Metabolite Profiling in Arabidopsisthaliana with Moderately Impaired Photorespiration Reveals Novel Metabolic Links and Compensatory Mechanisms of Photorespiration
Abstract
:1. Introduction
2. Results
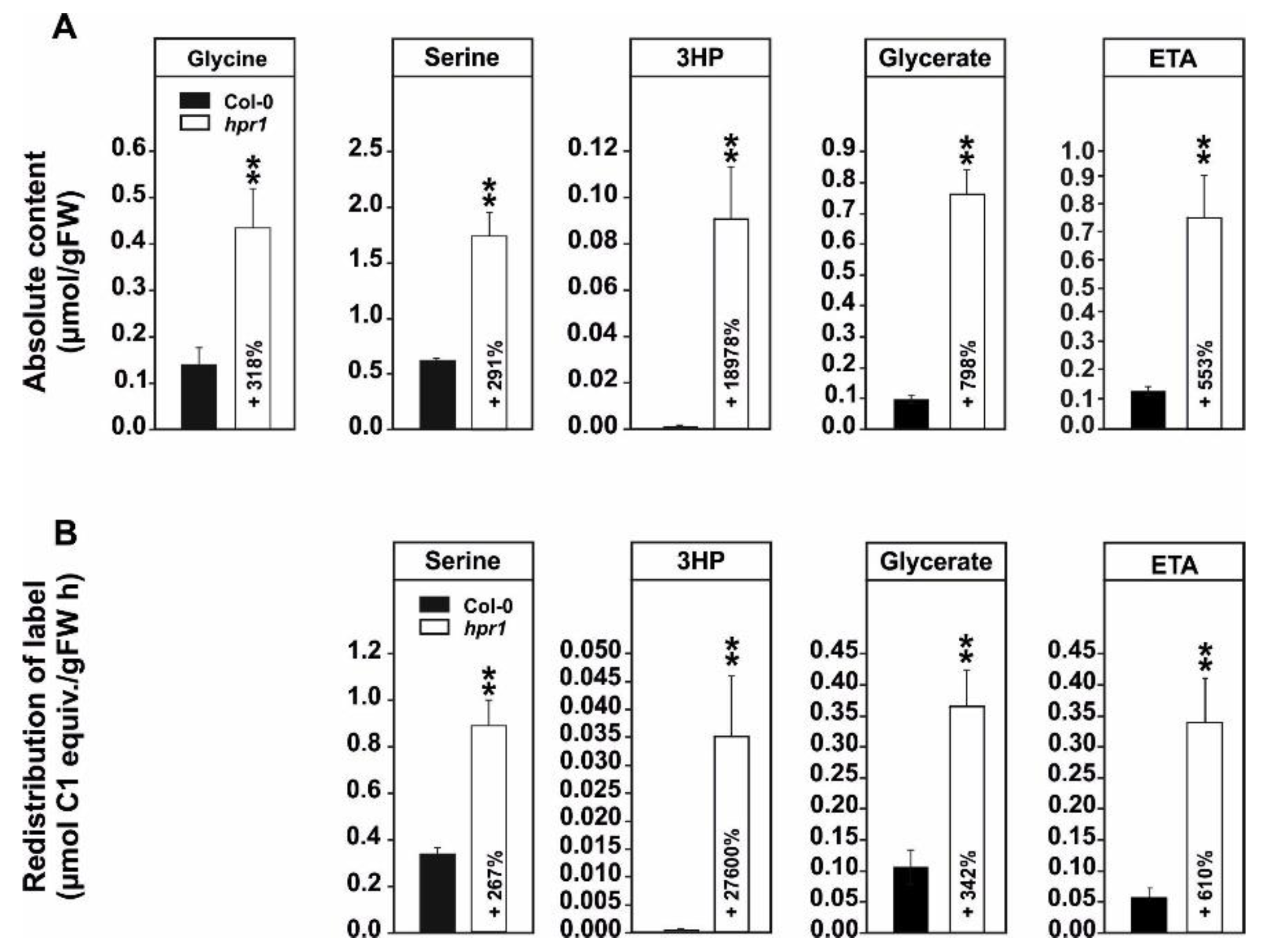
2.1. Absolute Metabolite Quantification and 13C-Glucose Feeding Suggests Enhanced Non-Enzymatic 3HP Decarboxylation and Increased Flux towards Ethanolamine in hpr1
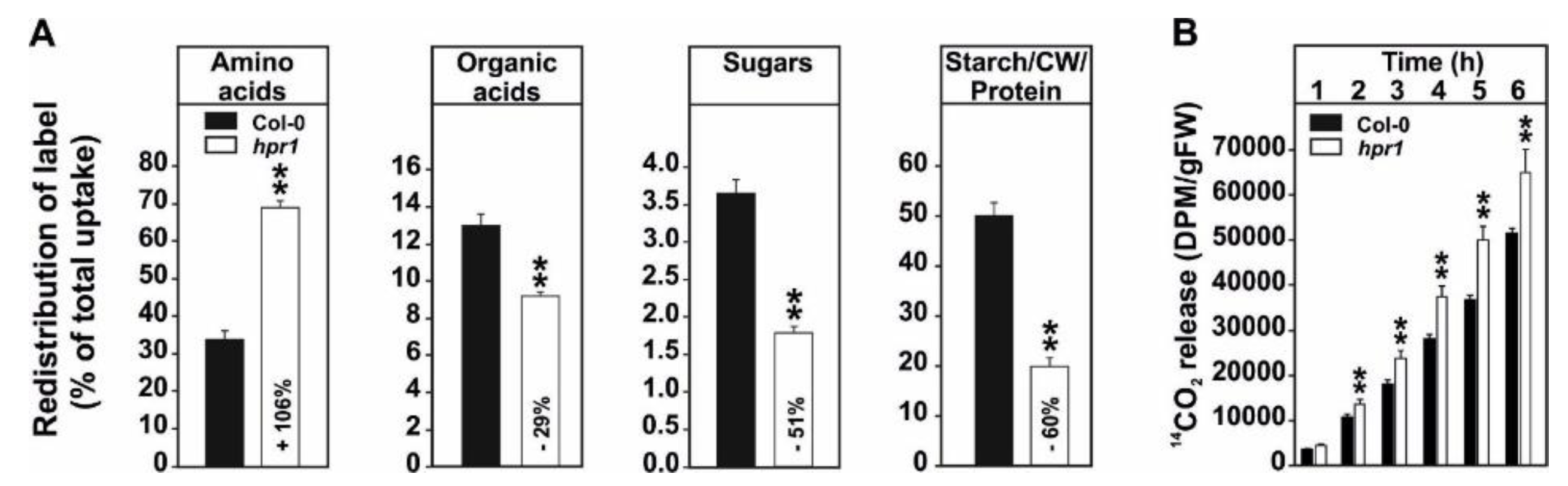
2.2. 14C-Glycine Labeling Reveals a Strong Reduction of the Leaf Carbohydrate and Organic Acid Status and an Increased CO2 Release from hpr1 Leaves
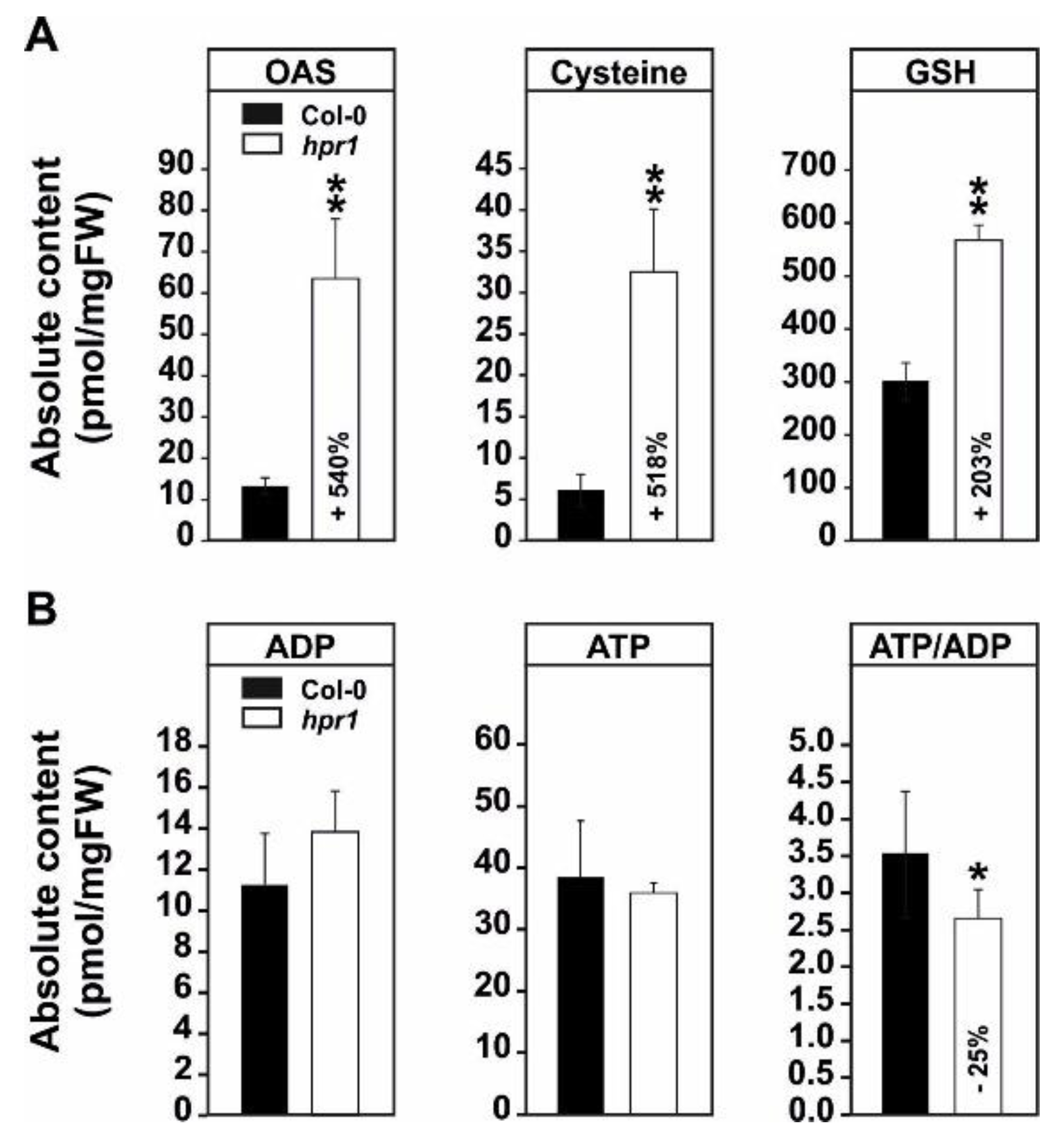
2.3. The Elevated Serine Content in hpr1 Enhances Glutathione Biosynthesis
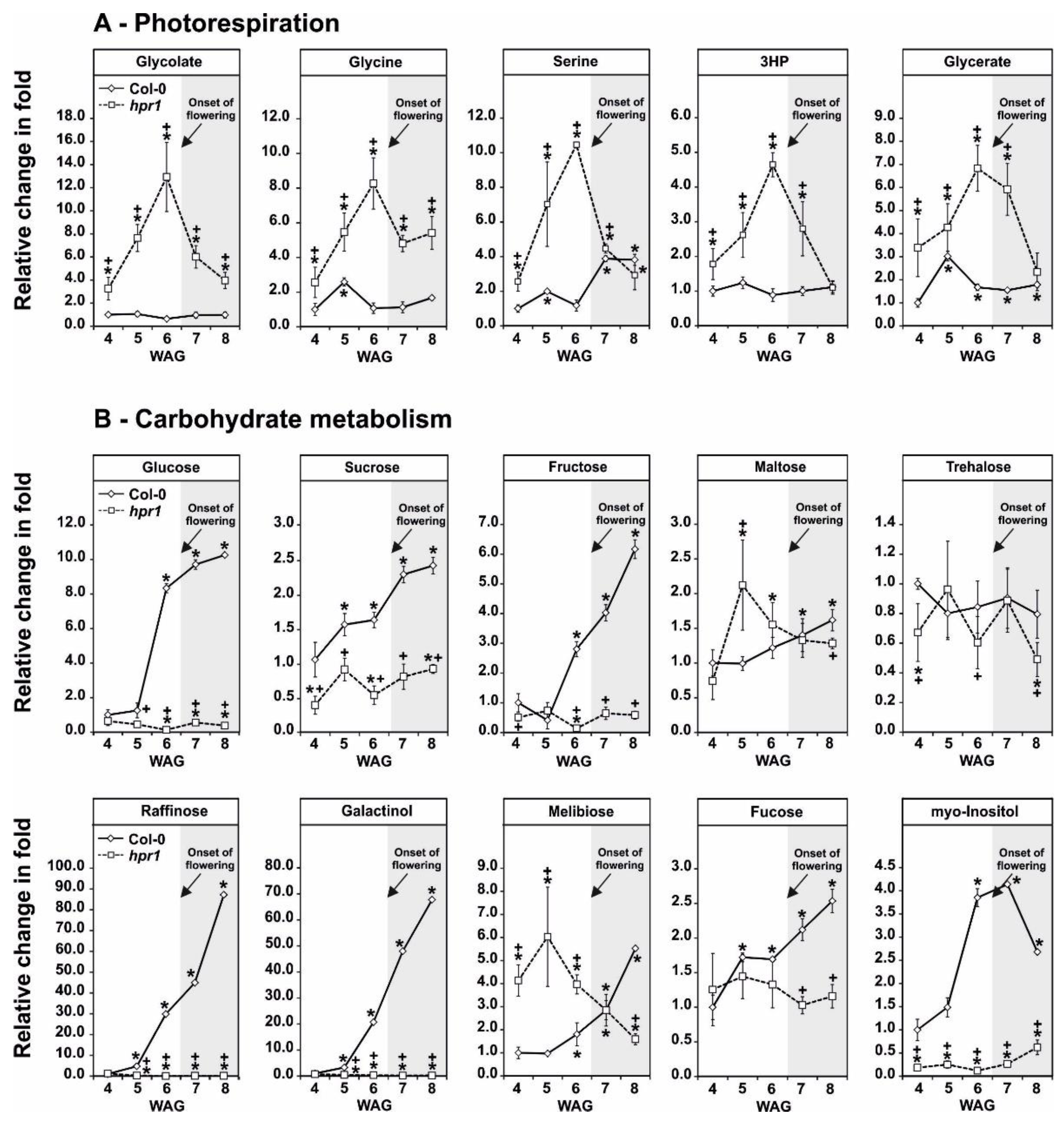
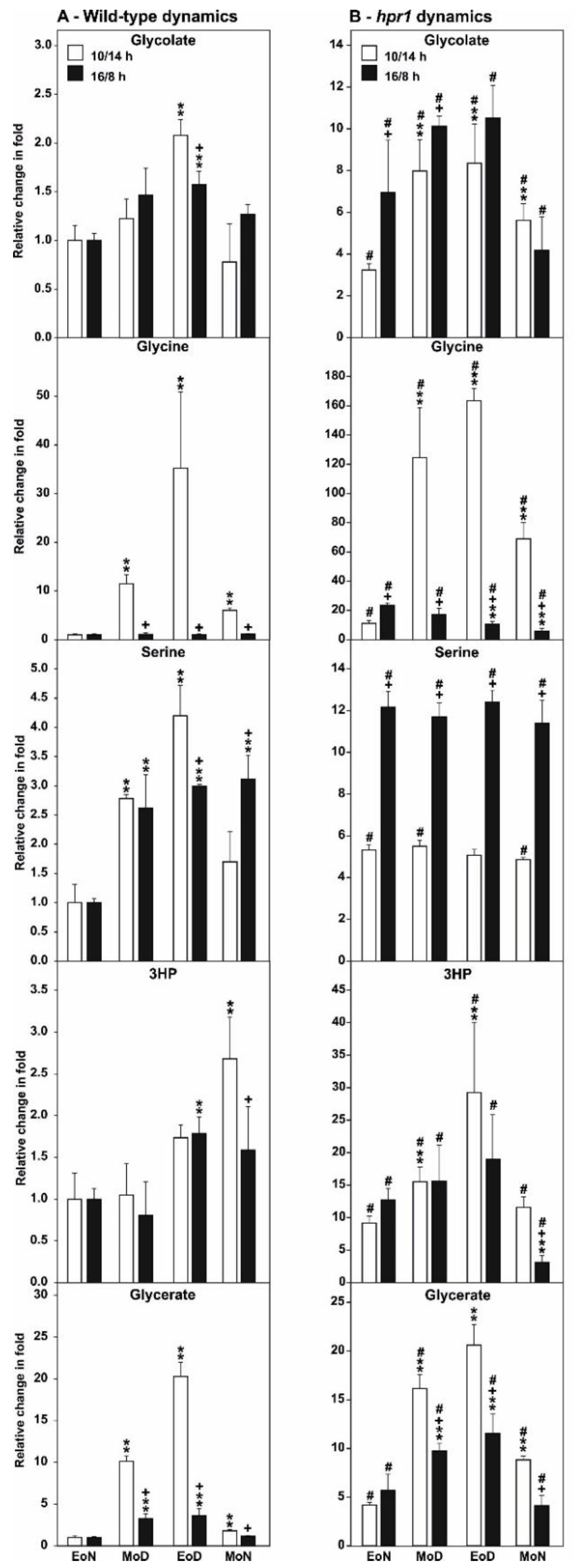
2.4. HPR1 Deficiency Increasingly Impairs Photorespiration during Vegetative Growth
2.5. Impaired Photorespiration Lowers the Accumulation of Sugars
2.6. HPR1 Deficiency Impacts on the TCA Cycle and Amino Acid Metabolism during Development
2.7. Short Day Growth seems to Slowdown the Photorespiratory Flux Mainly at the GDC and GLYK Steps
3. Discussion
3.1. HPR1 Deficiency Causes Enhanced Leaf CO2 Release by Both, Increased Non-Enzymatic 3HP Decarboxylation and Higher Flux from Serine to ETA
3.2. Even a Moderate Impairment in the Photorespiratory Flux Reduces the Leaf-Carbohydrate Status including a Reduction in Metabolites Related to Cell Wall Biosynthesis
3.3. HPR1 Deficiency Impacts on Glutathione Biosynthesis
3.4. Impairment of the Photorespiratory Flux in hpr1 Is Strongest during Vegetative Growth in a Fully Developed Rosette
3.5. A Shorter Photoperiod Intensifies the Photorespiratory Defect, Mainly at the GDC Step
3.6. Conclusion
4. Materials and Methods
4.1. Plant Material and Growth
4.2. Metabolite Analysis
4.2.1. Gas-Chromatography Coupled to Mass Spectrometry (GC-MS)
4.2.2. High-Performance Liquid Chromatography (HPLC)
4.3. 14C-Glycine and 13C-Glucose-Feeding and Determination of Isotope Accumulation
