1. Introduction
Magnetic resonance spectroscopy (MRS) is a complementary technique to magnetic resonance imaging (MRI) for the study of brain pathologies and, in particular, brain tumors. Tumor classification, grade determination and tumor metabolism remain essential questions for prognosis and therapeutic management (radiotherapy, neurosurgical resection, etc.).
As a matter of fact,
H MRS is a valuable method to assess the main brain metabolic biomarkers: N-acetylaspartate (NAA), total choline (tCho) and total creatine (tCr) compounds, lactate (Lac), and mobile lipids; and the observation [
1] that brain tumors present a very different spectrum from the healthy brain [
2] has allowed the positioning of
H MRS as a solid contribution to tumor diagnosis. Möller-Hartman et al. [
3] showed that
H MRS increased diagnostic relevance and efficiency of a brain tumor by 16% compared to morphological MRI alone and Doblas study [
4] demonstrates the potential of
H MRS for the characterization and the differentiation of several rodent glioma models. However, nuclear magnetic resonance (NMR) spectroscopy remains a technique with marked limitations for the study of small volumes or finite quantities of materials, as may be the case for some primary tumors and metastases.
The use of miniaturized coils represents one of the most efficient solutions, with the increase of the static magnetic field, to perform spectroscopy or magnetic resonance imaging on these samples [
5]. Indeed, as a first approximation, the sensitivity of an NMR coil scales linearly with the inverse of the coil size [
6]. In addition, reducing the detection volume of the coil allows the filling factor of the coil to be optimized. Very few studies using implantable microcoils with diameters below several millimeters have been reported [
7,
8] and the in vivo one has focused on water signal detection due to very small volume of detection and limited level of signal-to-noise ratio (SNR) [
8]. When the dimensions and geometry of the microcoils are suitable, one can envision to take advantage of their sensitivity to perform MRI or MRS measurements in situ in biological tissues. The implantation of microcoils in situ would thus make it possible to acquire spectroscopic or imaging data with a sufficient signal-to-noise ratio (SNR) on a small volume of interest representative of a tumor.
The implantation of the microcoil imposes strong geometrical constraints to limit invasiveness and preserve the healthy and pathological tissues to be investigated. It is essential, for example, for implantable devices to have a needle shape with a diameter limited to a few hundred micrometers. In addition, the implantable device must be sufficiently rigid to allow its insertion into the tissue.
Thus, it is therefore important to find a compromise for the design of the implantable microcoil to satisfy the minimally invasive character, the size of the volume of interest and the available SNR. Finally, it is necessary to limit the distortions of the static magnetic field by ensuring the best possible match between the magnetic susceptibility of the surrounding tissue and that of the coil.
In this study, we evaluated the performance of an implantable microcoil designed to obtain diagnostic-quality in vivo proton NMR spectra in healthy rats and C6 glioma-bearing rats. In order to preserve tissue during the implantation of the microprobe, we opted for an elongated coil with a diameter limited to a few hundred micrometers. The implantable microcoils were manufactured using insulated copper microwires (150 micrometer diameter) adapted to match the magnetic susceptibilities of the tissue. In this article, we detail the manufacturing process of the implantable microcoils and the explorations of the results obtained in vitro and in vivo in rats.
3. Discussion
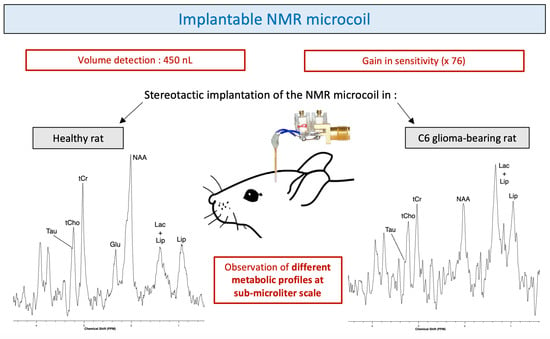
The compromise found between the design (minimally invasive feature) and the optimized use (best quality of NMR results) of the microcoil resulted in the ability of identifying, allowed by a sufficiently good spectral resolution, and quantifying, allowed by a high enough SNR, some metabolites such as NAA, Cho, Cr and Lac in healthy tissue and tumor tissue.
