Identification of Circulating Diagnostic Biomarkers for Coronary Microvascular Disease in Postmenopausal Women Using Machine-Learning Techniques
Abstract
1. Introduction
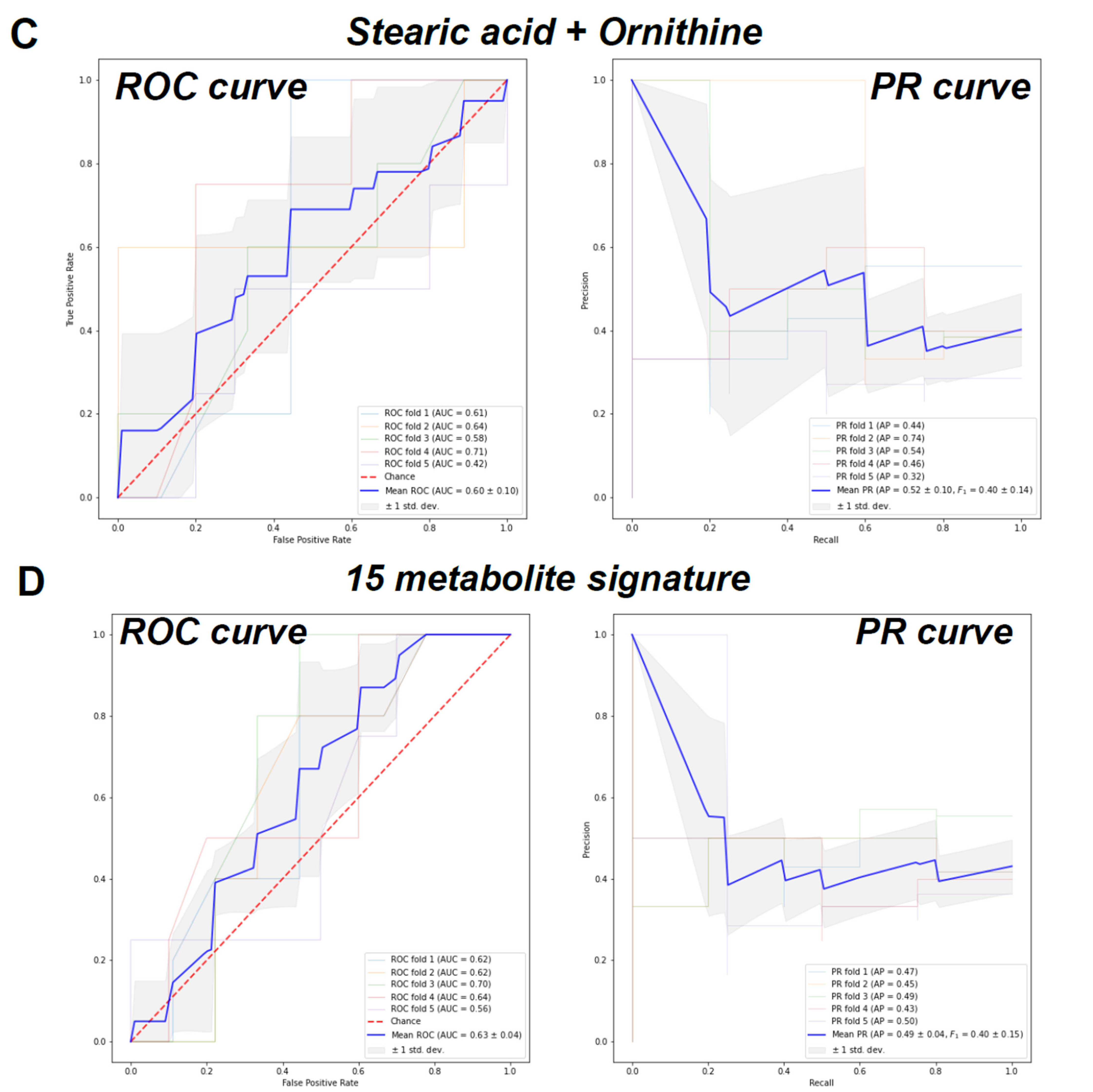
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. GC/MS Validation of the Metabolites by Whole Metabolite Profiling
4.3. Machine Learning Analysis: Data Preprocessing, Feature Selection and Classification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, B.; Wood, T.; Shaw, L.; Johnson, B.D.; Kelsey, S.; Anderson, R.D.; Pepine, C.J.; Merz, C.N.B. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: Findings from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am. Heart J. 2013, 166, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, P.; Tankó, L.B.; Bagger, Y.Z.; Qin, G.; Christiansen, C. The long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric 2006, 9, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Tunc, E.; Eve, A.A.; Madak-Erdogan, Z. Coronary Microvascular Dysfunction and Estrogen Receptor Signaling. Trends Endocrinol. Metab. 2020, 31, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.A.; Liu, X.; Zhao, Y.C.; Hieronymi, K.; Rossi, G.; Auvil, L.S.; Welge, M.; Bushell, C.; Smith, R.L.; Carlson, K.E.; et al. Long-Term Administration of Conjugated Estrogen and Bazedoxifene Decreased Murine Fecal beta-Glucuronidase Activity Without Impacting Overall Microbiome Community. Sci. Rep. 2018, 8, 8166. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Santaliz-Casiano, A.; Patel, M.; Marino, N.; Storniolo, A.M.V.; Torun, H.; Acar, B.; Madak Erdogan, Z. A Computational Statistics Approach to Evaluate Blood Biomarkers for Breast Cancer Risk Stratification. Horm. Cancer 2020, 11, 17–33. [Google Scholar] [CrossRef]
- Eve, A.A.; Liu, X.; Wang, Y.; Miller, M.J.; Jeffery, E.H.; Madak-Erdogan, Z. Biomarkers of Broccoli Consumption: Implications for Glutathione Metabolism and Liver Health. Nutrients 2020, 12, 2514. [Google Scholar] [CrossRef]
- Smith, B.P.; Auvil, L.S.; Welge, M.; Bushell, C.; Bhargava, R.; Elango, N.; Johnson, K.; Erdogan, Z.M. Identification of Early Liver Toxicity Gene Signatures Using Comparative Supervised Machine Learning. Sci. Rep. 2020, 10, 1–27. [Google Scholar] [CrossRef]
- Madak-Erdogan, Z.; Band, S.; Zhao, Y.C.; Smith, B.P.; Kulkoyluoglu-Cotul, E.; Zuo, Q.; Casiano, A.S.; Wrobel, K.; Rossi, G.; Smith, R.L.; et al. Free Fatty Acids Rewire Cancer Metabolism in Obesity-Associated Breast Cancer via Estrogen Receptor and mTOR Signaling. Cancer Res. 2019, 79, 2494–2510. [Google Scholar] [CrossRef]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2000, 1486, 1–17. [Google Scholar] [CrossRef]
- Violante, S.; Ijlst, L.; Te Brinke, H.; Koster, J.; de Almeida, I.T.; Wanders, R.J.A.; Ventura, F.V.; Houten, S.M. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obes. (Silver Spring) 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships Between Circulating Metabolic Intermediates and Insulin Action in Overweight to Obese, Inactive Men and Women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W.; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5, e003190. [Google Scholar] [CrossRef]
