Comparative Metabolic Profiling of Grape Pulp during the Growth Process Reveals Systematic Influences under Root Restriction
Abstract
:1. Introduction
2. Results
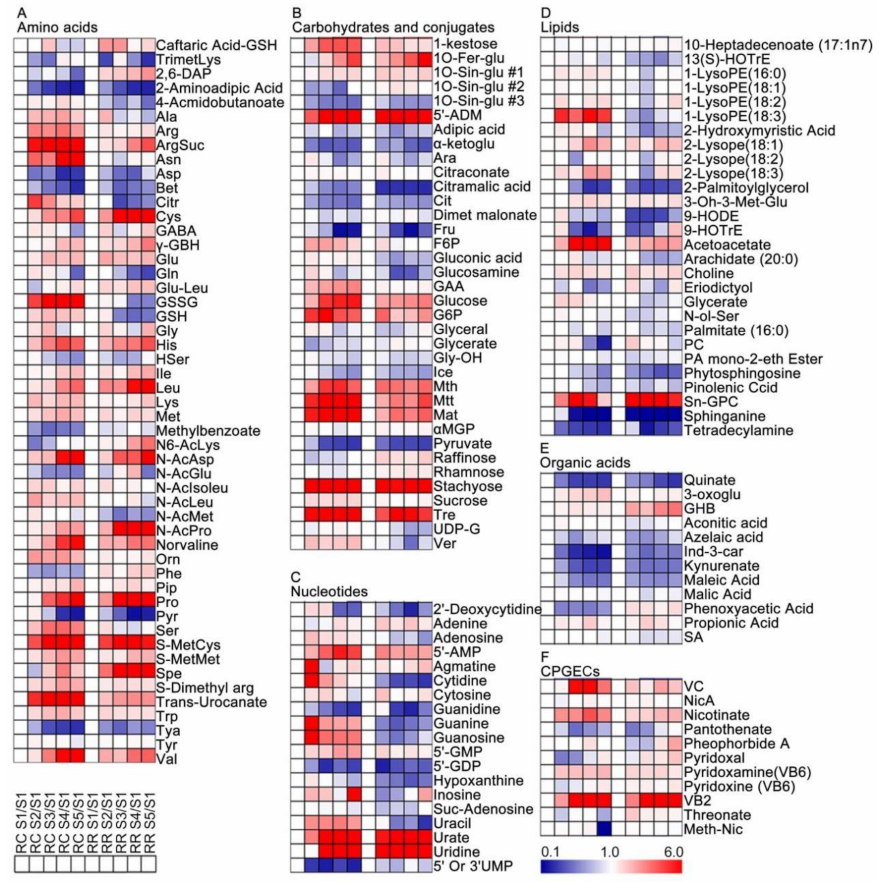
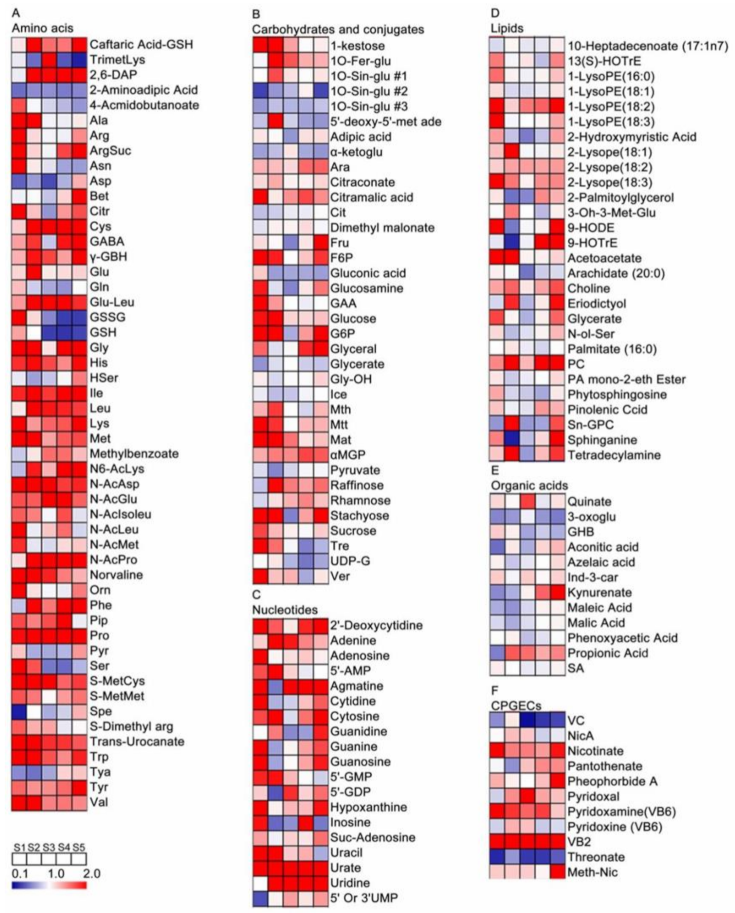
2.1. Metabolite Profiling of Grape Pulp Samples
2.2. Kinetic Patterns of Developing Grape Pulp Metabolomes
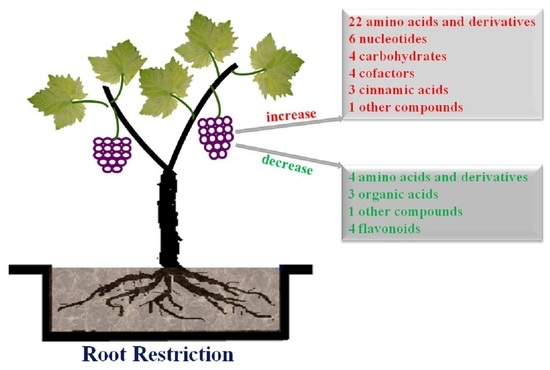
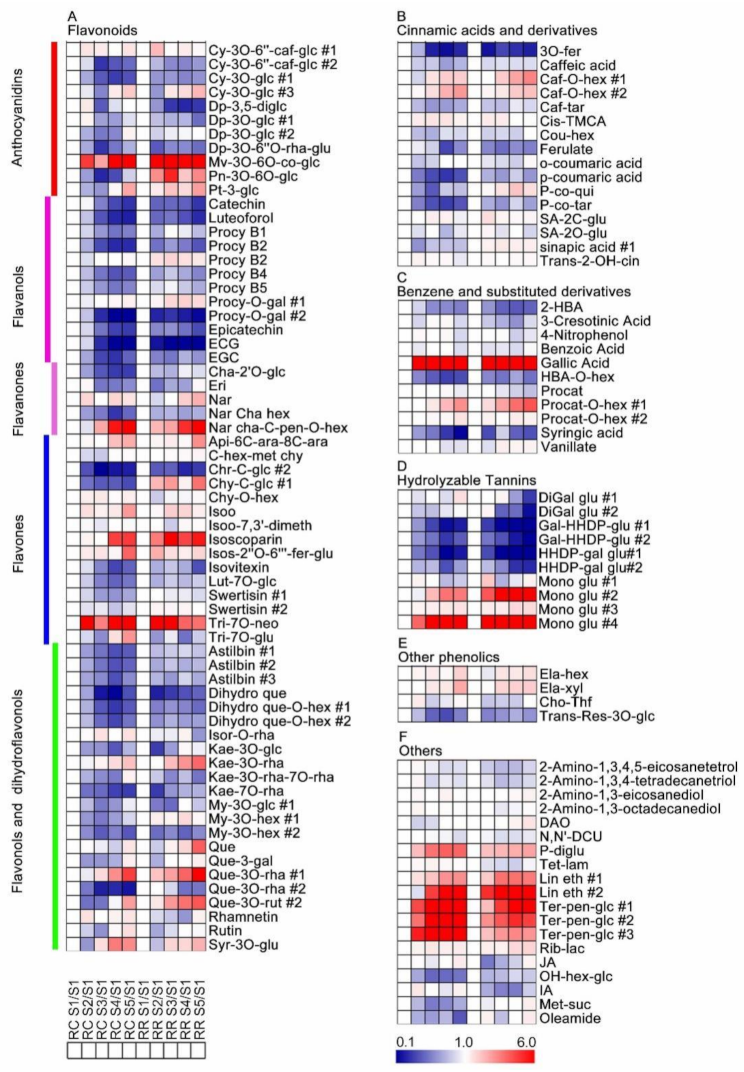
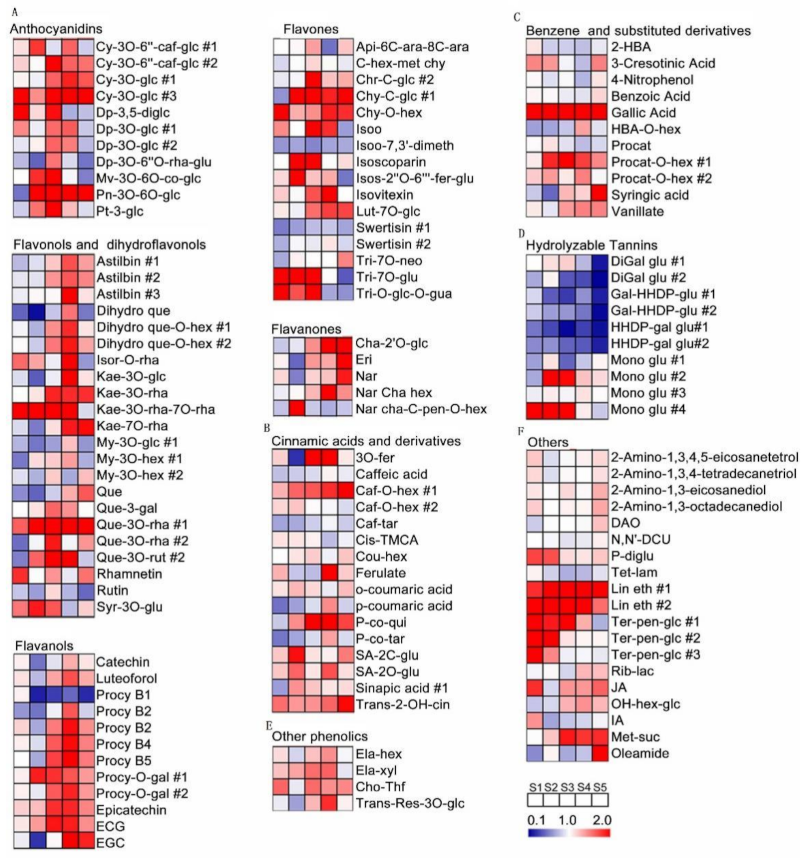
2.3. Metabolic Changes of Grape Pulp at Different Developmental Stages
3. Discussion
3.1. Primary Metabolites
3.2. Secondary Metabolites
4. Materials and Methods
4.1. Plant Material and Sample Collection
4.2. Metabolite Extraction and Profiling
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuadros-Inostroza, A.; Ruíz-Lara, S.; González, E.; Eckardt, A.; Willmitzer, L.; Peña-Cortés, H. GC–MS metabolic profiling of Cabernet Sauvignon and Merlot cultivars during grapevine berry development and network analysis reveals a stage- and cultivar-dependent connectivity of primary metabolites. Metabolomics 2016, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, F.; Lin, Q.; Wu, D.; Wang, S.P.; Wang, D.L.; Sun, C.D. Comparative transcriptomic analysis of grape berry in re-sponse to root restriction during developmental stages. Molecules 2016, 21, 1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The Transcription Factor VvMYB5b Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Developing Grape Berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2009, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Anesi, A.; Stocchero, M.; Santo, S.D.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.B.; Siebert, T.E.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant Biol. 2015, 15, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight Modulates Fruit Metabolic Profile and Shapes the Spatial Pattern of Compound Accumulation within the Grape Cluster. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, A.; Regalado, A.; Rodrigues, D.; Costa, J.M.; Blumwald, E.; Chaves, M.M.; Gerós, H. Polyols in grape berry: Transport and metabolic adjustments as a physiological strategy for water-deficit stress tolerance in grapevine. J. Exp. Bot. 2014, 66, 889–906. [Google Scholar] [CrossRef] [Green Version]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2006, 7, 111–134. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; María, E.S.