The brain implantation of the microcoil did not produce any bleeding. No inflammation or hemorrhage was observed in the MRI images and spectra obtained with the implanted microcoils were similar to those obtained with conventional external coils. The quality of spectra obtained with the microcoil is actually a good sign of the limited impact of the implantation of the coil on the tissue integrity. These observations are consistent with those reported during implantations of microdialysis probes [
11] or intracranial electrodes [
12]. Physiological changes such as gliosis are significant several days or weeks after implantation. In this study, microcoils are implanted for a very short period of time relative to the duration of the experiment (approximately 2–3 h). Note that, contrary to the microdialysis where gliosis on the membrane can be redhibitory in chronic implantation, the sensitive region of the coil extends at some distance from the microwires (few hundreds of micrometers) where the tissues are less likely to be impacted by the microcoil implantation.
The use of copper wire, with a magnetic volumic susceptibility (−9.63 ppm) close to human tissue (−9.2 to −8.8 ppm), allowed to limit the distortion of the magnetic field caused by the difference in susceptibility of the two media.
The gain in sensitivity obtained through the use of microcoils facilitates the detection of metabolic variations characteristic of tumor metabolism at submicroliter scale.
The mitotic activity of glioma cells can be assessed by the tCho/tCr ratio and necrosis by the increase in lipids and decrease in tCr. For C6 gliomas, Coquery et al. showed that the concentration of tCr in the tumor and in the healthy contralateral side remained relatively stable. The decrease of tCr in the spectrum of the C6 glioma-bearing rat seems to demonstrate that there was an onset of necrosis within the tumor. In general, in case of tumor proliferation, an increase in the tCho/NAA ratio is observed and is explained by the replacement of normal neurons by glial tumor cells. Particular metabolic and spectroscopic differences also occur in the differentiation of low and high-grade gliomas [
13].
The gain in sensitivity obtained through the use of microcoils enables the detection of metabolic variations characteristic of tumor metabolism at submicroliter scale which can be a real asset in the context of tumors with a heterogeneous appearance. Indeed, tumors do not consist of a set of homogeneous cells but of a complex and very heterogeneous system [
14]. The possibility of performing MRS or MRI in very small volumes of interest would make it possible to study different aspects of the tumor without having to perform a biopsy of the tumor, for example, which is more damaging to the integrity of the tissue. In the case of gliomas, there is a classical tumor organization in different layers—necrotic heart, inner crown of quiescent cells (which usually do not multiply) and proliferating cells on the outer surface. There is also an area, visible on MRI, that extends all around the tumor. This area commonly corresponds to an edema that also contains cancer cells but at a much lower density than in the tumor. The spectroscopic analysis, performed by these implantable microcoils, would allow to guide and evaluate therapeutic treatments to the most metabolically active areas, such as the tumor growth crown, with a high precision of the volume concerned, regardless of the anatomical aspect of the tumor.
In the case of metastases (often smaller than primary tumors) [
15], the use of implantable NMR microcoils could also be advantageous. In fact, the spread of one or more metastases is very common in the progression of cancer. Approximately 20–40% of cancer patients are susceptible to develop a brain metastasis. According to several studies, the majority of deaths (at least 2/3) due to cancer are caused by metastasis [
16]. A better understanding of the molecular processes involved in the formation of these metastases by using MRS and MRI would further help the development of therapeutic strategies such as immunotherapy [
17], stereotactic radiosurgery [
18] or combined techniques such as concomitant chemoradiotherapy [
19] and thus reduce cancer mortality.
The main limitation of this study is the limited number of glioma-bearing animals investigated. This limited number of glioma-bearing rats does not allow to assess the reproducibility of metabolite quantification in tumor tissue. The reproducibility study of the use of implantable microcoils is, however, validated by the data obtained in the five healthy rats. In fact, the results obtained and presented in this article represent a feasibility and proof-of-concept study.
Following the positive results obtained and the validation of the approach and its applicability for the investigation of tumor metabolism on a very small scale, more advanced studies with modifications of protocols and instrumentation (active decoupling to improve the homogeneity of the RF excitation field, pre-amp integration) are being developed in the laboratory.
Author Contributions
Y.C. conceptualized the study. Y.C., J.D. and N.P. participated to the MRI and MRS investigations with the microcoil and to the corresponding data analysis. N.P. realized the NMR microcoils used in the study. J.D. and Y.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was achieved within the context of the Laboratory of Excellence TRAIL ANR-10-LABX-57 with funding of the research program Insight. The authors acknowledge a financial support from France Life Imaging (FLI) network.
