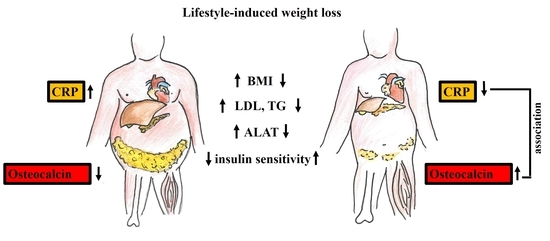Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome
Abstract
:1. Introduction
2. Results
2.1. Clinical and Laboratory Parameters
2.2. Correlation Analysis of Osteocalcin and Parameters of the Metabolic Syndrome
2.3. Multiple Regression Analysis between Osteocalcin and Parameters of the Metabolic Syndrome
3. Discussion
4. Materials and Methods
4.1. Research Design
4.2. Dual Energy X-ray Absorptiometry
4.3. Clinical and Laboratory Parameters
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Goldberg, R.B.; Mather, K. Targeting the Consequences of the Metabolic Syndrome in the Diabetes Prevention Program. Arter. Thromb. Vasc. Biol. 2012, 32, 2077–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.-Y.; Chen, F.-Q.; Hong, H.; Lv, X.-J.; Dong, M.; Wang, Q.-Y. The Relationship between Serum Osteocalcin Concentration and Glucose and Lipid Metabolism in Patients with Type 2 Diabetes Mellitus—The Role of Osteocalcin in Energy Metabolism. Ann. Nutr. Metab. 2015, 66, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine Regulation of Energy Metabolism by the Skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, P.; Arrieta, F.; Piñera, M.; Botella-Carretero, J.I.; Balsa, J.A.; Zamarrón, I.; Menacho, M.; Díez, J.J.; Muñoz, T.; Vázquez, C. Serum concentrations of osteocalcin, procollagen type 1 N-terminal propeptide and beta-CrossLaps in obese subjects with varying degrees of glucose tolerance. Clin. Endocrinol. 2011, 75, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Tubic, B.; Magnusson, P.; Mårild, S.; Leu, M.; Schwetz, V.; Sioen, I.; Herrmann, D.; Obermayer-Pietsch, B.; Lissner, L.; Swolin-Eide, D. Different osteocalcin forms, markers of metabolic syndrome and anthropometric measures in children within the IDEFICS cohort. Bone 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Laurent, M.; EMAS Group; Cook, M.; Gielen, E.; Ward, K.; Antonio, L.; Adams, J.E.; Decallonne, B.; Bartfai, G.; Casanueva, F.F.; et al. Lower bone turnover and relative bone deficits in men with metabolic syndrome: A matter of insulin sensitivity? The European Male Ageing Study. Osteoporos. Int. 2016, 27, 3227–3237. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yeap, B.B.; Brock, K.E.; Levinger, I.; Golledge, J.; Flicker, L.; Brennan-Speranza, T.C. Associations of Osteocalcin Forms With Metabolic Syndrome and Its Individual Components in Older Men: The Health In Men Study. J. Clin. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef]
- Mohammad Rahimi, G.R.; Bijeh, N.; Rashidlamir, A. Effects of exercise training on serum preptin, undercarboxylated osteocal-cin and high molecular weight adiponectin in adults with metabolic syndrome. Exp. Physiol. 2020, 105, 449–459. [Google Scholar] [CrossRef]
- Nakamura, A.; Dohi, Y.; Akahane, M.; Ohgushi, H.; Nakajima, H.; Funaoka, H.; Takakura, Y. Osteocalcin Secretion as an Early Marker ofIn VitroOsteogenic Differentiation of Rat Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2009, 15, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nat. Cell Biol. 1996, 382, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Rifai, O.; Julien, C.; Lacombe, J.; Faubert, D.; Lira-Navarrete, E.; Narimatsu, Y.; Clausen, H.; Ferron, M. The half-life of the bone-derived hormone osteocalcin is regulated through O-glycosylation in mice, but not in humans. eLife 2020, 9, e61174. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; McKee, M.D.; Levine, R.L.; Ducy, P.; Karsenty, G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012, 50, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, M.; Wu, Y.; Quarles, L.D. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J. Bone Miner. Res. 2011, 26, 1680–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geserick, M.; Vogel, M.; Eckelt, F.; Schlingmann, M.; Hiemisch, A.; Baber, R.; Thiery, J.; Körner, A.; Kiess, W.; Kratzsch, J. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations—Results derived from new pediatric reference ranges. Bone 2020, 132, 115124. [Google Scholar] [CrossRef]
- Hill, H.S.; Gramß, J.; Walton, R.G.; Liu, J.; Moellering, D.R.; Garvey, W.T. Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm. Metab. Res. 2014, 46, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Biemann, R.; Penner, M.; Borucki, K.; Westphal, S.; Luley, C.; Rönicke, R.; Biemann, K.; Weikert, C.; Lux, A.; Goncharenko, N.; et al. Serum bile acids and GLP-1 decrease following telemetric induced weight loss: Results of a randomized controlled trial. Sci. Rep. 2016, 6, 30173. [Google Scholar] [CrossRef]
