, J.J.; Johnson, E.J.; Dieter, B.; Tuttle, K.R. Sodium-Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes 2019, 68, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD Chronic Kidney Disease Collaboration; Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Manns, B.; Hemmelgarn, B.; Tonelli, M.; Au, F.; Chiasson, T.C.; Dong, J.; Klarenbach, S.; Alberta Kidney Disease, N. Population based screening for chronic kidney disease: Cost effectiveness study. BMJ 2010, 341, c5869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkler, D.; Gao, P.; Lee, S.F.; Heinze, G.; Clase, C.M.; Tobe, S.; Teo, K.K.; Gerstein, H.; Mann, J.F.; Oberbauer, R.; et al. Risk Prediction for Early CKD in Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2015, 10, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; Hrabe de Angelis, M.; Kronenberg, F.; Meitinger, T.; Mewes, H.W.; Wichmann, H.E.; Weinberger, K.M.; Adamski, J.; et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef] [Green Version]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wagele, B.; Altmaier, E.; CardioGram; Deloukas, P.; Erdmann, J.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Huang, J.; Huth, C.; Covic, M.; Troll, M.; Adam, J.; Zukunft, S.; Prehn, C.; Wang, L.; Nano, J.; Scheerer, M.F.; et al. Machine Learning Approaches Reveal Metabolic Signatures of Incident Chronic Kidney Disease in Individuals with Prediabetes and Type 2 Diabetes. Diabetes 2020, 69, 2756–2765. [Google Scholar] [CrossRef]
- Fornoni, A.; Merscher, S.; Kopp, J.B. Lipid biology of the podocyte--new perspectives offer new opportunities. Nat. Rev. Nephrol. 2014, 10, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Russo, S.B.; Ross, J.S.; Cowart, L.A. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb. Exp. Pharmacol. 2013, 373–401. [Google Scholar] [CrossRef] [Green Version]
- Lisowska-Myjak, B. Uremic toxins and their effects on multiple organ systems. Nephron Clin. Pract. 2014, 128, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol Renal Physiol 2003, 284, F1138–F1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Hyeon, J.S.; Kim, N.H.; Cho, A.; Lee, G.; Jang, S.Y.; Kim, M.K.; Lee, E.Y.; Chung, C.H.; Ha, H.; et al. Metabolic changes in urine and serum during progression of diabetic kidney disease in a mouse model. Arch. Biochem. Biophys. 2018, 646, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Maeshima, Y.; Kitayama, H.; Kitamura, S.; Takazawa, Y.; Sugiyama, H.; Yamasaki, Y.; Makino, H. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 2004, 53, 1831–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingel-Ristić, V.; Schrijvers, B.F.; van Vliet, A.K.; Rasch, R.; Han, V.K.; Drop, S.L.; Flyvbjerg, A. Kidney growth in normal and diabetic mice is not affected by human insulin-like growth factor binding protein-1 administration. Exp. Biol. Med. (Maywood) 2005, 230, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.P.; Lautenslager, G.T.; Shearman, C.W. Increased urinary type IV collagen marks the development of glomerular pathology in diabetic d/db mice. Metabolism 2001, 50, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Trak-Smayra, V.; Paradis, V.; Massart, J.; Nasser, S.; Jebara, V.; Fromenty, B. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int. J. Exp. Pathol. 2011, 92, 413–421. [Google Scholar] [CrossRef]