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Betti, M.; Bauwe, H.; Busch, F.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry, M.A.J.; Sage, R.; Timm, S.; et al. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; López-Calcagno, P.; Raines, C. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the energy balancing network of photosynthesis enables safe op-eration under changing environmental conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nat. Cell Biol. 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Wingler, A.; Quick, W.P.; Bungard, R.A.; Bailey, K.J.; Lea, P.J.; Leegood, R.C. The role of photorespiration during drought stress: An analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 1999, 22, 361–373. [Google Scholar] [CrossRef]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A. Photorespiration is crucial to the dynamic response of photosynthetic metabolism to altered CO2 availability. Mol. Plant 2017, 10, 437–461. [Google Scholar] [CrossRef] [Green Version]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S. The impact of photorespiration on plant primary metabolism through metabolic and redox regulation. Biochem. Soc. Trans. 2020, 48, 2495–2504. [Google Scholar] [CrossRef]
- Shi, X.; Bloom, A. Photorespiration: The futile cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef]
- Anderson, L.E. Chloroplast and cytoplasmic enzymes II. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta Enzym. 1971, 235, 237–244. [Google Scholar] [CrossRef]
- Kelly, G.; Latzko, E. Inhibition of spinach-leaf phosphofructokinase by 2-phosphoglycollate. FEBS Lett. 1976, 68, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Flügel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Hagemann, M. Photorespiration—How is it regulated and how does it regulate overall plant metabolism? J. Exp. Bot. 2020, 71, 3955–3965. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Arrivault, S.; Stitt, M.; Fernie, A.R.; Bauwe, H. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 2012, 586, 3692–3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Wittmiß, M.; Gamlien, S.; Ewald, R.; Florian, A.; Frank, M.; Wirtz, M.; Hell, R.; Fernie, A.R.; Bauwe, H. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. Plant Cell 2015, 27, 1968–1984. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Woitschach, F.; Heise, C.; Hagemann, M.; Bauwe, H. Faster removal of 2-phosphoglycolate through photorespiration improves abiotic stress tolerance of arabidopsis. Plants 2019, 8, 563. [Google Scholar] [CrossRef] [Green Version]
- Collakova, E.; Goyer, A.; Naponelli, V.; Krassovskaya, I.; Gregory, J.; Hanson, A.D.; Shachar-Hill, Y. Arabidopsis 10-formyl tetrahydrofolate deformylases are essential for photorespiration. Plant Cell 2008, 20, 1818–1832. [Google Scholar] [CrossRef] [Green Version]
- Keys, A.J. The re-assimilation of ammonia produced by photorespiration and the nitrogen economy of C3 higher plants. Photosynth. Res. 2006, 87, 165–175. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Abadie, C.; Tcherkez, G. Plant sulphur metabolism is stimulated by photorespiration. Commun. Biol. 2019, 2, 379. [Google Scholar] [CrossRef]
- Lim, S.-L.; Voon, C.P.; Guan, X.; Yang, Y.; Gardeström, P.; Lim, B.L. In planta study of photosynthesis and photorespiration using NADPH and NADH/NAD+ fluorescent protein sensors. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Timm, S.; Mielewczik, M.; Florian, A.; Frankenbach, S.; Dreissen, A.; Hocken, N.; Fernie, A.R.; Walter, A.; Bauwe, H. High-to-low CO2 acclimation reveals plasticity in the photorespiratory pathway and indicates regulatory links to other me-tabolism of Arabidopsis. PLoS ONE 2012, 7, e42009. [Google Scholar] [CrossRef] [Green Version]
- Florian, A.; Nikoloski, Z.; Sulpice, R.; Timm, S.; Araújo, W.L.; Tohge, T.; Bauwe, H.; Fernie, A.R. Analysis of short-term met-abolic alterations in Arabidopsis following changes in the prevailing environmental conditions. Mol. Plant 2014, 7, 893–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florian, A.; Timm, S.; Nikoloski, Z.; Tohge, T.; Bauwe, H.; Araújo, W.; Fernie, A.R. Analysis