- Strand, E.; Pedersen, E.R.; Svingen, G.F.T.; Olsen, T.; Bjørndal, B.; Karlsson, T.; Dierkes, J.; Njølstad, P.R.; Mellgren, G.; Tell, G.S.; et al. Serum Acylcarnitines and Risk of Cardiovascular Death and Acute Myocardial Infarction in Patients With Stable Angina Pectoris. J. Am. Heart Assoc. 2017, 6, e003620. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility With Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.-F.; Huang, T.; Luo, H.-H.; Chen, J.-X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.-Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, F.; Song, Z.; Han, D.; Zhang, J.; Chen, L.; Na, L. A high fat diet with a high C18:0/C16:0 ratio induced worse metabolic and transcriptomic profiles in C57BL/6 mice. Lipids Health Dis. 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.A.; Walker, C.L.; Xu, Z.; Whitley, P.; Pavlina, T.M.; Hise, M.; Zaloga, G.P.; Siddiqui, R.A. Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J. Lipid Res. 2010, 51, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jarne, E.; Martínez-Losa, E.; Prado-Santamaría, M.; Brugarolas-Brufau, C.; Serrano-Martínez, M.; Martínez-González, M.A. Risk of first non-fatal myocardial infarction negatively associated with olive oil consumption: A case-control study in Spain. Int. J. Epidemiol. 2002, 31, 474–480. [Google Scholar] [CrossRef]
- Maria, A.C.; Massaro, M.; Bonfrate, C.; Siculella, L.; Maffia, M.; Nicolardi, G.; Distante, A.; Storelli, C.; De Caterina, R. Oleic Acid Inhibits Endothelial Activation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 220–228. [Google Scholar] [CrossRef]
- Zhang, C.; Hein, T.W.; Wang, W.; Chang, C.-I.; Kuo, L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001, 15, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Moon, J.; Lee, Y.S.; Chung, H.K.; Lee, S.M.; Shin, M.J. Arginase inhibition restores endothelial function in diet-induced obesity. Biochem. Biophys. Res. Commun. 2014, 451, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kubo, M.; Nagaoka, K.; Funakubo, N.; Setiawan, H.; Takemoto, K.; Eguchi, E.; Fujikura, Y.; Ogino, K. Early obesity leads to increases in hepatic arginase I and related systemic changes in nitric oxide and l-arginine metabolism in mice. J. Physiol. Biochem. 2018, 74, 9–16. [Google Scholar] [CrossRef]
- Yao, L.; Bhatta, A.; Xu, Z.; Chen, J.; Toque, H.A.; Chen, Y.; Xu, Y.; Bagi, Z.; Lucas, R.; Huo, Y.; et al. Obesity-induced vascular inflammation involves elevated arginase activity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 313, R560–R571. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Fuster, V. New aspects in the pathogenesis of diabetic atherothrombosis. J. Am. Coll. Cardiol. 