- Hofmann, A.; Peitzsch, M.; Brunssen, C.; Mittag, J.; Jannasch, A.; Frenzel, A.; Brown, N.; Weldon, S.M.; Eisenhofer, G.; Bornstein, S.R.; et al. Elevated Steroid Hormone Production in the db/db Mouse Model of Obesity and Type 2 Diabetes. Horm. Metab. Res. 2017, 49, 43–49. [Google Scholar] [CrossRef]
- Chocian, G.; Chabowski, A.; Zendzian-Piotrowska, M.; Harasim, E.; Łukaszuk, B.; Górski, J. High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol. Cell Biochem. 2010, 340, 125–131. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigruener, A.; Kleber, M.E.; Heimerl, S.; Liebisch, G.; Schmitz, G.; Maerz, W. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE 2014, 9, e85724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofte, N.; Suvitaival, T.; Trost, K.; Mattila, I.M.; Theilade, S.; Winther, S.A.; Ahluwalia, T.S.; Frimodt-Moller, M.; Legido-Quigley, C.; Rossing, P. Metabolomic Assessment Reveals Alteration in Polyols and Branched Chain Amino Acids Associated With Present and Future Renal Impairment in a Discovery Cohort of 637 Persons with Type 1 Diabetes. Front. Endocrinol. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferre, M.; et al. Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floegel, A.; Kuhn, T.; Sookthai, D.; Johnson, T.; Prehn, C.; Rolle-Kampczyk, U.; Otto, W.; Weikert, C.; Illig, T.; von Bergen, M.; et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: A targeted metabolomic approach in two German prospective cohorts. Eur. J. Epidemiol. 2018, 33, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagnac, A.; Zingerman, B.; Rozen-Zvi, B.; Herman-Edelstein, M. Consequences of Glomerular Hyperfiltration: The Role of Physical Forces in the Pathogenesis of Chronic Kidney Disease in Diabetes and Obesity. Nephron 2019, 143, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Gartner, K. Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db). Diabetologia 1978, 15, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campion, C.G.; Sanchez-Ferras, O.; Batchu, S.N. Potential Role of Serum and Urinary Biomarkers in Diagnosis and Prognosis of Diabetic Nephropathy. Can. J. Kidney Health Dis. 2017, 4, 2054358117705371. [Google Scholar] [CrossRef]
- Ostler, J.E.; Maurya, S.K.; Dials, J.; Roof, S.R.; Devor, S.T.; Ziolo, M.T.; Periasamy, M. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E592–E605. [Google Scholar] [CrossRef] [Green Version]
- Kashima, S.; Inoue, K.; Matsumoto, M.; Akimoto, K. Low serum creatinine is a type 2 diabetes risk factor in men and women: The Yuport Health Checkup Center cohort study. Diabetes Metab. 2017, 43, 460–464. [Google Scholar] [CrossRef]
