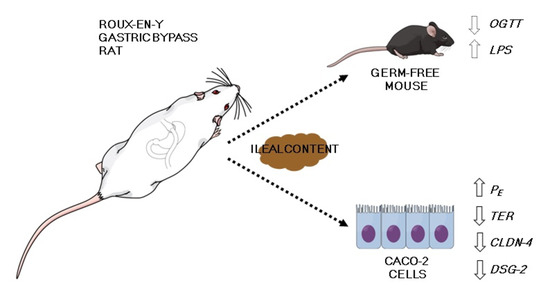
Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota
Abstract
:1. Introduction
2. Results
2.1. Ileal Content Transfer from RYGB-Treated Rats to Germ-Free Mice Modestly Impairs Oral Glucose Tolerance and Heightens Systemic Endotoxemia Development
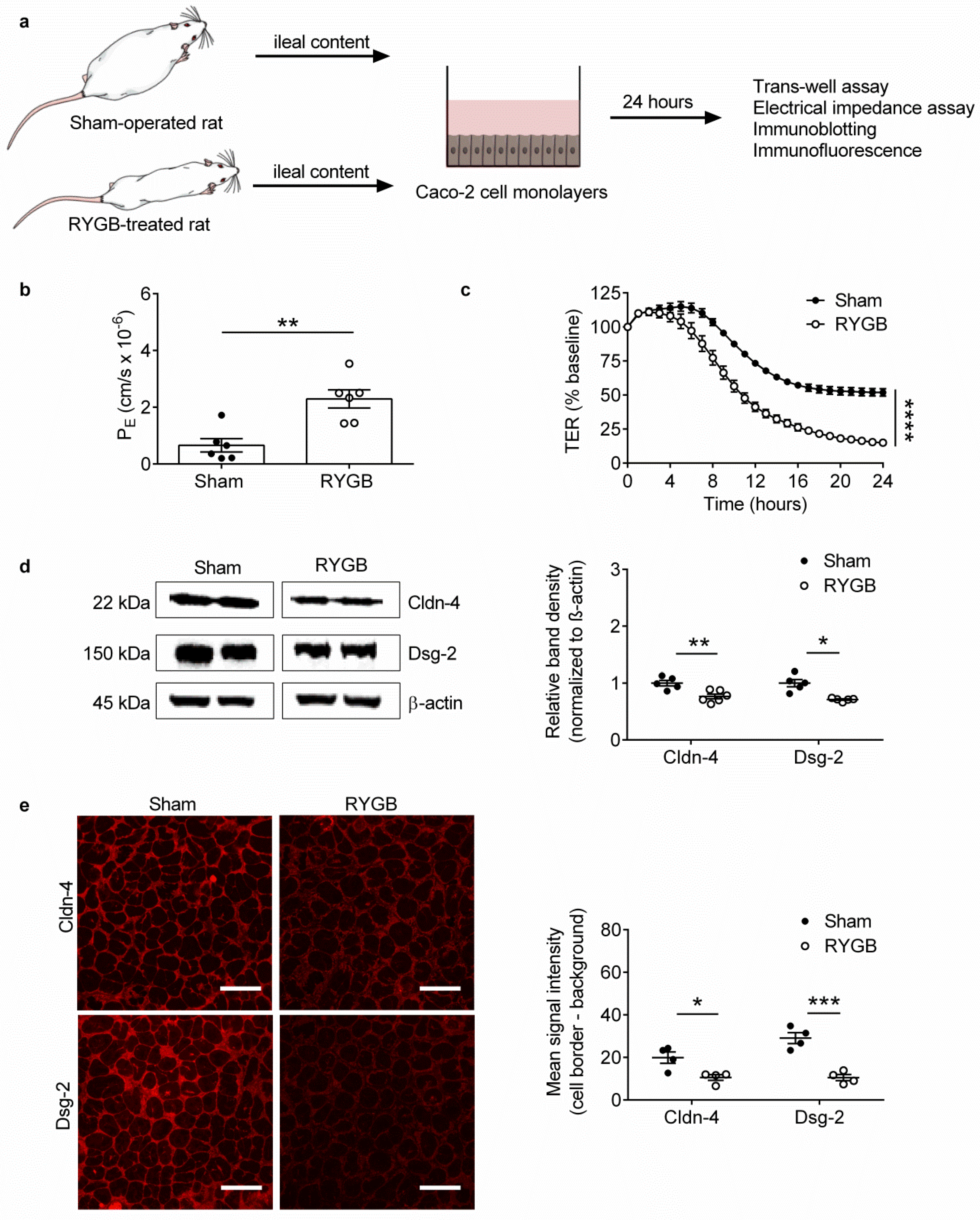
2.2. Ileal Content of RYGB-Treated Rats Contains Soluble Metabolites Which Disrupt Barrier Function and Structure in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Ileal Content Transfer Experiments
4.2. Immunoassays
4.3. Cell Culture
4.4. Ileal Content Preparation for Cell Culture Experiments
4.5. Measurement of TER
4.6. Measurement of 4 kDa FITC–Dextran Flux
4.7. Immunoblotting
4.8. Immunofluorescence
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Capristo, E.; Chamseddine, G.; Bornstein, S.R.; Rubino, F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021, 397, 293–304. [Google Scholar] [CrossRef]
- Arora, T.; Seyfried, F.; Docherty, N.G.; Tremaroli, V.; le Roux, C.W.; Perkins, R.; Backhed, F. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017, 11, 2035–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra141. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, L.; Chen, Y.; Lian, G.; Wang, J.; Zhang, J.; Shan, K.; Shang, L.; Tian, F.; Jing, C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes. Surg. 2021, 31, 327–336. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Stahlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fandriks, L.; le Roux, C.W.; Nielsen, J.; Backhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.C., Jr.; White, D.W.; Wu-Peng, X.S.; Liu, S.M.; Okada, N.; Kershaw, E.E.; Chung, W.K.; Power-Kehoe, L.; Chua, M.; Tartaglia, L.A.; et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes 1996, 45, 1141–1143. [Google Scholar] [CrossRef] [Green Version]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [Green Version]
- Luck, H.; Tsai, S.; Chung, J.; Clemente-Casares, X.; Ghazarian, M.; Revelo, X.S.; Lei, H.; Luk, C.T.; Shi, S.Y.; Surendra, A.; et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015, 21, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Troseid, M.; Nestvold, T.K.; Rudi, K.; Thoresen, H.; Nielsen, E.W.; Lappegard, K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care 2013, 36, 3627–3632. [Google Scholar] [CrossRef] [Green Version]
