Chronic Effect of a Cafeteria Diet and Intensity of Resistance Training on the Circulating Lysophospholipidome in Young Rats
Abstract
:1. Introduction
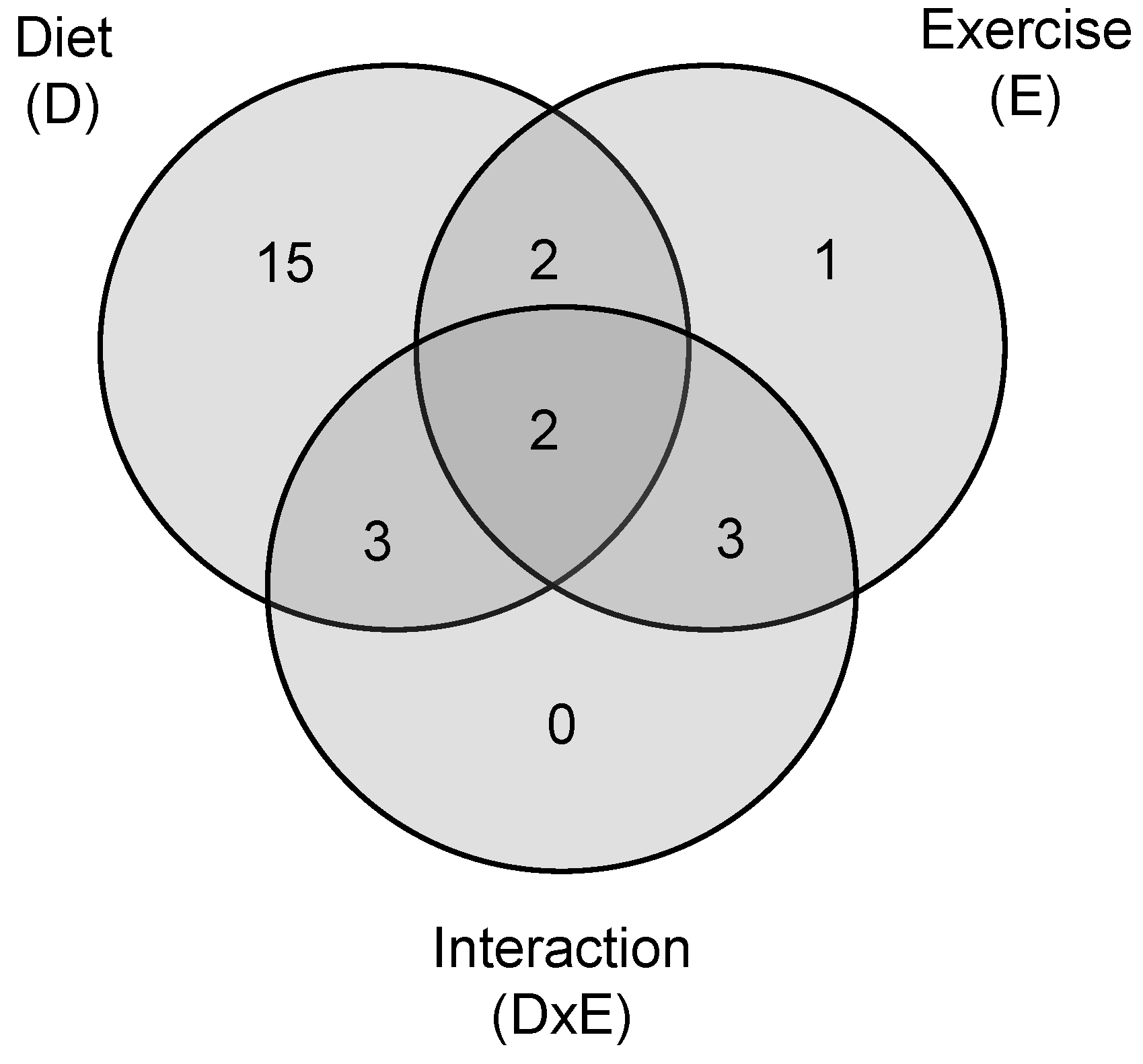
2. Results
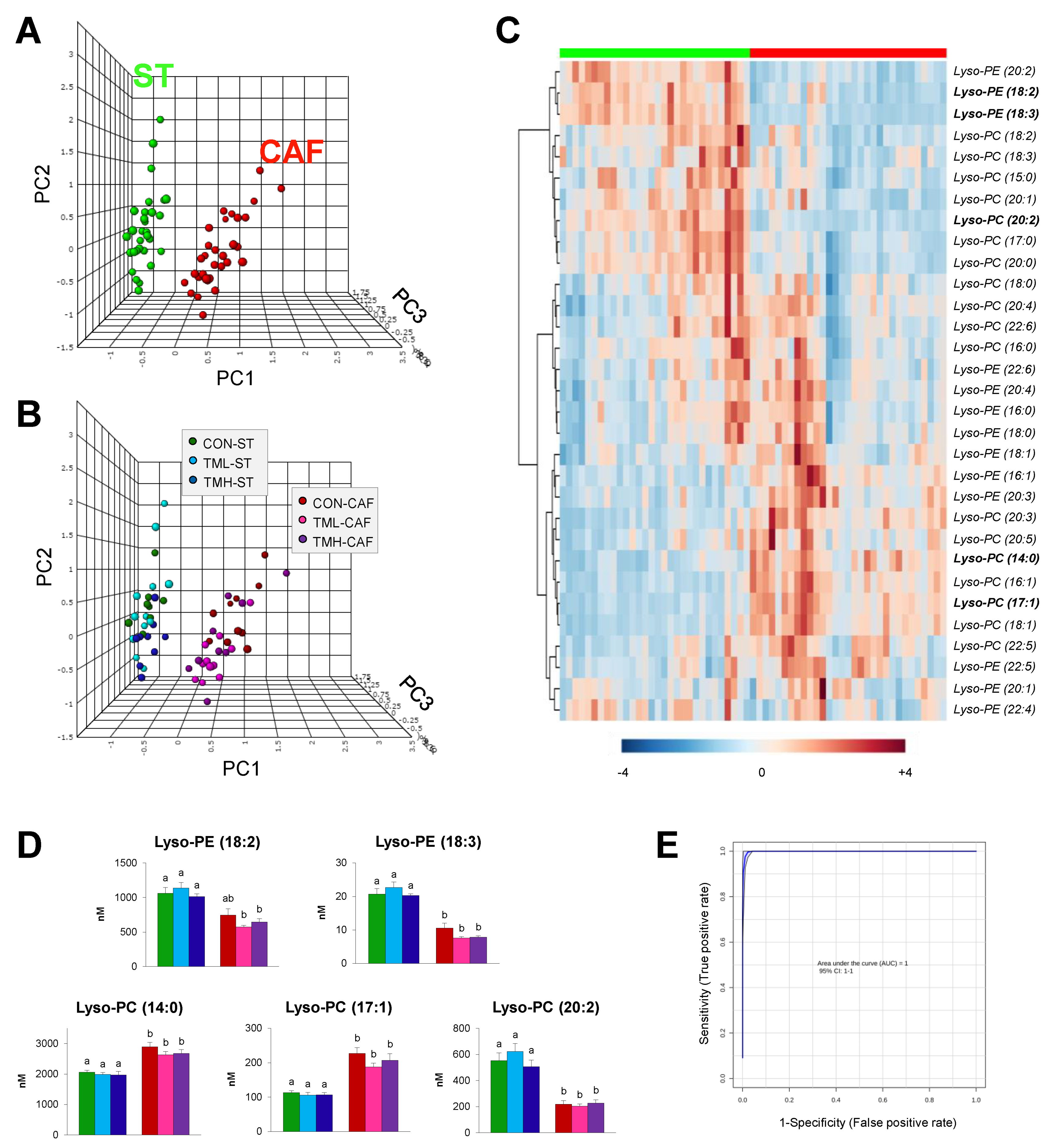
2.1. Determination of the Circulating Levels of Lyso-PLs
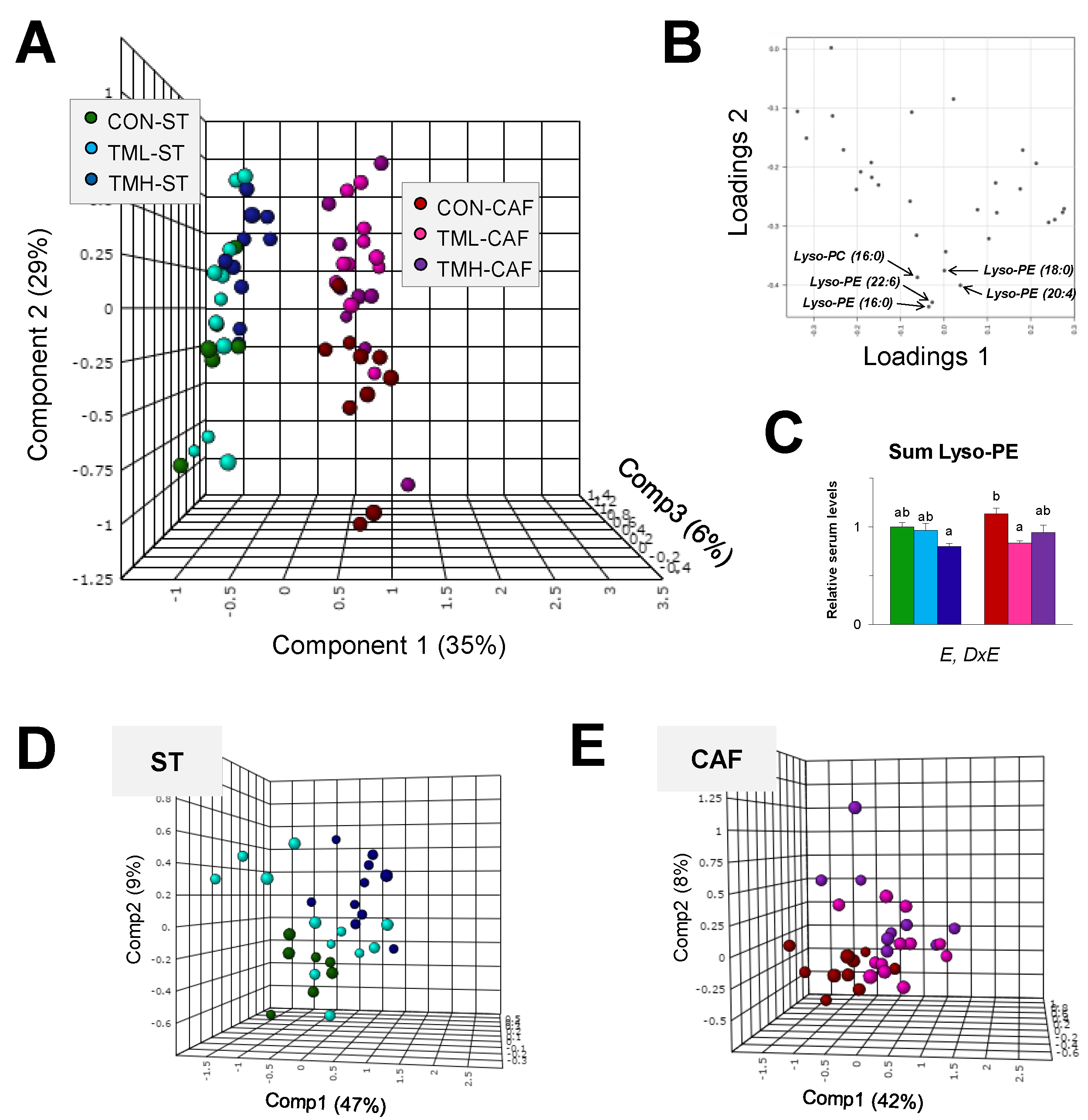
2.2. Multivariate Statistics Reveals Lyso-PLs Alteration Association with Cafeteria Diet
2.3. Lyso-PLs as Poor Indicators of the Practice of Daily Exercise
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Chemicals
4.4. Preparation of Standard Curves
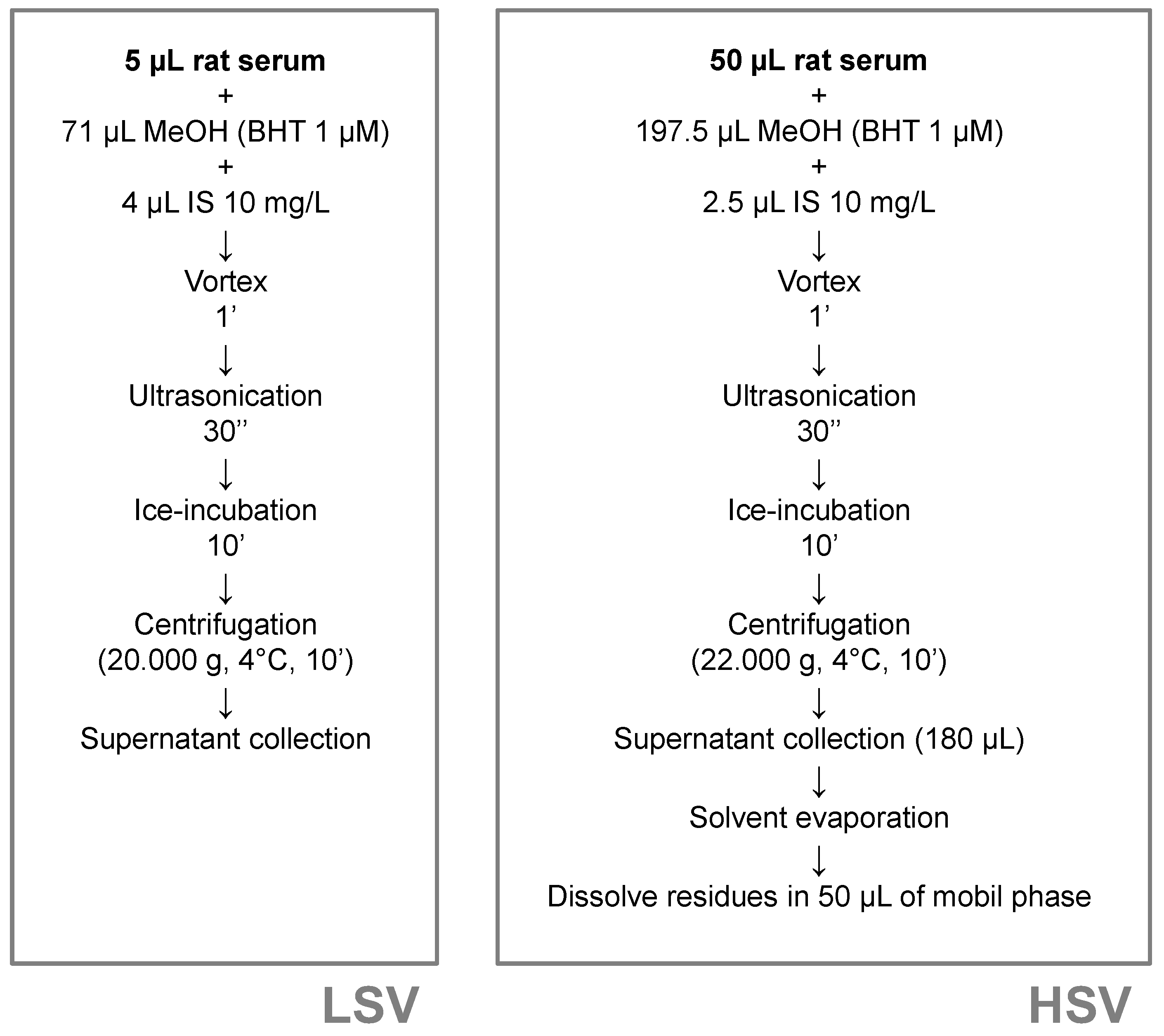
4.5. Sample Processing
4.6. Targeted Metabolomics Analysis of Lyso-PLs
4.7. Statistical Analyses
4.7.1. Univariate Analysis
4.7.2. Multivariate Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Salpea, K.D.; Mikhailidis, D.P. The Prevalence of Metabolic Syndrome in Various Populations. Am. J. Med. Sci. 2007, 333, 362–371. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Boyce, W.F.; Vereecken, C.; Mulvihill, C.; Roberts, C.; Currie, C.; Pickett, W. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes. Rev. 2005, 6, 123–132. [Google Scholar] [CrossRef]
- Chi, C.H.; Wang, Y.; Wilson, D.M.; Robinson, T.N. Definition of metabolic syndrome in preadolescent girls. J. Pediatr. 2006, 148, 788–792.e2. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Román, J.; Arreaza-Gil, V.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Mulero, M.; Muguerza, B.; Arola-Arnal, A.; Arola, L.; Torres-Fuentes, C. Impact of gut microbiota on plasma oxylipins profile under healthy and obesogenic conditions. Clin. Nutr. 2021, 40, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Del Bas, J.M.; Guirro, M.; Boqué, N.; Cereto, A.; Ras, R.; Crescenti, A.; Caimari, A.; Canela, N.; Arola, L. Alterations