- Harita, N.; Hayashi, T.; Sato, K.K.; Nakamura, Y.; Yoneda, T.; Endo, G.; Kambe, H. Lower serum creatinine is a new risk factor of type 2 diabetes: The Kansai healthcare study. Diabetes Care 2009, 32, 424–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallman, M.; Spragg, R.; Harrell, J.H.; Moser, K.M.; Gluck, L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J. Clin. Invest. 1982, 70, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Papinska, A.M.; Soto, M.; Meeks, C.J.; Rodgers, K.E. Long-term administration of angiotensin (1-7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol. Res. 2016, 107, 372–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.L.; Johnston, R.A.; Flynt, L.; Theman, T.A.; Terry, R.D.; Schwartzman, I.N.; Lee, A.; Shore, S.A. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L856–L865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, S.; Yeang, C.; Wadgaonkar, S.; Anjum, F.; Grinkina, N.; Cutaia, M.; Jiang, X.C.; Wadgaonkar, R. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L430–L440. [Google Scholar] [CrossRef] [Green Version]
- Mukai, H.; Ming, P.; Lindholm, B.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Lung Dysfunction and Mortality in Patients with Chronic Kidney Disease. Kidney Blood Press Res. 2018, 43, 522–535. [Google Scholar] [CrossRef]
- Kolahian, S.; Leiss, V.; Nürnberg, B. Diabetic lung disease: Fact or fiction? Rev. Endocr. Metab. Disord. 2019, 20, 303–319. [Google Scholar] [CrossRef]
- Giesbertz, P.; Padberg, I.; Rein, D.; Ecker, J.; Höfle, A.S.; Spanier, B.; Daniel, H. Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia 2015, 58, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Dahik, V.D.; Frisdal, E.; Le Goff, W. Rewiring of Lipid Metabolism in Adipose Tissue Macrophages in Obesity: Impact on Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5505. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802. [Google Scholar] [CrossRef] [Green Version]
- Edgel, K.A.; McMillen, T.S.; Wei, H.; Pamir, N.; Houston, B.A.; Caldwell, M.T.; Mai, P.O.; Oram, J.F.; Tang, C.; Leboeuf, R.C. Obesity and weight loss result in increased adipose tissue ABCG1 expression in db/db mice. Biochim. Biophys. Acta 2012, 1821, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dong, J.; Ding, T.; Kuo, M.S.; Cao, G.; Jiang, X.C.; Li, Z. Sphingomyelin synthase 2 activity and liver steatosis: An effect of ceramide-mediated peroxisome proliferator-activated receptor gamma2 suppression. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, H.; Li, Z.; Hailemariam, T.K.; Chakraborty, M.; Jiang, K.; Qiu, D.; Bui, H.H.; Peake, D.A.; Kuo, M.S.; et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 850–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsutake, S.; Zama, K.; Yokota, H.; Yoshida, T.; Tanaka, M.; Mitsui, M.; Ikawa, M.; Okabe, M.; Tanaka, Y.; Yamashita, T.; et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 2011, 286, 28544–28555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, H.; Liu, J.; Liang, C.P.; Li, Y.; Li, Y.; Teitelman, G.; Beyer, T.; Bui, H.H.; Peake, D.A.; et al. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell Biol. 2011, 31, 4205–4218. [Google Scholar] [CrossRef] [Green Version]
