A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox
Abstract
:1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Food/Nutrient Intake Data
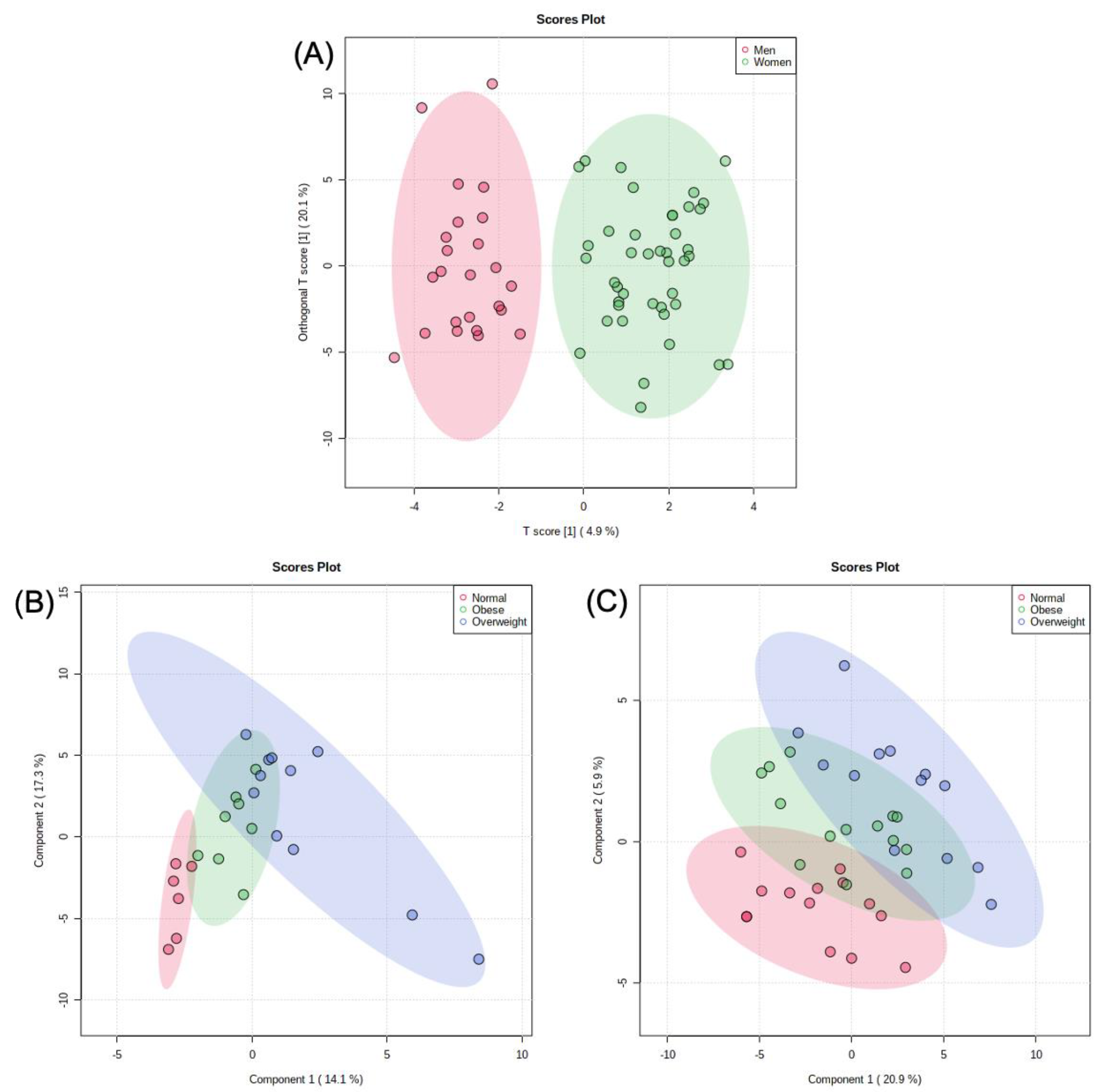
2.3. Metabolomic Analysis
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Anthropometric Assessment
4.3. Biological Markers Assessment
4.4. Diet/Lifestyle Assessment
4.5. Sample Preparation for Metabolomic Analyses
4.6. LC-MS/MS Analyses
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22 (Suppl. 7), s176–s185. [Google Scholar]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Fontaine, K.R.; McCubrey, R.; Mehta, T.; Pajewski, N.M.; Keith, S.W.; Bangalore, S.S.; Crespo, C.J.; Allison, D.B. Body mass index and mortality rate among Hispanic adults: A pooled analysis of multiple epidemiologic data sets. Int. J. Obes. 2012, 36, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Teruya, S.A.; Bazargan-Hejazi, S. The Immigrant and Hispanic Paradoxes: A Systematic Review of Their Predictions and Effects. Hisp. J. Behav. Sci. 2013, 35, 486–509. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA 2006, 295, 1549. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Eberhardt, M.S.; Flegal, K.M.; Engelgau, M.M.; Saydah, S.H.; Williams, D.E.; Geiss, L.S.; Gregg, E.W. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the U.S. Population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 2006, 29, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, L.B.; Sundquist, J.; Winkleby, M. Differences in energy, nutrient, and food intakes in a US sample of Mexican-American women and men: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2000, 152, 548–557. [Google Scholar] [CrossRef]
- Montez, J.K.; Eschbach, K. Country of birth and language are uniquely associated with intakes of fat, fiber, and fruits and vegetables among Mexican-American women in the United States. J. Am. Diet. Assoc. 2008, 108, 473–480. [Google Scholar] [CrossRef]
- Din-Dzietham, R.; Liu, Y.; Bielo, M.-V.; Shamsa, F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 2007, 116, 1488–1496. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. Trend in the prevalence of the metabolic syndrome and its impact on cardiovascular disease incidence: The San Antonio Heart Study. Diabetes Care 2006, 29, 625–630. [Google Scholar] [CrossRef] [Green Version]
- McNaughton, S.A.; Mishra, G.D.; Stephen, A.M.; Wadsworth, M.E.J. Dietary patterns throughout adult life are associated with body mass index, waist circumference, blood pressure, and red cell folate. J. Nutr. 2007, 137, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.N.; Horino, M.; McCarthy, W.J. Adult Intake of Minimally Processed Fruits and Vegetables: Associations with Cardiometabolic Disease Risk Factors. J. Acad. Nutr. Diet. 2016, 116, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009, 33, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [Green Version]