of metabolic alterations in Ara-bidopsis following changes in the carbon dioxide and oxygen partial pressures. J. Integr. Plant Biol. 2014, 56, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Araújo, W.L.; Fernie, A.R. New insights into photorespiration obtained from metabolomics. Plant Biol. 2013, 15, 656–666. [Google Scholar] [CrossRef]
- Obata, T.; Florian, A.; Timm, S.; Bauwe, H.; Fernie, A.R. On the metabolic interactions of (photo)respiration. J. Exp. Bot. 2016, 67, 3003–3014. [Google Scholar] [CrossRef] [Green Version]
- Blume, C.; Ost, J.; Mühlenbruch, M.; Peterhänsel, C.; Laxa, M. Low CO2 induces urea cycle intermediate accumulation in Arabidopsis thaliana. PLoS ONE 2019, 14, e0210342. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Bauwe, H.; Eisenhut, M.; Florian, A.; Hanson, D.T.; Hagemann, M.; Keech, O.; Mielewczik, M.; Nikoloski, Z.; Peterhansel, C.; et al. Perspectives on plant photorespiratory metabolism. Plant Biol. 2013, 15, 748–753. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bauwe, H. Wasteful, essential, evolutionary stepping stone? The multiple personalities of the photorespiratory pathway. Plant J. 2020, 102, 666–677. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Vanloocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Nunes-Nesi, A.; Pärnik, T.; Morgenthal, K.; Wienkoop, S.; Keerberg, O.; Weckwerth, W.; Kleczkowski, L.A.; Fernie, A.R.; Bauwe, H. A cytoplasmic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in arabidopsis. Plant Cell 2008, 20, 2848–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Florian, A.; Jahnke, K.; Nunes-Nesi, A.; Fernie, A.R.; Bauwe, H. The hydroxypyruvate-reducing system in Ara-bidopsis: Multiple enzymes for the same end. Plant Physiol. 2011, 155, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in arabidopsis. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawand, S.; Dorne, A.