- Monte, S.V.; Caruana, J.A.; Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Schentag, J.J.; Dandona, P. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 2012, 151, 587–593. [Google Scholar] [CrossRef]
- Yang, P.J.; Lee, W.J.; Tseng, P.H.; Lee, P.H.; Lin, M.T.; Yang, W.S. Bariatric surgery decreased the serum level of an endotoxin-associated marker: Lipopolysaccharide-binding protein. Surg. Obes. Relat. Dis. 2014, 10, 1182–1187. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.Q.; Liu, G.P.; Huang, Z.P.; Zou, D.J. Roux-en-Y gastric bypass decreases endotoxemia and inflammatory stress in association with improvements in gut permeability in obese diabetic rats. J. Diabetes 2019, 11, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, P.B.; Ren, Z.Q.; Zhou, F.; Hu, H.H.; Zhang, H.; Xue, K.K.; Xu, P.; Shao, X.Q. Changes of serum lipopolysaccharide, inflammatory factors, and cecal microbiota in obese rats with type 2 diabetes induced by Roux-en-Y gastric bypass. Nutrition 2019, 67–68, 110565. [Google Scholar] [CrossRef]
- Taylor, J.A.; Sommerfeld-Sager, J.M.; Meng, C.X.; Nagel, S.C.; Shioda, T.; Vom Saal, F.S. Reduced body weight at weaning followed by increased post-weaning growth rate interacts with part-per-trillion fetal serum concentrations of bisphenol A (BPA) to impair glucose tolerance in male mice. PLoS ONE 2018, 13, e0208846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef]
- Schlegel, N.; Boerner, K.; Waschke, J. Targeting desmosomal adhesion and signalling for intestinal barrier stabilization in inflammatory bowel diseases-Lessons from experimental models and patients. Acta Physiol. 2020, 231, e13492. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Andres, S.; Fromm, A.; van Tol, E.A.; Amasheh, M.; Mankertz, J.; Fromm, M.; Schulzke, J.D. Transforming growth factor-beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J. Nutr. 2011, 141, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, N.; Meir, M.; Heupel, W.M.; Holthofer, B.; Leube, R.E.; Waschke, J. Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G774–G783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, Q.; Bai, J.; Zhao, N.; Wang, Y.; Zhou, R.; Kong, W.; Zeng, T.; Tao, K.; Xia, Z. Upregulation of Intestinal NLRP6 Inflammasomes After Roux-en-Y Gastric Bypass Promotes Gut Immune Homeostasis. Obes. Surg. 2019, 30, 327–335. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Gonzalez, F.J.