- Butler, T.J.; Ashford, D.; Seymour, A.M. Western diet increases cardiac ceramide content in healthy and hypertrophied hearts. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 991–998. [Google Scholar] [CrossRef]
- Dhungana, S.; Carlson, J.E.; Pathmasiri, W.; McRitchie, S.; Davis, M.; Sumner, S.; Appt, S.E. Impact of a western diet on the ovarian and serum metabolome. Maturitas 2016, 92, 134–142. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The western diet–microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Kautzky-Willer, A.; Harreiter, J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017, 131, 230–241. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; López-Grueso, R.; Gambini, J.; Monleón, D.; Mas-Bargues, C.; Abdelaziz, K.M.; Viña, J.; Borrás, C. Sex differences in age-associated type 2 diabetes in rats—Role of estrogens and oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 6734836. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zhai, G.; Macgregor, A.; Prehn, C.; Römisch-Margl, W.; Suhre, K.; Adamski, J.; Cassidy, A.; Illig, T.; Spector, T.D.; et al. Targeted metabolomics profiles are strongly correlated with nutritional patterns in women. Metabolomics 2013, 9, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, S.; Navarro, S.L.; Buas, M.F.; Schwarz, Y.; Gu, H.; Djukovic, D.; Raftery, D.; Kratz, M.; Neuhouser, M.L.; Lampe, J.W. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct. 2015, 6, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, P.; Zhu, J.; Raftery, D. Globally Optimized Targeted Mass Spectrometry: Reliable Metabolomics Analysis with Broad Coverage. Anal. Chem. 2015, 87, 12355–12362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Wang, S.; Jasbi, P.; Turner, C.; Hrovat, J.; Wei, Y.; Liu, J.; Gu, H. Database Assisted Globally Optimized Targeted Mass Spectrometry (dGOT-MS): Broad and Reliable Metabolomics Analysis with Enhanced Identification. Anal. Chem. 2019, 91, 13737–13745. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Sarmidi, M.R.; Cheng, K.-K.; Ali Khan, A.; Suan, C.L.; Zaman Huri, H.; Yaakob, H. Metabolomics—The complementary field in systems biology: A review on obesity and type 2 diabetes. Mol. Biosyst. 2015, 11, 1742–1774. [Google Scholar] [CrossRef]
- Jasbi, P.; Wang, D.; Cheng, S.L.; Fei, Q.; Cui, J.Y.; Liu, L.; Wei, Y.; Raftery, D.; Gu, H. Breast cancer detection using targeted plasma metabolomics. J. Chromatogr. B 2019, 1105, 26–37. [Google Scholar] [CrossRef]
- Parent, B.A.; Seaton, M.; Djukovic, D.; Gu, H.; Wheelock, B.; Navarro, S.L.; Raftery, D.; O’Keefe, G.E. Parenteral and enteral nutrition in surgical critical care: Plasma metabolomics demonstrates divergent effects on nitrogen, fatty-acid, ribonucleotide, and oxidative metabolism. J. Trauma Acute Care Surg. 2017, 82, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Brennan, L. Metabolomics in nutrition research-a powerful window into nutritional metabolism. Essays Biochem. 2016, 60, 451–458. [Google Scholar] [CrossRef]