-J.; Long, D.; Coupland, G.; Mache, R.; Carol, P. Arabidopsis a bout de souffle, which is homologous with mammalian carnitine acyl carrier, is required for postembryonic growth in the light. Plant Cell 2002, 14, 2161–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhut, M.; Planchais, S.; Cabassa, C.; Guivarc’H, A.; Justin, A.-M.; Taconnat, L.; Renou, J.-P.; Linka, M.; Gagneul, D.; Timm, S.; et al. Arabidopsis a bout de souffle is a putative mitochondrial transporter involved in photorespiratory metabolism and is required for meristem growth at ambient CO2 levels. Plant J. 2013, 73, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Florian, A.; Fernie, A.R.; Bauwe, H. The regulatory interplay between photorespiration and photosynthesis. J. Exp. Bot. 2016, 67, 2923–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, M.; Timm, S.; Mettler-Altmann, T.; Borghi, G.L.; Koczor, M.; Arrivault, S.; Weber, A.P.; Bauwe, H.; Gowik, U.; Westhoff, P. Efficient 2-phosphoglycolate degradation is required to maintain carbon assimilation and allocation in the C4 plant Flaveria bidentis. J. Exp. Bot. 2019, 70, 575–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, N.A. Myo-inositol biosynthesis genes in arabidopsis: Differential patterns of gene expression and role in cell death. Plant Cell 2010, 22, 537. [Google Scholar] [CrossRef] [Green Version]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hofman, N.E.; Davis, K.R.; Gorlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [Green Version]
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.A.; Vandorpe, M.; Gakière, B.; Vanacker, H.; Miginiac-Maslow, M.; Van Breusegem, F.; Noctor, G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007, 52, 640–657. [Google Scholar]
- Queval, G.; Neukermans, J.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ. 2012, 35, 374–387. [Google Scholar] [CrossRef]