- Igal, R.A.; Mandon, E.C.; de Gómez Dumm, I.N. Abnormal metabolism of polyunsaturated fatty acids in adrenal glands of diabetic rats. Mol. Cell Endocrinol. 1991, 77, 217–227. [Google Scholar] [CrossRef]
- Gross, I.; Ballard, P.L.; Ballard, R.A.; Jones, C.T.; Wilson, C.M. Corticosteroid stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung: Evidence for de novo protein synthesis mediated by glucocorticoid receptors. Endocrinology 1983, 112, 829–837. [Google Scholar] [CrossRef]
- Decleves, A.E.; Zolkipli, Z.; Satriano, J.; Wang, L.; Nakayama, T.; Rogac, M.; Le, T.P.; Nortier, J.L.; Farquhar, M.G.; Naviaux, R.K.; et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 2014, 85, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Hsu, C.C.; Hamm, G.; Darshi, M.; Diamond-Stanic, M.; Declèves, A.E.; Slater, L.; Pennathur, S.; Stauber, J.; Dorrestein, P.C.; et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine 2016, 7, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Soler, M.J.; Riera, M.; Batlle, D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: Efforts to replicate human nephropathy. Exp. Diabetes Res. 2012, 2012, 616313. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.A.; Riethmuller, J.; Seitz, A.P.; Gardner, A.; Boudreau, R.; Kamler, M.; Kleuser, B.; Schuchman, E.; Caldwell, C.C.; Edwards, M.J.; et al. Sphingolipids as targets for inhalation treatment of cystic fibrosis. Adv. Drug Deliv. Rev. 2018, 133, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, P.E.; Levin, A.; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neschen, S.; Scheerer, M.; Seelig, A.; Huypens, P.; Schultheiss, J.; Wu, M.; Wurst, W.; Rathkolb, B.; Suhre, K.; Wolf, E.; et al. Metformin supports the antidiabetic effect of a sodium glucose cotransporter 2 inhibitor by suppressing endogenous glucose production in diabetic mice. Diabetes 2015, 64, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukunft, S.; Prehn, C.; Röhring, C.; Möller, G.; Hrabě de Angelis, M.; Adamski, J.; Tokarz, J. High-throughput extraction and quantification method for targeted metabolomics in murine tissues. Metabolomics 2018, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Naimi, A.I.; Moodie, E.E.; Auger, N.; Kaufman, J.S. Constructing inverse probability weights for continuous exposures: A comparison of methods. Epidemiology 2014, 25, 292–299. [Google Scholar] [CrossRef]
- Robins, J.M.; Hernán, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef]


| Clinical Variables | Hyperglycemic Participants | NGT Participants | ||||
|---|---|---|---|---|---|---|
| CKD n = 83 | Non-CKD n = 427 | p-Value | CKD n = 85 | Non-CKD n = 1312 | p-Value | |
| Age, years | 74.36 ± 7.66 | 64.32 ± 10.53 | 1.003 × 10−12 | 72.05 ± 8.23 | 55.47 ± 10.53 | 3.255 × 10−27 |
| Sex, male, % | 49.4 | 57.61 | 1.686 × 10−1 | 48.24 | 43.9 | 4.361 × 10−1 |
| BMI, kg/m2 | 29.25 ± 4.3 | 30.16 ± 5.02 | 1.228 × 10−1 | 28.11 ± 4.94 | 26.52 ± 4.37 | 1.415 × 10−3 |
| HbA1C (%) | 5.74 ± 0.42 | 5.73 ± 0.54 | 7.552 × 10−1 | 5.56 ± 0.32 | 5.3 ± 0.32 | 7.958 × 10−12 |