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance Responses in Ripening Berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Jia, H.; Wu, W.; Wang, X.; Fang, J.; Wang, C. Functional conservation analysis and expression modes of grape anthocyanin synthesis genes responsive to low temperature stress. Gene 2015, 574, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA Are Involved in Phenol Metabolism of Vitis vinifera L. Increasing Biosynthesis of Berry Skin Polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated conse-quences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Chitarrini, G.; Soini, E.; Riccadonna, S.; Franceschi, P.; Zulini, L.; Masuero, D.; Vecchione, A.; Stefanini, M.; Di Gaspero, G.; Mattivi, F.; et al. Identification of biomarkers for defense response to plasmoparaviticola in a resistant grape variety. Front. Plant Sci. 2017, 8, 1524. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Tang, D.D.; Lin, Q.; Cao, J.P.; Wu, D.; Wang, S.P.; Sun, C.D. Transcriptomic analyses of ascorbic acid and carot-enoid metabolites influenced by root restriction during grape berry development and ripening. J. Agr. Food Chem. 2017, 65, 2008–2016. [Google Scholar] [CrossRef]
- Leng, F.; Cao, J.P.; Wang, S.P.; Jiang, L.; Li, X.; Sun, C.D. Transcriptomic analyses of root restriction effects on phytohor-mone content and signal transduction during grape berry development and ripening. Int. J. Mol. Sci. 2018, 19, 2300. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Li, B.; Forney, C.F.; Xu, W.; Wang, S. Changes in sugar content and relative enzyme activity in grape berry in response to root restriction. Sci. Hortic. 2009, 123, 39–45. [Google Scholar] [CrossRef]
- Wang, B.; He, J.J.; Duan, C.Q.; Yu, X.M.; Zhu, L.N.; Xie, Z.S.; Zhang, C.X.; Xu, W.P.; Wang, S.P. Root restriction affects anthocyanin accumulation and composition in berry skin of ’Kyoho’ grape (Vitis vinifera L. × Vitis labrusca L.) during ripening. Sci. Hortic. 2012, 137, 20–28. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, L.; Wang, S.; Gu, W.; Huang, D.; Xu, W.; Jiang, A.; Li, S. Nitrate uptake kinetics of grapevine under root restriction. Sci. Hortic. 2007, 111, 358–364. [Google Scholar] [CrossRef]
- Wang, B.; He, J.J.; Bai, Y.; Yu, X.M.; Li, J.F.; Zhang, C.X.; Xu, W.P.; Bai, X.J.; Cao, X.J.; Wang, S.P. Root restriction af-fected anthocyanin composition and up-regulated the transcription of their biosynthetic genes during berry development in ’Summer Black’ grape. Acta Physiol. Plant. 2013, 35, 2205–2217. [Google Scholar] [CrossRef]