- Jasbi, P.; Mitchell, N.M.; Shi, X.; Grys, T.E.; Wei, Y.; Liu, L.; Lake, D.F.; Gu, H. Coccidioidomycosis Detection Using Targeted Plasma and Urine Metabolic Profiling. J. Proteome Res. 2019, 18, 2791–2802. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Kluttig, A.; Giegling, I.; Prehn, C.; Adamski, J.; Kastenmüller, G.; Lacruz, M.E. The human metabolic profile reflects macro- and micronutrient intake distinctly according to fasting time. Sci. Rep. 2018, 8, 12262. [Google Scholar] [CrossRef]
- Collet, T.-H.; Sonoyama, T.; Henning, E.; Keogh, J.M.; Ingram, B.; Kelway, S.; Guo, L.; Farooqi, I.S. A Metabolomic Signature of Acute Caloric Restriction. J. Clin. Endocrinol. Metab. 2017, 102, 4486–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Yoo, H.J.; Kim, M.; Kim, M.; Lee, J.H. Metabolomics identifies increases in the acylcarnitine profiles in the plasma of overweight subjects in response to mild weight loss: A randomized, controlled design study. Lipids Health Dis. 2018, 17, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirulli, E.T.; Guo, L.; Leon Swisher, C.; Shah, N.; Huang, L.; Napier, L.A.; Kirkness, E.F.; Spector, T.D.; Caskey, C.T.; Thorens, B.; et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019, 29, 488–500.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Shen, J.; Djukovic, D.; Daniel-MacDougall, C.; Gu, H.; Wu, X.; Chow, W.-H. Metabolomics-identified metabolites associated with body mass index and prospective weight gain among Mexican American women. Obes. Sci. Pract. 2016, 2, 309–317. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- Daviglus, M.L.; Pirzada, A.; Talavera, G.A. Cardiovascular risk factors in the Hispanic/Latino population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog. Cardiovasc. Dis. 2014, 57, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.E.; Martin, O.J.; Noland, R.C.; Slentz, D.H.; DeBalsi, K.L.; Ilkayeva, O.R.; An, J.; Newgard, C.B.; Koves, T.R.; Muoio, D.M. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J. Lipid Res. 2014, 55, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Dudek, M.; Knutelska, J.; Bednarski, M.; Nowiński, L.; Zygmunt, M.; Kazek, G.; Mordyl, B.; Głuch-Lutwin, M.; Zaręba, P.; Kulig, K.; et al. Pyrrolidin-2-one derivatives may reduce body weight in rats with diet-induced obesity. Eur. J. Pharmacol. 2016, 776, 146–155. [Google Scholar] [CrossRef]
- Xu, W.Y.; Shen, Y.; Zhu, H.; Gao, J.; Zhang, C.; Tang, L.; Lu, S.Y.; Shen, C.L.; Zhang, H.X.; Li, Z.; et al. 2-Aminoadipic acid protects against obesity and diabetes. J. Endocrinol. 2019, 243, 111–123. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. Landmark 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [Green Version]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, D.; Yang, Q.; Wang, H.; Melick, C.H.; Navlani, R.; Frank, A.R.; Jewell, J.L. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 2020, 295, 2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through the use as an amino acid exchange factor. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The physiological action of picolinic acid in the human brain. Int. J. Tryptophan Res. 2009, 2, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Y.; Zhong, Z.S.; Ye, Z.H.; Zhang, W.; He, G.H.; Zheng, Y.F.; Huang, S.P. D-galacturonic acid ameliorates the intestinal mucosal permeability and inflammation of functional dyspepsia in rats. Ann. Palliat. Med. 2021, 10, 538–548. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Zhao, W.; Song, J.; Yin, L.; Wang, K.; Wei, L.; Xu, Y.; Li, H.; Min, B.; Tang, N.; et al. The Relationship of Large-Artery Atherothrombotic Stroke with Plasma Trimethylamine N-Oxide Level and Blood Lipid-Related Indices: A Cross-Sectional Comparative Study. Biomed Res. Int. 2021, 2021, 5549796. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; Debosch, B.; Softic, S. Pathogenesis of Type 2 Diabetes and Insulin Resistance (M-E Patti, Section Editor) Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diabetes Rep. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.H.; Chung, B.C. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids 2016, 111, 54–59. [Google Scholar] [CrossRef]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transpl. 2018, 33, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Zisman, A.L. Effectiveness of Treatment Modalities on Kidney Stone Recurrence. Clin. J. Am. Soc. Nephrol. 2017, 12, 1699. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018, 27, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Sekine, A.; Okamoto, M.; Kanatani, Y.; Sano, M.; Shibata, K.; Fukuwatari, T. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. Springerplus 2015, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.; Fairlie, D.P.; Brown, L. Lysine acetylation in obesity, diabetes and metabolic disease. Immunol. Cell Biol. 2012, 90, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.F.; Sun, X.Y.; Han, L.; Li, S.N.; Gu, W.Q.; Song, M.; Jiang, C.T.; Yang, X.; Fang, Z.Z. Plasma tyrosine and its interaction with low high-density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J. Diabetes Investig. 2019, 10, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acidsare predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Tobias, D.K.; Mora, S.; Verma, S.; Lawler, P.R. Altered branched chain amino acid metabolism: Toward a unifying cardiometabolic hypothesis. Curr. Opin. Cardiol. 2018, 33, 558–564. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Vargas-Morales, J.M.; Méndez-García, A.L.; López-Barradas, A.M.; Granados-Portillo, O.; Ordaz-Nava, G.; Rocha-Viggiano, A.K.; Gutierrez-Leyte, C.A.; Medina-Cerda, E.; Rosado, J.L.; et al. Amino acid profiles of young adults differ by sex, body mass index and insulin resistance. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 393–401. [Google Scholar] [CrossRef]
- Tochikubo, O.; Nakamura, H.; Jinzu, H.; Nagao, K.; Yoshida, H.; Kageyama, N.; Miyano, H. Weight loss is associated with plasma free amino acid alterations in subjects with metabolic syndrome. Nutr. Diabetes 2016, 6, e197. [Google Scholar] [CrossRef] [Green Version]