2004, 44, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Kövamees, O.; Shemyakin, A.; Checa, A.; Wheelock, C.E.; Lundberg, J.O.; Östenson, C.-G.; Pernow, J. Arginase Inhibition Improves Microvascular Endothelial Function in Patients With Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Wang, H.; Wu, W.; Ye, Y. Arginine methylation dysfunction increased risk of acute coronary syndrome in coronary artery disease population: A case-control study. Medicine 2017, 96, e6074. [Google Scholar] [CrossRef]
- Malik, A.; Sharma, U.; Lakshmy, R.; Narang, R.; Jagannathan, N.R. Biochemical characterization of blood plasma of coronary artery disease patients by in vitro high-resolution proton NMR spectroscopy. J. Biosci. 2015, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Young, M.E.; Taegtmeyer, H. Anaplerosis of the citric acid cycle: Role in energy metabolism of heart and skeletal muscle. Acta Physiol. Scand. 2000, 168, 657–665. [Google Scholar] [CrossRef]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite Profiling and Cardiovascular Event Risk. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Granger, C.B.; Craig, D.; Haynes, C.; Bain, J.; Stevens, R.D.; Hauser, E.R.; Newgard, C.B.; Kraus, W.E.; Newby, L.K.; et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 2014, 232, 191–196. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Palazoglu, M.; Fiehn, O. Metabolite Identification in Blood Plasma Using GC/MS and the Agilent Fiehn GC/MS Metabolomics RTL Library. Agil. Appl. Note 2009, 97–109. [Google Scholar]
- Borgogna, J.-L.C.; Shardell, M.D.; Yeoman, C.J.; Ghanem, K.G.; Kadriu, H.; Ulanov, A.V.; Gaydos, C.A.; Hardick, J.; Robinson, C.K.; Bavoil, P.M.; et al. The association of Chlamydia trachomatis and Mycoplasma genitalium infection with the vaginal metabolome. Sci. Rep. 2020, 10, 3420. [Google Scholar] [CrossRef]


| Characteristics | Control (n = 26) | CMD (n = 23) | CAD (n = 21) | p Value a |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, mean (SD), Y | 62(8) | 58 (7) | 62 (7) | 0.2057 |
| BMI, median (IQR) b | 31 (30) | 30 (30) | 30 (29) | 0.8036 |
| Hypertension, no.(%) | 17 (65) | 8 (34) | 11 (52) | 0.101 |
| Diabetes, no.(%) | 5 (19) | 6 (26) | 11 (52) | 0.0412 |
| Smoking, no.(%) | 2 (7) | 0 (0) | 1 (4) | ND |
| COPD or asthma, no. (%) | 2 (7) | 4 (17) | 3 (14) | ND |
| HL, no. (%) | 5 (19) | 5 (21) | 5 (23) | ND |
| Rheumatology, no. (%) | 0 (0) | 1 (4) | 1 (4) | ND |
| Thyroid, no. (%) | 4 (15) | 0 (0) | 3 (14) | ND |
| Medication | ||||
| Antithrombotic, no. (%) | 6 (23) | 14 (60) | 20 (95) | <0.0001 |
| ACE-ARB diuretics, no. (%) | 14 (53) | 6 (26) | 13 (61) | 0.0408 |
| CA channel blockers, no. (%) | 8 (30) | 3 (13) | 6 (28) | 0.3035 |
| Beta blocker, no. (%) | 4 (15) | 6 (26) | 7 (33) | 0.3507 |
| Antianginal, no. (%) | 0 (0) | 3 (13) | 11 (52) | <0.0001 |
| Antihyperlipidemic, no. (%) | 4 (15) | 10 (43) | 12 (57) | 0.0097 |
| Blood test results | ||||
| Total cholesterol, mean (median), mg/dL | 237 (234) | 213 (202) | 192 (185) | 0.0072 |