| Fasting glucose, mg/dl | 112.55 ± 20.44 | 111.3 ± 16.37 b | 6.440 × 10−1 | 96.79 ± 7.68 | 94.02 ± 7.3 | 1.130 × 10−3 |
| 2-h glucose, mg/dl | 164.43 ± 38.98 b | 160.63 ± 46.66 b | 3.724 × 10−1 | 103.16 ± 21.18 | 95.89 ± 19.9 | 3.232 × 10−3 |
| Systolic BP, mmHg | 120.31 ± 22.27 | 124.78 ± 18.03 | 4.847 × 10−2 | 116.65 ± 18.23 | 115.85 ± 16.06 | 6.617 × 10−1 |
| Diastolic BP, mmHg | 68.27 ± 11.15 | 74.93 ± 10.55 | 5.054 × 10−7 | 69.41 ± 10.14 | 73.06 ± 8.95 | 3.048 × 10−4 |
| Triglyceride, mg/dl a | 121.11 (93.44–157.4) | 128 (92.98–178.27) | 9.711 × 10−1 | 109 (78–143.79) | 93 (70–127.46) | 1.492 × 10−2 |
| Total cholesterol, mg/dl | 208.58 ± 41.45 | 220.93 ± 42.16 | 1.533 × 10−2 | 211.48 ± 43.58 | 218.59 ± 37.7 | 9.597 × 10−2 |
| HDL cholesterol, mg/dl | 61.12 ± 18.42 | 59.78 ± 17.54 | 5.303 × 10−1 | 65.63 ± 18.42 | 68.57 ± 18.75 | 1.612 × 10−1 |
| LDL cholesterol, mg/dl | 126.3 ± 35.49 | 140.65 ± 37.2 | 1.456 × 10−3 | 130.94 ± 37.34 | 135.83 ± 34.05 | 2.025 × 10−1 |
| Creatinine, mg/dl | 1.24 ± 0.21 | 0.89 ± 0.15 | 3.916 × 10−21 | 1.25 ± 0.28 | 0.86 ± 0.16 | 6.345 × 10−31 |
| eGFR, mL/min/1.73 m² | 50.5 ± 7.87 | 81.33 ± 11.9 | 3.645 × 10−47 c | 50.97 ± 8.01 | 86.92 ± 12.69 | 5.563 × 10−54 c |
| UACR, mg/g a | 9.76 (5.73–26.07) | 5.43 (3.39–9.86) | 1.180 × 10−7 | 7.33 (4.44–15.38) | 4.26 (2.94–7.07) | 1.604 × 10−8 |
| Smoking, % | 7.394 × 10−3 | 8.080 × 10−5 | ||||
| Nonsmoker | 55.42 | 43.79 | Ref. | 40 | 41.54 | Ref. |
| Former smoker | 40.96 | 42.39 | 2.789 × 10−1 | 56.47 | 40.4 | 1.086 × 10−1 |
| Current smoker | 3.61 | 13.82 | 1.028 × 10−2 | 3.53 | 18.06 | 8.628 × 10−3 |
| Medication usage, % | ||||||
| Lipid-lowering | 34.94 | 22.48 | 1.684 × 10−2 | 32.94 | 7.7 | 2.377 × 10−12 |
| Antihypertensive | 84.34 | 47.07 | 1.367 × 10−8 | 69.41 | 19.97 | 2.272 × 10−19 |
| Models | SM C18:1 | PC aa C38:0 | ||
|---|---|---|---|---|
| Effect Estimate (95% CI) | p-Value | Effect Estimate (95% CI) | p-Value | |
| Adjusted imbalanced covariates | −1.51 (−2.92 to −0.1) a | 3.624 × 10−2 | −1.82 (−3.04 to −0.59) b | 3.757 × 10−3 |
| Basic model | −1.83 (−2.98 to −0.68) | 1.879 × 10−3 | −1.91 (−3.11 to −0.72) | 1.784 × 10−3 |
| Full model | −1.76 (−2.9 to −0.62) | 2.499 × 10−3 | −1.81 (−2.99 to −0.63) | 2.607 × 10−3 |
| Metabolites | Models | NGT Participants | Hyperglycemic Participants | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| SM C18:1 | Adjusted imbalance covariates | 0.96 (0.77–1.21) a | 7.233 × 10−1 | 1.46 (1.09–1.97) b | 1.169 × 10−2 |
| Basic model | 1.05 (0.82–1.35) | 6.986 × 10−1 | 1.93 (1.38–2.78) | 2.251 × 10−4 | |
| Full model | 1.14 (0.86–1.51) | 3.733 × 10−1 | 1.99 (1.37–2.96) | 4.482 × 10−4 | |
| PC aa C38:0 | Adjusted imbalance covariates | 0.98 (0.78–1.23) c | 8.438 × 10−1 | 1.61 (1.2–2.17) d | 1.487 × 10−3 |
| Basic model | 1.12 (0.87–1.46) | 3.752 × 10−1 | 1.68 (1.24–2.29) | 8.723 × 10−4 | |
| Full model | 1.19 (0.91–1.58) | 2.142 × 10−1 | 1.71 (1.23–2.41) | 1.578 × 10−3 | |
| Clinical Variables | db/db Mice n = 10 | Wild Type Mice n = 10 | p-Value |
|---|---|---|---|