- Ivy, J.L. Effect of pyruvate and dihydroxyacetone on metabolism and aerobic endurance capacity. Med. Sci. Sports Exerc. 1998, 30, 837–843. [Google Scholar]
- Mero, A.A.; Ojala, T.; Hulmi, J.J.; Puurtinen, R.; Karila, T.A.; Seppälä, T. Effects of alfa-hydroxy-isocaproic acid on body composition, DOMS and performance in athletes. J. Int. Soc. Sports Nutr. 2010, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Hootman, K.C.; Trezzi, J.-P.; Kraemer, L.; Burwell, L.S.; Dong, X.; Guertin, K.A.; Jaeger, C.; Stover, P.J.; Hiller, K.; Cassano, P.A. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc. Natl. Acad. Sci. USA 2017, 114, E4233–E4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; O’Brien, S.V.; Hayden, K.; Pandya, B.; Lisboa, P.J.G.; Hardy, K.J.; Wilding, J.P.H. Effect of a cooked meat meal on serum creatinine and estimated glomerular filtration rate in diabetes-related kidney disease. Diabetes Care 2014, 37, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashima, S.; Inoue, K.; Matsumoto, M.; Akimoto, K. Low serum creatinine is a type 2 diabetes risk factor in men and women: The Yuport Health Checkup Center cohort study. Diabetes Metab. 2017, 43, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Leung, L.H. Pantothenic acid as a weight-reducing agent: Fasting without hunger, weakness and ketosis. Med. Hypotheses 1995, 44, 403–405. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, X.; Zhong, W. Peptide Sequence Influence on the Differentiation of Valine and Norvaline by Hot Electron Capture Dissociation. Anal. Chem. 2019, 91, 4381–4387. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Meng, Q.; Zhang, Q.; Guo, F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012, 43, 725–734. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Bene, J.; Hadzsiev, K.; Melegh, B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 2018, 8, 8. [Google Scholar] [CrossRef]
- Chmielewski, M.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B. Uremic Toxicity. Nutr. Manag. Ren. Dis. 2013, 49–77. [Google Scholar] [CrossRef]
- Gupta, P.; Ibrahim, A.; Butany, J. The Pericardium and its Diseases. Cell. Mol. Pathobiol. Cardiovasc. Dis. 2014, 297–314. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: Intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Senyilmaz-Tiebe, D.; Pfaff, D.H.; Virtue, S.; Schwarz, K.V.; Fleming, T.; Altamura, S.; Muckenthaler, M.U.; Okun, J.G.; Vidal-Puig, A.; Nawroth, P.; et al. Dietary stearic acid regulates mitochondria in vivo in humans. Nat. Commun. 2018, 9, 3129. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, M.; Yamaguchi, R.; Hide, I.; Chen, Z.; Hirai, Y.; Sugimoto, A.; Yasuhara, T.; Nakata, Y. Possible involvement of long chain fatty acids in the spores of Ganoderma lucidum (Reishi Houshi) to its anti-tumor activity. Biol. Pharm. Bull. 2008, 31, 1933–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, K.; Jiang, L.; Zhu, S.; Feng, C.; Zhao, Y.; Zhang, L.; Wang, T. Dimethylglycine sodium salt protects against oxidative damage and mitochondrial dysfunction in the small intestines of mice. Int. J. Mol. Med. 2019, 43, 2199–2211. [Google Scholar] [CrossRef]