in gut microbiota associated with a cafeteria diet and the physiological consequences in the host. Int. J. Obes. 2018, 42, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, S.; Del Bas, J.M.; Caimari, A.; Escorihuela, R.M.; Arola, L.; Suárez, M. Impact of a cafeteria diet & daily physical training on the rat serum metabolome. PLoS ONE 2017, 12, e0171970. [Google Scholar] [CrossRef]
- Cigarroa, I.; Lalanza, J.F.; Caimari, A.; del Bas, J.M.; Capdevila, L.; Arola, L.; Escorihuela, R.M. Treadmill Intervention Attenuates the Cafeteria Diet-Induced Impairment of Stress-Coping Strategies in Young Adult Female Rats. PLoS ONE 2016, 11, e0153687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data—New insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Murakami, M. Lipid Mediators in Life Science. Exp. Anim. 2011, 60, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Yin, P.; Tang, L.; Xing, W.; Huang, Q.; Cao, D.; Zhao, X.; Wang, W.; Lu, X.; Xu, Z.; et al. Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: Potential biomarkers effective for small hepatocellular carcinoma diagnosis. Mol. Cell. Proteom. 2012, 11, M111.010694. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Matsubara, T.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012, 56, 118–129. [Google Scholar] [CrossRef]
- Lehmann, R.; Franken, H.; Dammeier, S.; Rosenbaum, L.; Kantartzis, K.; Peter, A.; Zell, A.; Adam, P.; Li, J.; Xu, G.; et al. Circulating Lysophosphatidylcholines Are Markers of a Metabolically Benign Nonalcoholic Fatty Liver. Diabetes Care 2013, 36, 2331–2338. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.J.; Kwon, D.Y.; Jang, Y.; Lee, J.H. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF Mass spectrometry (UPLC-Q-TOF MS). J. Proteome Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef]