- Dellero, Y.; Lamothe-Sibold, M.; Jossier, M.; Hodges, M. Arabidopsis thaliana ggt1photorespiratory mutants maintain leaf carbon/nitrogen balance by reducing RuBisCO content and plant growth. Plant J. 2015, 83, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Boldt, R.; Edner, C.; Kolukisaoglu, Ü.; Hagemann, M.; Weckwerth, W.; Wienkoop, S.; Morgenthal, K.; Bauwe, H. D-glycerate 3-kinase, the last unknown enzyme in the photorespiratory cycle in arabidopsis, belongs to a novel kinase family. Plant Cell 2005, 17, 2413–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howitz, K.T.; McCarty, R.E. d-Glycerate transport by the pea chloroplast glycolate carrier: Studies on [1-C]d-glycerate uptake and d-glycerate dependent O(2) evolution. Plant Physiol. 1986, 80, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, N.J.; Butt, V.S. Metabolism and decarboxylation of glycollate and serine in leaf peroxisomes. Planta 1981, 153, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Weraduwage, S.M.; Preiser, A.L.; Tietz, S.; Weise, S.E.; Strand, D.D.; Froehlich, J.E.; Kramer, D.M.; Hu, J.; Sharkey, T.D. A cytoplasmic bypass and G6P shunt in plants lacking peroxisomal hydroxypyruvate reductase. Plant Physiol. 2019, 180, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sharkey, T.D.; Sachar-Hill, Y.; Walker, B.J. The metabolic origins of non-photorespiratory CO2 release during photo-synthesis: A metabolic flux analysis. Plant Physiol. 2021, 186, 297–314. [Google Scholar] [CrossRef]
- Rontein, D.; Nishida, I.; Tashiro, G.; Yoshioka, K.; Wu, W.I.; Voelker, D.R.; Basset, G.; Hanson, A.D. Plants synthesize ethan-olamine by direct decarboxylation of serine using a pyridoxal phosphate enzyme. J. Biol. Chem. 2001, 276, 35523–35529. [Google Scholar] [CrossRef] [Green Version]
- Rontein, D.; Rhodes, D.; Hanson, A.D. Evidence from engineering that decarboxylation of free serine is the major source of ethanolamine moieties in plants. Plant Cell Physiol. 2003, 44, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Yu, S.-I.; Lee, H.; Yim, J.H.; Zhu, J.-K.; Lee, B.