| Body weight, g | 47.87 ± 2.37 | 21.97 ± 0.58 | 1.796 × 10−4 |
| Kidney weight, g | 0.20 ± 0.02 | 0.16 ± 0.02 | 2.057 × 10−4 |
| Liver weight, g | 2.56 ± 0.3 | 1.02 ± 0.09 | 1.083 × 10−5 |
| Heart weight, g | 0.14 ± 0.01 | 0.12 ± 0.01 | 4.871 × 10−4 |
| Blood glucose, mg/dL | 421.60 ± 41.24 | 106.7 ± 16.88 | 1.806 × 10−4 |
| Plasma insulin, µg/L | 7.76 ± 2.33 | 1.03 ± 0.4 | 1.083 × 10−5 |
| Triglyceride, mg/dL | 224.78 ± 106.51 | 122.24 ± 24.52 | 5.869 × 10−2 |
| Total cholesterol, mg/dL | 153.24 ± 16.14 | 100.58 ± 12.16 | 1.817 × 10−4 |
| HDL cholesterol, mg/dL | 125.28 ± 13.12 | 84.28 ± 8.65 | 1.083 × 10−5 |
| LDL cholesterol, mg/dL | 18.76 ± 3.67 | 14.5 ± 2.08 | 8.127 × 10−3 |
| C-reactive protein, mg/L | 13.12 ± 3.27 | 5.36 ± 1.12 | 1.786 × 10−4 |
| Plasma creatinine a, mg/dL | 0.05 ± 0.01 | 0.08 ± 0.01 | 2.076 × 10−4 |
| Plasma albumin, g/dL | 3.10 ± 0.34 | 2.56 ± 0.13 | 5.509 × 10−4 |
| Tissue | Creatinine | SM C18:1 | PC aa C38:0 | |||
|---|---|---|---|---|---|---|
| t Statistic | p-Value | t Statistic | p-Value | t Statistic | p-Value | |
| Plasma | −5.68 | 2.284 × 10−5 | 4.71 | 3.160 × 10−4 | 0.35 | 7.327 × 10−1 |
| Urine a | −9.20 | 9.396 × 10−8 | −2.39 | 4.193 × 10−2 | −4.56 | 4.516 × 10−4 |
| Liver | −9.21 | 5.298 × 10−8 | 6.00 | 1.288 × 10−5 | 0.19 | 8.499 × 10−1 |
| Lung | −3.54 | 2.531 × 10−3 | 2.46 | 2.440 × 10−2 | 3.60 | 2.173 × 10−3 |
| Adrenal glands b | 1.33 | 2.098 × 10−1 | 0.16 | 8.745 × 10−1 | 4.11 | 9.695 × 10−4 |
| Adipose tissue c | −0.49 | 6.308 × 10−1 | −3.70 | 1.763 × 10 −3 | −2.36 | 3.856 × 10−2 |
| Cerebellum | −0.37 | 7.164 × 10−1 | 1.18 | 2.543 ×10−1 | 1.46 | 1.605 × 10−1 |
| Testis | 2.05 | 5.560 × 10−2 | −0.52 | 6.069 × 10−1 | −0.28 | 7.849 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Covic, M.; Huth, C.; Rommel, M.; Adam, J.; Zukunft, S.; Prehn, C.; Wang, L.; Nano, J.; Scheerer, M.F.; et al. Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient db/db Mouse. Metabolites 2021, 11, 89. https://doi.org/10.3390/metabo11020089
Huang J, Covic M, Huth C, Rommel M, Adam J, Zukunft S, Prehn C, Wang L, Nano J, Scheerer MF, et al. Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient db/db Mouse. Metabolites. 2021; 11(2):89. https://doi.org/10.3390/metabo11020089
Chicago/Turabian StyleHuang, Jialing, Marcela Covic, Cornelia Huth, Martina Rommel, Jonathan Adam, Sven Zukunft, Cornelia Prehn, Li Wang, Jana Nano, Markus F. Scheerer, and et al. 2021. "Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient db/db Mouse" Metabolites 11, no. 2: 89. https://doi.org/10.3390/metabo11020089
APA StyleHuang, J., Covic, M., Huth, C., Rommel, M., Adam, J., Zukunft, S., Prehn, C., Wang, L., Nano, J., Scheerer, M. F., Neschen, S., Kastenmüller, G., Gieger, C., Laxy, M., Schliess, F., Adamski, J., Suhre, K., de Angelis, M. H., Peters, A., & Wang-Sattler, R. (2021). Validation of Candidate Phospholipid Biomarkers of Chronic Kidney Disease in Hyperglycemic Individuals and Their Organ-Specific Exploration in Leptin Receptor-Deficient db/db Mouse. Metabolites, 11(2), 89. https://doi.org/10.3390/metabo11020089