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Römisch-Margl, W.; et al. Childhood obesity is associated with changes in the serum metabolite profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, Z.; Ding, S.; Jiang, H.; Li, J.; Shen, J.; Xia, X. Simultaneous determination of nitroimidazoles, benzimidazoles, and chloramphenicol components in bovine milk by ultra-high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2016, 192, 280–287. [Google Scholar] [CrossRef]
- Del Bas, J.M.; Caimari, A.; Rodriguez-Naranjo, M.I.; Childs, C.; Paras Chavez, C.; West, A.; Miles, E.; Arola, L.; Calder, P. Impairment of lysophospholipid metabolism in obesity: Altered plasma profile and desensitization to the modulatory properties of n-3 polyunsaturated fatty acids in a randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 266–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietiläinen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppänen-Laakso, T.; Yki-Järvinen, H.; Kaprio, J.; Oresic, M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects—A monozygotic twin study. PLoS ONE 2007, 2, e218. [Google Scholar] [CrossRef]
- Suárez-García, S.; Caimari, A.; Del Bas, J.M.; Suárez, M.; Arola, L. Serum lysophospholipid levels are altered in dyslipidemic hamsters. Sci. Rep. 2017, 7, 10431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondia-Pons, I.; Martinez, J.A.; de la Iglesia, R.; Lopez-Legarrea, P.; Poutanen, K.; Hanhineva, K.; Zulet, M.D. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Cartron, E.; Carbonneau, M.A.; Fouret, G.; Descomps, B.; Léger, C.L. Specific Antioxidant Activity of Caffeoyl Derivatives and Other Natural Phenolic Compounds: LDL Protection against Oxidation and Decrease in the Proinflammatory Lysophosphatidylcholine Production. J. Nat. Prod. 2001, 64, 480–486. [Google Scholar] [CrossRef]