-H. Arabidopsis serine decarboxylase mutants implicate the roles of ethanolamine in plant growth and development. Int. J. Mol. Sci. 2012, 13, 3176–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein degradation—An alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate valves: Old shuttles with new perspectives. Plant Biol. 2018, 21, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environ-mental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Bourguignon, J.; Neuburger, M.; Douce, R. Resolution and characterization of the glycine cleavage reaction in pea leaf mito-chondria. Properties of the forward reaction catalyzed by glycine decarboxylase and serine hydroxymethyltransferase. Biochem. J. 1988, 255, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartsch, O.; Mikkat, S.; Hagemann, M.; Bauwe, H. An Autoinhibitory domain confers redox regulation to maize glycerate kinase. Plant Physiol. 2010, 153, 832–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernie, A.R.; Aharoni, A.; Willmitzer, L.; Stitt, M.; Tohge, T.; Kopka, J.; Carroll, A.J.; Saito, K.; Fraser, P.D.; DeLuca, V. Rec-ommendations for reporting metabolite data. Plant Cell 2011, 23, 2477–2482. [Google Scholar] [CrossRef] [Green Version]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Engel, N.; Daele, K.V.D.; Kolukisaoglu, U.; Morgenthal, K.; Weckwerth, W.; Pärnik, T.; Keerberg, O.; Bauwe, H. Deletion of glycine decarboxylase in arabidopsis is lethal under nonphotorespiratory conditions. Plant Physiol. 2007, 144, 1328–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzewuski, G.; Cornell, K.A.; Rooney, L.; Bürstenbinder, K.; Wirtz, M.; Hell, R.; Sauter, M. OsMTN encodes a 5′-methylthioadenosine nucleosidase that is up-regulated during submergence-induced ethylene synthesis in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1505–1514. [Google Scholar] [CrossRef] [Green Version]
- Heeg, C.; Kruse, C.; Jost, R.; Gutensohn, M.; Ruppert, T.; Wirtz, M.; Hell, R. Analysis of the Arabidopsis o-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 2008, 20, 168–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernie, A.R.; Roessner, U.; Trethewey, R.N.; Willmitzer, L. The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 