Institutional Review Board Statement
The study protocol was approved by the local animal welfare committee (University of Bordeaux, reference number 04490.02) and complied with EU guidelines (directive 2010/63/EU).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to colleagues Alan Wong, Luisa Ciobanu and Fawzi Boumezbeur from CEA Saclay for their advices and fruitful discussions. They thank Véronique Bouchaud, from the CRMSB laboratory, for providing C6 glioma cells.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruhn, H.; Frahm, J.; Gyngell, M.L.; Merboldt, K.D.; Hänicke, W.; Sauter, R.; Hamburger, C. Noninvasive differentiation of tumors with use of localized 1H MR spectroscopy in vivo: Initial experience in patients with cerebral tumors. Radiology 1989, 172, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Howe, F.; Barton, S.; Cudlip, S.; Stubbs, M.; Saunders, D.; Murphy, M.; Wilkins, P.; Opstad, K.; Doyle, V.; McLean, M.; et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 2003, 49, 223–232. [Google Scholar] [CrossRef]
- Möller-Hartman, W.; Herminghaus, S.; Krings, T.; Marquardt, G.; Lanfermann, H.; Pilatus, U.; Zanella, F.E. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002, 44, 371–381. [Google Scholar] [CrossRef]
- Doblas, S.; He, T.; Saunders, D.; Hoyle, J.; Smith, N.; Pye, Q.; Lerner, M.; Jensen, R.L.; Towner, R.A. In vivo characterization of several rodent glioma models by 1H MRS. NMR Biomed. 2012, 25, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Webb, A. Radiofrequency microcoils for magnetic resonance imaging and spectroscopy. J. Magn. Reson. 2013, 229, 55–66. [Google Scholar] [CrossRef]
- Hoult, D.; Richards, R. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. 1976, 24, 71–85. [Google Scholar] [CrossRef]
- Baxan, N.; Rabeson, H.; Pasquet, G.; Châteaux, J.F.; Briguet, A.; Morin, P.; Graveron-Demilly, D.; Fakri-Bouchet, L. Limit of detection of cerebral metabolites by localized NMR spectroscopy using microcoils. Comptes Rendus Chim. 2008, 11, 448–456. [Google Scholar] [CrossRef]
- Handwerker, J.; Perez Rodas, M.; Beyerlein, M.; Vincent, F.; Beck, A.; Freytag, N.; Yu, X.; Pohmann, R.; Anders, J.; Scheffler, K. A CMOS NMR needle for probing brain physiology with high spatial and temporal resolution. Nat. Methods 2020, 17. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Coquery, N.; Stupar, V.; Farion, R.; Maunoir-Regimbal, S.; Barbier, E.; Remy, C.; Fauvelle, F. The three glioma rat models C6, F98 and RG2 exhibit different metabolic profiles: In vivo 1H MRS and ex vivo 1 H HRMAS combined with multivariate statistics. Metabolomics 2015. [Google Scholar] [CrossRef]
- Hascup, E.R.; af Bjerkén, S.; Hascup, K.N.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009, 1291, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirshler, Y.K.; Polat, U.; Biegon, A. Intracranial electrode implantation produces regional neuroinflammation and memory deficits in rats. Exp. Neurol. 2010, 222, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naser, R.K.A.; Hassan, A.A.K.; Shabana, A.M.; Omar, N.N. Role of magnetic resonance spectroscopy in grading of primary brain tumors. Egypt. J. Radiol. Nucl. Med. 2016, 47, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Perus, L.J.M.; Walsh, L.A. Microenvironmental Heterogeneity in Brain Malignancies. Front. Immunol. 2019, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.; Rennert, R.; Anders, C.; Soffietti, R.; Ahluwalia, M.; Nayak, L.; Peters, S.; Arvold, N.; Harsh, G.; Steeg, P.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Nieblas-Bedolla, E.; Nayyar, N.; Singh, M.; Sullivan, R.J.; Brastianos, P.K. Emerging Immunotherapies in the Treatment of Brain Metastases. Oncologist 2020. [Google Scholar] [CrossRef]
- Lippitz, B.; Lindquist, C.; Paddick, I.; Peterson, D.; O’Neill, K.; Beaney, R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat. Rev. 2014, 40, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Matsutani, M. Chemoradiotherapy for brain tumors: Current status and perspectives. Int. J. Clin. Oncol. 2004, 9, 471–474. [Google Scholar] [CrossRef]
- Gruetter, R. Automatic, localized in Vivo adjustment of all first-and second-order shim coils. Magn. Reson. Med. 1993, 29, 804–811. [Google Scholar] [CrossRef]
- Tkáč, I.; Starčuk, Z.; Choi, I.Y.; Gruetter, R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999, 41, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.; Reynolds, G.; Kauppinen, R.A.; Arvanitis, T.N.; Peet, A.C. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn. Reson. Med. 2011, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pijnappel, W.; van den Boogaart, A.; de Beer, R.; van Ormondt, D. SVD-based quantification of magnetic resonance signals. J. Magn. Reson. 1992, 97, 122–134. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).