- Yu, S.; Peng, M.; Ronis, M.; Badger, T.; Fang, N. Analysis of polar lipids in the serum from rats fed shiitake by liquid chromatography-mass spectrometry/mass spectrometry. J. Agric. Food Chem. 2010, 58, 12650–12656. [Google Scholar] [CrossRef]
- Bispo, K.; Piovezan, M.; García-seco, D.; Dudzik, D.; Ramos-solano, B. Blackberry (Rubus sp. var. Loch Ness) extract reduces obesity induced by a cafeteria diet and affects the lipophilic metabolomic profile in rats. J. Food Nutr. Disord. 2014, 3, 4. [Google Scholar]
- Kim, H.J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.T.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y.; et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2010, 10, 722–731. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [Green Version]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2015, 15, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, C.Y.; Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Paik, Y.H.; Lee, E.J.; Lee, J.H. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin. Endocrinol. 2012, 76, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Duggan, G.E.; Hittel, D.S.; Sensen, C.W.; Weljie, A.M.; Vogel, H.J.; Shearer, J. Metabolomic response to exercise training in lean and diet-induced obese mice. J. Appl. Physiol. 2011, 110, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.; Kim, I.Y.; Son, Y.; Byeon, S.K.; Yoon, D.H.; Son, J.S.; Song, H.S.; Song, W.; Seong, J.K.; Moon, M.H. Evaluation of treadmill exercise effect on muscular lipid profiles of diabetic fatty rats by nanoflow liquid chromatography–tandem mass spectrometry. Sci. Rep. 2016, 6, 29617. [Google Scholar] [CrossRef] [Green Version]