| HDL, mean (median), mg/dL | 55 (53) | 49 (47) | 43 (44) | 0.0039 |
| LDL, mean (median), mg/dL | 150 (147) | 128 (126) | 113 (99) | 0.0145 |
| Triglyceride, mean (median), mg/dL | 162 (146) | 172 (141) | 179 (151) | 0.815 |
| Glucose, mean (median), mg/dL | 113 (104) | 133 (114) | 127 (120) | 0.2305 |
| Urea, mean (median), mg/dL | 14 (13) | 13 (13) | 14 (13) | 0.5087 |
| Creatinine, mean (median), mg/dL | 0.8 (0.8) | 0.7 (0.7) | 0.7 (0.7) | 0.0491 |
| AST, mean (median), U/L | 19 (18) | 19 (15) | 18 (17) | 0.7581 |
| ALT, mean (median), U/L | 18 (17) | 17 (16) | 20 (17) | 0.5324 |
| Na⁺, mean (median), meq/L | 140 (140) | 139 (139) | 140 (139) | 0.6461 |
| K⁺, mean (median), meq/L | 4.3 (4.3) | 4.3 (4.4) | 4.4 (4.5) | 0.6139 |
| WBC, mean (median), ×109/L | 7 (7) | 7 (7) | 8 (8) | 0.3099 |
| HB, mean (median), g/dL | 23 (13) | 20 (13) | 18 (13) | 0.8631 |
| PLT, mean (median) ×109/L | 283 (270) | 275 (254) | 307 (303) | 0.4216 |
| MCV, mean (median), fL | 87 (87) | 85 (83) | 83 (82) | 0.3882 |
| Characteristics | Control (n = 26) | CMD (n = 23) | CAD (n = 21) |
|---|---|---|---|
| Transthoracic echocardiography | |||
| Systolic dysfunction | 1 | 1 | 0 |
| Diastolic dysfunction | 15 | 11 | 13 |
| Valve disorder | 10 | 12 | 8 |
| LV hypertrophy | 4 | 1 | 2 |
| Pulmonary hypertension | 1 | 1 | 0 |
| Coronary angiography | |||
| Normal | 22 | ||
| Atherosclerotic heart disease | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arredondo Eve, A.; Tunc, E.; Liu, Y.-J.; Agrawal, S.; Erbak Yilmaz, H.; Emren, S.V.; Akyıldız Akçay, F.; Mainzer, L.; Žurauskienė, J.; Madak Erdogan, Z. Identification of Circulating Diagnostic Biomarkers for Coronary Microvascular Disease in Postmenopausal Women Using Machine-Learning Techniques. Metabolites 2021, 11, 339. https://doi.org/10.3390/metabo11060339
Arredondo Eve A, Tunc E, Liu Y-J, Agrawal S, Erbak Yilmaz H, Emren SV, Akyıldız Akçay F, Mainzer L, Žurauskienė J, Madak Erdogan Z. Identification of Circulating Diagnostic Biomarkers for Coronary Microvascular Disease in Postmenopausal Women Using Machine-Learning Techniques. Metabolites. 2021; 11(6):339. https://doi.org/10.3390/metabo11060339
Chicago/Turabian StyleArredondo Eve, Alicia, Elif Tunc, Yu-Jeh Liu, Saumya Agrawal, Huriye Erbak Yilmaz, Sadık Volkan Emren, Filiz Akyıldız Akçay, Luidmila Mainzer, Justina Žurauskienė, and Zeynep Madak Erdogan. 2021. "Identification of Circulating Diagnostic Biomarkers for Coronary Microvascular Disease in Postmenopausal Women Using Machine-Learning Techniques" Metabolites 11, no. 6: 339. https://doi.org/10.3390/metabo11060339
APA StyleArredondo Eve, A., Tunc, E., Liu, Y.-J., Agrawal, S., Erbak Yilmaz, H., Emren, S. V., Akyıldız Akçay, F., Mainzer, L., Žurauskienė, J., & Madak Erdogan, Z. (2021). Identification of Circulating Diagnostic Biomarkers for Coronary Microvascular Disease in Postmenopausal Women Using Machine-Learning Techniques. Metabolites, 11(6), 339. https://doi.org/10.3390/metabo11060339