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine Deficiency and the Development of Diabetes. Diabetes 2015, 64, 3010–3016. [Google Scholar] [CrossRef] [Green Version]
- Chalvatzi, S.; Papadopoulos, G.A.; Tsiouris, V.; Giannenas, I.; Karapanagiotidis, I.T.; Theodoridis, A.; Georgopoulou, I.; Fortomaris, P.D. Dimethylglycine Supplementation in Reduced Energy Broilers’ Diets Restores Performance by Improving Nutrient Digestibility. Animals 2020, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Garcia, R.A.; Taren, D.; Teufel, N.I. Factors associated with the reproducibility of specific food items from the Southwest Food Frequency Questionnaire. Ecol. Food Nutr. 2000, 38, 549–561. [Google Scholar] [CrossRef]
- Martínez, M.E.; Marshall, J.R.; Graver, E.; Whitacre, R.C.; Woolf, K.; Ritenbaugh, C.; Alberts, D.S. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol. Biomark. Prev. 1999, 8, 941–946. [Google Scholar]
- Taren, D.; de Tobar, M.; Ritenbaugh, C.; Graver, E.; Whitacre, R.; Aickin, M. Evaluation of the Southwest Food Frequency Questionnaire. Ecol. Food Nutr. 2000, 38, 515–547. [Google Scholar] [CrossRef]
- Carroll, P.A.; Diolaiti, D.; McFerrin, L.; Gu, H.; Djukovic, D.; Du, J.; Cheng, P.F.; Anderson, S.; Ulrich, M.; Hurley, J.B.; et al. Deregulated Myc requires MondoA/Mlx for metabolic reprogramming and tumorigenesis. Cancer Cell 2015, 27, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Carroll, P.A.; Du, J.; Zhu, J.; Neto, F.C.; Eisenman, R.N.; Raftery, D. Quantitative Method to Investigate the Balance between Metabolism and Proteome Biomass: Starting from Glycine. Angew. Chem. Int. Ed. Engl. 2016, 55, 15646–15650. [Google Scholar] [CrossRef]
- Eghlimi, R.; Shi, X.; Hrovat, J.; Xi, B.; Gu, H. Triple Negative Breast Cancer Detection Using LC–MS/MS Lipidomic Profiling. J. Proteome Res. 2020, 19, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]



| Measurement | BMI Categories | p-Value | ||||
|---|---|---|---|---|---|---|
| Normal | Overweight | Obese | Normal vs. Overweight | Normal vs. Obese | Overweight vs. Obese | |
| Gender (M/F) | 5/16 | 11/11 | 10/17 | |||
| Age (years) | 35.82 ± 10.91 | 39.91 ± 9.42 | 37.00 ± 7.93 | 0.146 | 0.653 | 0.264 |
| BMI (kg/m2) | 22.78 ± 1.62 | 28.05 ± 1.46 | 34.05 ± 3.50 | <0.001 | <0.001 | <0.001 |
| Height (cm) | 162.78 ± 7.11 | 166.78 ± 8.85 | 162.82 ± 7.15 | 0.086 | 0.986 | 0.067 |
| Weight (kg) | 60.54 ± 7.56 | 78.18 ± 9.10 | 90.38 ± 12.05 | <0.001 | <0.001 | <0.001 |
| Waist Circumference (cm) | 80.54 ± 6.08 | 93.47 ± 6.75 | 106.72 ± 8.28 | <0.001 | <0.001 | <0.001 |
| Hip Circumference (cm) | 96.25 ± 4.61 | 106.12 ± 5.07 | 116.58 ± 9.21 | <0.001 | <0.001 | <0.001 |
| Systolic BP (mmHg) | 114.77 ± 12.86 | 116.62 ± 10.26 | 117.83 ± 10.87 | 0.585 | 0.338 | 0.701 |
| Diastolic BP (mmHg) | 71.92 ± 10.88 | 72.97 ± 8.86 | 75.00 ± 7.94 | 0.703 | 0.235 | 0.427 |