- Garvey, S.M.; Russ, D.W.; Skelding, M.B.; Dugle, J.E.; Edens, N.K. Molecular and metabolomic effects of voluntary running wheel activity on skeletal muscle in late middle-aged rats. Physiol. Rep. 2015, 3, e12319. [Google Scholar] [CrossRef]
- Hoene, M.; Li, J.; Li, Y.; Runge, H.; Zhao, X.; Häring, H.U.; Lehmann, R.; Xu, G.; Weigert, C. Muscle and liver-specific alterations in lipid and acylcarnitine metabolism after a single bout of exercise in mice. Sci. Rep. 2016, 6, 22218. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Goto, T.; Yamazaki, Y.; Kamakari, K.; Hirata, M.; Suzuki, H.; Shibata, D.; Nakata, R.; Inoue, H.; Takahashi, N.; et al. Metabolomics reveal 1-palmitoyl lysophosphatidylcholine production by peroxisome proliferator-activated receptor α. J. Lipid Res. 2015, 56, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Sossdorf, M.; Fischer, J.; Meyer, S.; Dahlke, K.; Wissuwa, B.; Seidel, C.; Schrepper, A.; Bockmeyer, C.L.; Lupp, A.; Neugebauer, S.; et al. Physical exercise induces specific adaptations resulting in reduced organ injury and mortality during severe polymicrobial sepsis. Crit. Care Med. 2013, 41, e246–e255. [Google Scholar] [CrossRef]
- Lalanza, J.F.; Sanchez-Roige, S.; Cigarroa, I.; Gagliano, H.; Fuentes, S.; Armario, A.; Capdevila, L.; Escorihuela, R.M. Long-term moderate treadmill exercise promotes stress-coping strategies in male and female rats. Sci. Rep. 2015, 5, 16166. [Google Scholar] [CrossRef] [Green Version]
- Suárez-García, S.; Arola, L.; Pascual-Serrano, A.; Arola-Arnal, A.; Aragonès, G.; Bladé, C.; Suárez, M. Development and validation of a UHPLC-ESI-MS/MS method for the simultaneous quantification of mammal lysophosphatidylcholines and lysophosphatidylethanolamines in serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1055, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Metabolite (µM) | CON-ST | TML-ST | TMH-ST | CON-CAF | TML-CAF | TMH-CAF | 2-Way ANOVA |