2001, 213, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Porzucek, A.; Nunes-Nesi, A.; Sulpice, R.; Lisec, J.; Centeno, D.C.; Carillo, P.; Leisse, A.; Urbanczyk-Wochniak, E.; Fernie, A.R. Mild reductions in mitochondrial citrate synthase activity result in a compromised nitrate assimilation and reduced leaf pigmentation but have no effect on photosynthetic performance or growth. Plant Physiol. 2008, 147, 115–127. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timm, S.; Nunes-Nesi, A.; Florian, A.; Eisenhut, M.; Morgenthal, K.; Wirtz, M.; Hell, R.; Weckwerth, W.; Hagemann, M.; Fernie, A.R.; et al. Metabolite Profiling in Arabidopsisthaliana with Moderately Impaired Photorespiration Reveals Novel Metabolic Links and Compensatory Mechanisms of Photorespiration. Metabolites 2021, 11, 391. https://doi.org/10.3390/metabo11060391
Timm S, Nunes-Nesi A, Florian A, Eisenhut M, Morgenthal K, Wirtz M, Hell R, Weckwerth W, Hagemann M, Fernie AR, et al. Metabolite Profiling in Arabidopsisthaliana with Moderately Impaired Photorespiration Reveals Novel Metabolic Links and Compensatory Mechanisms of Photorespiration. Metabolites. 2021; 11(6):391. https://doi.org/10.3390/metabo11060391
Chicago/Turabian StyleTimm, Stefan, Adriano Nunes-Nesi, Alexandra Florian, Marion Eisenhut, Katja Morgenthal, Markus Wirtz, Rüdiger Hell, Wolfram Weckwerth, Martin Hagemann, Alisdair R. Fernie, and et al. 2021. "Metabolite Profiling in Arabidopsisthaliana with Moderately Impaired Photorespiration Reveals Novel Metabolic Links and Compensatory Mechanisms of Photorespiration" Metabolites 11, no. 6: 391. https://doi.org/10.3390/metabo11060391
APA StyleTimm, S., Nunes-Nesi, A., Florian, A., Eisenhut, M., Morgenthal, K., Wirtz, M., Hell, R., Weckwerth, W., Hagemann, M., Fernie, A. R., & Bauwe, H. (2021). Metabolite Profiling in Arabidopsisthaliana with Moderately Impaired Photorespiration Reveals Novel Metabolic Links and Compensatory Mechanisms of Photorespiration. Metabolites, 11(6), 391. https://doi.org/10.3390/metabo11060391