- Duan, S.Y.; Wu, Y.S.; Fu, R.F.; Wang, L.; Chen, Y.J.; Xu, W.P.; Zhang, C.X.; Ma, C.; Shi, J.X.; Wang, S.P. Comparative metabolic profiling of grape skin tissue along grapevine berry developmental stages reveals systematic influences of root re-striction on skin metabolome. Int. J. Mol. Sci. 2019, 20, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Esteso, M.J.; Vilella-Anton, M.T.; Pedreno, M.A.; Valero, M.L.; Bru-Martinez, R. iTRAQ-based protein profiling provides insights into the central metabolism changes driving grape berry development and ripening. BMC Plant Biol. 2013, 13, 167. [Google Scholar] [CrossRef] [Green Version]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Niculcea, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Sánchez-Díaz, M.; Morales, F.; Ayestarán, B.; Antolín, M.C. Effects of Water-Deficit Irrigation on Hormonal Content and Nitrogen Compounds in Developing Berries of Vitis vinifera L. cv. Tempranillo. J. Plant Growth Regul. 2013, 32, 551–563. [Google Scholar] [CrossRef]
- Zoccatelli, G.; Zenoni, S.; Savoi, S.; Santo, S.D.; Tononi, P.; Zandonà, V.; Cin, A.D.; Guantieri, V.; Pezzotti, M.; Tornielli, G. Skin pectin metabolism during the postharvest dehydration of berries from three distinct grapevine cultivars. Aust. J. Grape Wine Res. 2013, 19, 171–179. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dornyei, A.; Mark, L.; Dimovska, V.; Stafilov, T.; Kilar, F. Identification of polyphe-nolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Dai, Z.W.; Léon, C.; Feil, R.; Lunn, J.E.; Delrot, S.; Gomès, E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 2013, 64, 1345–1355. [Google Scholar] [CrossRef]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.-M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212–233. [Google Scholar] [CrossRef] [Green Version]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.L.; Weiszmann, J.; Weckwerth, W. System-level and granger network analysis of integrated proteomic and metabolomic dynamics identifies key points of grape berry development at the interface of primary and secondary metabo-lism. Front. Plant Sci. 2017, 8, 1066. [Google Scholar] [CrossRef] [Green Version]