|---|---|---|---|---|---|---|---|
| Lyso-PCs | |||||||
| (14:0) | 2.062 ± 0.059 a | 1.988 ± 0.064 a | 1.969 ± 0.113 a | 2.894 ± 0.143 b | 2.632 ± 0.108 b | 2.674 ± 0.129 b | D |
| (15:0) | 1.530 ± 0.075 a | 1.323 ± 0.057 a,b | 1.298 ± 0.064 a,b | 1.220 ± 0.044 b | 1.113 ± 0.038 b | 1.168 ± 0.051 b | D, E |
| (16:0) | 154.774 ± 0.740 a | 138.183 ± 7.787 a,b | 134.710 ± 4.210 b | 145.448 ± 3.447 a,b | 126.934 ± 4.947 b | 131.106 ± 7.666 a,b | E |
| (16:1) | 2.298 ± 0.135 a | 2.405 ± 0.176 a,c | 2.191 ± 0.122 a | 4.378 ± 0.326 b | 3.405 ± 0.127 b | 3.744 ± 0.342 b,c | D, DxE |
| (17:0) | 3.374 ± 0.209 a | 3.521 ± 0.278 a,b | 2.962 ± 0.207 ac | 2.517 ± 0.122 b,c | 2.289 ± 0.116 c | 2.195 ± 0.177 c | D |
| (17:1) | 0.113 ± 0.006 a | 0.106 ± 0.008 a | 0.107 ± 0.007 a | 0.227 ± 0.017 b | 0.188 ± 0.012 b | 0.207 ± 0.020 b | D |
| (18:0) | 223.830 ± 8.906 | 254.579 ± 16.940 | 220.397 ± 10.399 | 235.556 ± 12.324 | 211.205 ± 9.884 | 223.689 ± 10.308 | |
| (18:1) | 10.704 ± 0.282 a | 12.041 ± 1.212 a | 10.943 ± 0.652 a | 22.178 ± 1.427 b | 17.845 ± 1.032 b | 21.538 ± 1.940 b | D, DxE |
| (18:2) | 31.608 ± 1.393 a | 31.560 ± 2.673 a,b | 28.4467 ± 1.703 a,b | 23.611 ± 1.627 b,c | 21.097 ± 1.051 c | 24.427 ± 2.059 a,c | D |
| (18:3) | 0.471 ± 0.039 | 0.579 ± 0.074 | 0.512 ± 0.075 | 0.382 ± 0.049 | 0.318 ± 0.039 | 0.317 ± 0.048 | D |
| (20:0) | 0.504 ± 0.031 a | 0.530 ± 0.045 a | 0.434 ± 0.013 a,b | 0.308 ± 0.022 b,c | 0.271 ± 0.021 c | 0.276 ± 0.028 c | D |
| (20:1) | 1.425 ± 0.079 a | 1.441 ± 0.106 a | 1.122 ± 0.089 a,b | 0.888 ± 0.048 b,c | 0.763 ± 0.056 c | 1.109 ± 0.157 a,c | D, DxE |
| (20:2) | 0.552 ± 0.059 a | 0.622 ± 0.062 a | 0.506 ± 0.052 a | 0.219 ± 0.028 b | 0.203 ± 0.018 b | 0.227 ± 0.025 b | D |