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Mendonca Machado, N.; Torrinhas, R.S.; Sala, P.; Ishida, R.K.; Guarda, I.; Moura, E.G.H.; Sakai, P.; Santo, M.A.; Linetzky Waitzberg, D. Type 2 Diabetes Metabolic Improvement After Roux-en-Y Gastric Bypass May Include a Compensatory Mechanism That Balances Fatty Acid beta and omega Oxidation. JPEN J. Parenter. Enteral Nutr. 2020, 44, 1417–1427. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Hankir, M.K.; Langseder, T.; Bankoglu, E.E.; Ghoreishi, Y.; Dischinger, U.; Kurlbaum, M.; Kroiss, M.; Otto, C.; le Roux, C.W.; Arora, T.; et al. Simulating the Post-gastric Bypass Intestinal Microenvironment Uncovers a Barrier-Stabilizing Role for FXR. iScience 2020, 23, 101777. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, F.; Miras, A.D.; Bueter, M.; Prechtl, C.G.; Spector, A.C.; le Roux, C.W. Effects of preoperative exposure to a high-fat versus a low-fat diet on ingestive behavior after gastric bypass surgery in rats. Surg. Endosc. 2013, 27, 4192–4201. [Google Scholar] [CrossRef] [Green Version]
- Robilliard, L.D.; Kho, D.T.; Johnson, R.H.; Anchan, A.; O’Carroll, S.J.; Graham, E.S. The Importance of Multifrequency Impedance Sensing of Endothelial Barrier Formation Using ECIS Technology for the Generation of a Strong and Durable Paracellular Barrier. Biosensors 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, P.; Gobbo, S.; Caputi, V.; Spagnol, L.; Schirato, G.; Pasqualin, M.; Levorato, E.; Palu, G.; Giron, M.C.; Castagliuolo, I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol. Cell. Neurosci. 2015, 68, 24–35. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankir, M.K.; Seyfried, F.; Schellinger, I.N.; Schlegel, N.; Arora, T. Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota. Metabolites 2021, 11, 153. https://doi.org/10.3390/metabo11030153
Hankir MK, Seyfried F, Schellinger IN, Schlegel N, Arora T. Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota. Metabolites. 2021; 11(3):153. https://doi.org/10.3390/metabo11030153
Chicago/Turabian StyleHankir, Mohammed K., Florian Seyfried, Isabel N. Schellinger, Nicolas Schlegel, and Tulika Arora. 2021. "Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota" Metabolites 11, no. 3: 153. https://doi.org/10.3390/metabo11030153
APA StyleHankir, M. K., Seyfried, F., Schellinger, I. N., Schlegel, N., & Arora, T. (2021). Leaky Gut as a Potential Culprit for the Paradoxical Dysglycemic Response to Gastric Bypass-Associated Ileal Microbiota. Metabolites, 11(3), 153. https://doi.org/10.3390/metabo11030153