- Agudelo-Romero, P.; Erban, A.; Sousa, L.; Pais, M.S.; Kopka, J.; Fortes, A.M. Search for Transcriptional and Metabolic Markers of Grape Pre-Ripening and Ripening and Insights into Specific Aroma Development in Three Portuguese Cultivars. PLoS ONE 2013, 8, e60422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and oth-er developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liang, Y.; An, X.; Kong, F.; Gao, D.; Yin, H. Changes in sugar content and related enzyme activities in table grape (Vitis viniferaL.) in response to foliar selenium fertilizer. J. Sci. Food Agric. 2017, 97, 4094–4102. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; Van Leeuwen, C.; et al. Ecophysiological, Genetic, and Molecular Causes of Variation in Grape Berry Weight and Composition: A Review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Forney, C.F.; Xu, W.; Wang, S. Effects of Root Restriction on Ultrastructure of Phloem Tissues in Grape Berry. HortScience 2009, 44, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.Y.; Wu, Y.S.; Gao, Z.; Luo, M.; Wang, S.P.; Song, S.R.; Zhang, C.X.; Xu, W.P. Effects of root restriction on contents of carbohydrates and nitrogen compouds in ‘Kyoho’ grapevine at veraison and maturation stage. Acta Hortic. Sin. 2016, 43, 431–440. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Grimplet, J.; Wheatley, M.D.; Ben Jouira, H.; Deluc, L.G.; Cramer, G.R.; Cushman, J.C. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deficit stress conditions. Proteomics 2009, 9, 2503–2528. [Google Scholar] [CrossRef] [Green Version]
- Somkuwar, R.G.; Bahetwar, A.; Khan, I.; Satisha, J.; Ramteke, S.D.; Itroutwar, P.; Bhongale, A.; Oulkar, D. Changes in growth, photosynthetic activities, biochemical parameters and amino acid profile of Thompson Seedless grapes (Vitis vinifera L.). J. Environ. Biol. 2014, 35, 1157–1163. [Google Scholar]
- Zaharah, S.S.; Razi, I.M. Growth, stomata aperture, biochemical changes and branch anatomy in mango (Mangiferaindica) cv. Chokanan in response to root restriction and water stress. Sci. Hortic. 2009, 123, 58–67. [Google Scholar] [CrossRef]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Degu, A.; Hochberg, U.; Sikron, N.; Venturini, L.; Buson, G.; Ghan, R.; Plaschkes, I.; Batushansky, A.; Chalifa-Caspi, V.; Mattivi, F.; et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, S.; Stefanini, M.; Stanstrup, J.; Perenzoni, D.; Mattivi, F.; Vrhovsek, U. The metabolomic profile of red non- V. vinifera genotypes. Food Res. Int. 2017, 98, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kai, G.-Y.; Lu, B.-B.; Zhang, H.-M.; Tang, K.-X.; Jiang, J.-H.; Chen, W.