| (20:3) | 0.757 ± 0.062 a,c | 0.803 ± 0.115 a,c | 0.722 ± 0.107 a | 1.724 ± 0.136 b | 1.647 ± 0.122 b | 1.814 ± 0.273 b,c | D |
| (20:4) | 53.596 ± 2.983 | 55.154 ± 5.681 | 46.297 ± 3.136 | 61.430 ± 2.813 | 51.069 ± 3.096 | 55.131 ± 4.538 | |
| (20:5) | 0.117 ± 0.027 a | 0.137 ± 0.029 a,b | 0.120 ± 0.033 a,b | 0.266 ± 0.035 b | 0.189 ± 0.025 a,b | 0.250 ± 0.074 a,b | D |
| (22:5) | 0.595 ± 0.128 a,b | 0.470 ± 0.064 a | 0.470 ± 0.047 a | 0.807 ± 0.059 b | 0.738 ± 0.110 a,b | 0.757 ± 0.141 a,b | D |
| (22:6) | 6.452 ± 0.675 | 6.622 ± 0.695 | 4.863 ± 0.244 | 6.357 ± 0.388 | 4.950 ± 0.415 | 5.253 ± 0.585 | |
| Lyso-PEs | |||||||
| (16:0) | 4.730 ± 0.291 a | 4.484 ± 0.319 a,b | 3.510 ± 0.165 b | 4.923 ± 0.215 a | 3.749 ± 0.183 b | 4.241 ± 0.355 a,b | E, DxE |
| (16:1) | 0.027 ± 0.002 a | 0.030 ± 0.003 a,c | 0.028 ± 0.002 a | 0.058 ± 0.005 b | 0.039 ± 0.002 b,c | 0.041 ± 0.002 b,c | D, E, DxE |
| (18:0) | 9.292 ± 0.427 a,b | 9.590 ± 0.819 a,b | 7.355 ± 0.290 a | 11.173 ± 0.641 b | 8.270 ± 0.265 a | 9.059 ± 0.779 a,b | E, DxE |
| (18:1) | 1.109 ± 0.055 a,b | 1.024 ± 0.041 a | 1.038 ± 0.059 a | 1.364 ± 0.095 b | 1.077 ± 0.046 a | 1.280 ± 0.067 a,b | D, E |
| (18:2) | 1.061 ± 0.086 a | 1.138 ± 0.080 a | 1.014 ± 0.040 a | 0.743 ± 0.092 a,b | 0.574 ± 0.024 b | 0.646 ± 0.048 b | D |
| (18:3) | 0.021 ± 0.002 a | 0.023 ± 0.002 a | 0.020 ± 0.001 a | 0.011 ± 0.001 b | 0.008 ± 0.000 b | 0.008 ± 0.000 b | D |
| (20:1) | 0.050 ± 0.003 | 0.047 ± 0.004 | 0.039 ± 0.002 | 0.056 ± 0.008 | 0.043 ± 0.004 | 0.056 ± 0.004 | |
| (20:2) | 0.031 ± 0.002 a | 0.032 ± 0.003 a | 0.028 ± 0.001 a | 0.018 ± 0.002 b | 0.014 ± 0.001 b | 0.017 ± 0.001 b | D |
| (20:3) | 0.027 ± 0.002 a,c | 0.026 ± 0.001 a | 0.027 ± 0.003 a,b,c | 0.043 ± 0.004 b,c | 0.036 ± 0.002 bc | 0.040 ± 0.003 b | D |