- Schett, G.; Kiechl, S.; Weger, S.; Pederiva, A.; Mayr, A.; Petrangeli, M.; Oberhollenzer, F.; Lorenzini, R.; Redlich, K.; Axmann, R.; et al. High-sensitivity C-reactive protein and risk of non-traumatic fractures in the Bruneck study. Arch. Intern. Med. 2006, 166, 2495–2501. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Huang, L.; Mao, Y.; Jiang, Y.; Yao, Z.; Lin, X.; Lu, Z.; Wu, C.; Qin, X.; Zhang, H.; et al. Serum Osteocalcin Is Associated with Inflammatory Factors in Meta-bolic Syndrome: A Population-Based Study in Chinese Males. Mediat. Inflamm. 2015, 2015, 683739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme-Gallego, B.; García-Molina, L.; Cano-Ibáñez, N.; Sánchez-Delgado, G.; Andújar-Vera, F.; García-Fontana, C.; González-Salvatierra, S.; García-Recio, E.; Martínez-Ruiz, V.; Bueno-Cavanillas, A.; et al. Circulating Undercarboxylated Osteocalcin as Estimator of Cardiovascular and Type 2 Diabetes Risk in Metabolic Syndrome Patients. Sci. Rep. 2020, 10, 1840. [Google Scholar] [CrossRef]
- Lucey, A.J.; Paschos, G.K.; Thorsdottir, I.; Martínéz, J.A.; Cashman, K.D.; Kiely, M. Young overweight and obese women with lower circulating osteocalcin concentrations exhibit higher insulin resistance and concentrations of C-reactive protein. Nutr. Res. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Sarkar, P.D.; Choudhury, A.B. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1631–1635. [Google Scholar]
- Chin, K.-Y.; Ima-Nirwana, S.; Mohamed, I.N.; Ahmad, F.; Ramli, E.S.M.; Aminuddin, A.; Ngah, W.Z.W. Serum Osteocalcin Is Signifi-cantly Related to Indices of Obesity and Lipid Profile in Malaysian Men. Int. J. Med. Sci. 2014, 11, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polgreen, L.E.; Jacobs, D.R., Jr.; Nathan, B.M.; Steinberger, J.; Moran, A.; Sinaiko, A.R. Association of Osteocalcin With Obesity, Insulin Resistance, and Cardiovascular Risk Factors in Young Adults. Obesity 2012, 20, 2194–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Confavreux, C.B.; Szulc, P.; Casey, R.; Varennes, A.; Goudable, J.; Chapurlat, R.D. Lower serum osteocalcin is associated with more severe metabolic syndrome in elderly men from the MINOS cohort. Eur. J. Endocrinol. 2014, 171, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Movahed, A.; Larijani, B.; Nabipour, I.; Kalantarhormozi, M.; Asadipooya, K.; Vahdat, K.; Akbarzadeh, S.; Farrokhnia, M.; Assadi, M.; Amirinejad, R.; et al. Reduced serum osteocalcin concen-trations are associated with type 2 diabetes mellitus and the metabolic syndrome components in postmenopausal women: The crosstalk between bone and energy metabolism. J. Bone Miner. Metab. 2012, 30, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Mezquita-Raya, P.; Muñoz-Torres, M.; Luna, J.D.D.; Luna, V.; Lopez-Rodriguez, F.; Torres-Vela, E.; Escobar-Jiménez, F. Relation Between Vitamin D Insufficiency, Bone Density, and Bone Metabolism in Healthy Postmenopausal Women. J. Bone Miner. Res. 2001, 16, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yamamoto, M.; Yamauchi, M.; Kurioka, S.; Yano, S.; Sugimoto, T. Serum Osteocalcin Level Is Associated with Glucose Metabolism and Atherosclerosis Parameters in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2009, 94, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Luley, C.; Blaik, A.; Götz, A.; Kicherer, F.; Kropf, S.; Isermann, B.; Stumm, G.; Westphal, S. Weight Loss by Telemonitoring of Nutrition and Physical Activity in Patients with Metabolic Syndrome for 1 Year. J. Am. Coll. Nutr. 2014, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, S.; Costa, M.B.W.; Mathew, A.; Krishnan, S.; Schneider, J.G.; Roomp, K.; Isermann, B.; Biemann, R. Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome. Metabolites 2021, 11, 526. https://doi.org/10.3390/metabo11080526
Zimmermann S, Costa MBW, Mathew A, Krishnan S, Schneider JG, Roomp K, Isermann B, Biemann R. Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome. Metabolites. 2021; 11(8):526. https://doi.org/10.3390/metabo11080526
Chicago/Turabian StyleZimmermann, Silke, Maria Beatriz Walter Costa, Akash Mathew, Shruthi Krishnan, Jochen G. Schneider, Kirsten Roomp, Berend Isermann, and Ronald Biemann. 2021. "Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome" Metabolites 11, no. 8: 526. https://doi.org/10.3390/metabo11080526
APA StyleZimmermann, S., Costa, M. B. W., Mathew, A., Krishnan, S., Schneider, J. G., Roomp, K., Isermann, B., & Biemann, R. (2021). Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome. Metabolites, 11(8), 526. https://doi.org/10.3390/metabo11080526