-S. Metabolic Engineering of Tropane Alkaloid Biosynthesis in Plants. J. Integr. Plant Biol. 2005, 47, 136–143. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.H.; Amar, O.; Lastochkin, E.; Larkov, O.; Ravid, U.; et al. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engi-neering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001, 127, 1256–1265. [Google Scholar] [CrossRef]
- Harborne, J.B. Twenty-five years of chemical ecology. Nat. Prod. Rep. 2001, 18, 361–379. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nat. Cell Biol. 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med. Hypotheses 2006, 67, 833–838. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Toffali, K.; Zamboni, A.; Anesi, A.; Stocchero, M.; Pezzotti, M.; Levi, M.; Guzzo, F. Novel aspects of grape berry ripening and post-harvest withering revealed by untargeted LC-ESI-MS metabolomics analysis. Metabolomics 2010, 7, 424–436. [Google Scholar] [CrossRef]
- Hu, C.Y.; Tohge, T.; Chan, S.A.; Song, Y.; Rao, J.; Cui, B.; Lin, H.; Wang, L.; Fernie, A.R.; Zhang, D.B.; et al. Identifica-tion of conserved and diverse metabolic shifts during rice grain development. Sci. Rep. 2016, 6, 20942. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN–A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, F.; Duan, S.; Song, S.; Zhao, L.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Comparative Metabolic Profiling of Grape Pulp during the Growth Process Reveals Systematic Influences under Root Restriction. Metabolites 2021, 11, 377. https://doi.org/10.3390/metabo11060377
Leng F, Duan S, Song S, Zhao L, Xu W, Zhang C, Ma C, Wang L, Wang S. Comparative Metabolic Profiling of Grape Pulp during the Growth Process Reveals Systematic Influences under Root Restriction. Metabolites. 2021; 11(6):377. https://doi.org/10.3390/metabo11060377
Chicago/Turabian StyleLeng, Feng, Shuyan Duan, Shiren Song, Liping Zhao, Wenping Xu, Caixi Zhang, Chao Ma, Lei Wang, and Shiping Wang. 2021. "Comparative Metabolic Profiling of Grape Pulp during the Growth Process Reveals Systematic Influences under Root Restriction" Metabolites 11, no. 6: 377. https://doi.org/10.3390/metabo11060377
APA StyleLeng, F., Duan, S., Song, S., Zhao, L., Xu, W., Zhang, C., Ma, C., Wang, L., & Wang, S. (2021). Comparative Metabolic Profiling of Grape Pulp during the Growth Process Reveals Systematic Influences under Root Restriction. Metabolites, 11(6), 377. https://doi.org/10.3390/metabo11060377