| (20:4) | 1.123 ± 0.046 a,b | 1.115 ± 0.094 a | 1.021 ± 0.077 a | 1.466 ± 0.099 b | 1.015 ± 0.048 a | 1.173 ± 0.090 a,b | D, E, DxE |
| (22:4) | 0.047 ± 0.002 | 0.045 ± 0.003 | 0.044 ± 0.002 | 0.045 ± 0.003 | 0.039 ± 0.002 | 0.043 ± 0.002 | |
| (22:5) | 0.046 ± 0.008 a,b | 0.037 ± 0.003 a | 0.039 ± 0.004 a | 0.061 ± 0.005 b | 0.048 ± 0.006 a,b | 0.050 ± 0.007 a,b | D |
| (22:6) | 0.337 ± 0.030 a,b | 0.336 ± 0.036 a,b | 0.237 ± 0.011 a | 0.379 ± 0.025 b | 0.250 ± 0.018 a | 0.280 ± 0.037 a,b | E, DxE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-García, S.; Caimari, A.; Bas, J.M.d.; Lalanza, J.; Escorihuela, R.M.; Suárez, M.; Torres-Fuentes, C.; Arola, L. Chronic Effect of a Cafeteria Diet and Intensity of Resistance Training on the Circulating Lysophospholipidome in Young Rats. Metabolites 2021, 11, 471. https://doi.org/10.3390/metabo11080471
Suárez-García S, Caimari A, Bas JMd, Lalanza J, Escorihuela RM, Suárez M, Torres-Fuentes C, Arola L. Chronic Effect of a Cafeteria Diet and Intensity of Resistance Training on the Circulating Lysophospholipidome in Young Rats. Metabolites. 2021; 11(8):471. https://doi.org/10.3390/metabo11080471
Chicago/Turabian StyleSuárez-García, Susana, Antoni Caimari, Josep M. del Bas, Jaume Lalanza, Rosa M. Escorihuela, Manuel Suárez, Cristina Torres-Fuentes, and Lluís Arola. 2021. "Chronic Effect of a Cafeteria Diet and Intensity of Resistance Training on the Circulating Lysophospholipidome in Young Rats" Metabolites 11, no. 8: 471. https://doi.org/10.3390/metabo11080471
APA StyleSuárez-García, S., Caimari, A., Bas, J. M. d., Lalanza, J., Escorihuela, R. M., Suárez, M., Torres-Fuentes, C., & Arola, L. (2021). Chronic Effect of a Cafeteria Diet and Intensity of Resistance Training on the Circulating Lysophospholipidome in Young Rats. Metabolites, 11(8), 471. https://doi.org/10.3390/metabo11080471