| Body Fat% | 26.25 ± 5.16 | 32.40 ± 7.33 | 40.86 ± 7.55 | 0.004 | <0.001 | <0.001 |
| Total Cholesterol (mg/dL) | 177.06 ± 39.69 | 189.51 ± 47.25 | 181.54 ± 30.52 | 0.345 | 0.647 | 0.461 |
| HDL (mg/dL) | 52.56 ± 11.46 | 41.38 ± 10.22 | 38.67 ± 7.82 | 0.001 | <0.001 | 0.279 |
| LDL (mg/dL) | 107.50 ± 36.39 | 115.92 ± 31.55 | 117.93 ± 27.21 | 0.411 | 0.242 | 0.804 |
| Triglycerides (mg/dL) | 85.03 ± 44.69 | 175.93 ± 228.04 | 124.65 ± 62.77 | 0.073 | 0.015 | 0.244 |
| Glucose (mg/dL) | 91.18 ± 7.44 | 97.14 ± 22.15 | 93.01 ± 8.80 | 0.237 | 0.435 | 0.355 |
| Insulin (mIU/mL) | 5.60 ± 3.59 | 6.86 ± 4.17 | 12.42 ± 6.22 | 0.298 | <0.001 | 0.001 |
| HOMA-IR (units) | 1.28 ± 0.84 | 1.64 ± 1.07 | 2.85 ± 1.42 | 0.301 | <0.001 | <0.001 |
| Men’s Intake | Intake Data | p-Value | ||||
|---|---|---|---|---|---|---|
| Normal | Overweight | Obese | Normal vs. Overweight | Normal vs. Obese | Overweight vs. Obese | |
| Total Calories (kcal/day) | 2849 ± 1416 | 2593 ± 1123 | 3888 ± 2784 | 0.7328 | 0.3559 | 0.1958 |
| Protein (g) | 130 ± 55 | 105 ± 41 | 168 ± 129 | 0.3870 | 0.4437 | 0.1667 |
| Total Fat (g) | 106 ± 64 | 88 ± 34 | 131 ± 89 | 0.5643 | 0.5510 | 0.1759 |
| Carbohydrates (g) | 341 ± 155 | 351 ± 200 | 525 ± 401 | 0.9139 | 0.2261 | 0.2382 |
| Fiber (g) | 29 ± 15 | 28 ± 17 | 42 ± 34 | 0.9270 | 0.3297 | 0.2701 |
| Women’s Intake | Intake Data | p-Value | ||||
|---|---|---|---|---|---|---|
| Normal | Overweight | Obese | Normal vs. Overweight | Normal vs. Obese | Overweight vs. Obese | |
| Total Calories (kcal/day) | 1920 ± 683 | 1938 ± 976 | 3326 ± 1295 | 0.9585 | 0.0006 | 0.0034 |
| Protein (g) | 82 ± 31 | 80 ± 38 | 140 ± 56 | 0.8371 | 0.0011 | 0.0022 |
| Total Fat (g) | 61 ± 23 | 64 ± 29 | 123 ± 50 | 0.7969 | 0.0001 | 0.0005 |
| Carbohydrates (g) | 268 ± 96 | 265 ± 152 | 429 ± 178 | 0.9494 | 0.0033 | 0.0157 |
| Fiber (g) | 24 ± 10 | 24 ± 10 | 38 ± 19 | 0.8605 | 0.0172 | 0.0176 |
| BMI | Sex | ||
|---|---|---|---|
| Metabolites | p-Value | Metabolites | p-Value |
| Acetohydroxamic acid | 0.009 | Glucose/Galactose | 0.005 |
| TMAO | 0.014 | Succinate | 0.032 |
| Acetylcarnitine | 0.004 | ||
| Asparagine | 0.018 | ||
| Creatinine | 0.003 | ||
| Glutamic acid | 0.028 | ||
| Pipecolic acid | 0.002 | Sex and BMI | |
| Cytidine | 0.048 | Metabolites | p-Value |
| Leucic acid | 0.044 | Fructose | 0.021 |
| D-Galacturonic acid | 0.042 | Glyceric acid | 0.039 |
| Picolinic acid | 0.044 | Pregnenolone sulfate | 0.016 |
| 2-Pyrrolidinone | 0.007 | Acetylornithine | 0.046 |
| Kynurenine | 0.032 | Phenylpyruvic acid | 0.023 |
| Nonadecanoic acid | 0.012 | ||
| Decanoylcarnitine | 0.035 | ||
| 2-Aminoadipic acid | 0.019 | ||
| Corrected Caloric Intake Model | |
|---|---|
| Metabolites | p-Value |
| Men | |
| Methylguanidine | 0.004 |
| 2-Hydroxyphenylacetic acid | 0.035 |
| Women | |
| Stearic acid | 0.002 |
| Nonadecanoic acid | 0.011 |
| Malic acid | 0.026 |
| Dimethylglycine | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patterson, J.; Shi, X.; Bresette, W.; Eghlimi, R.; Atlas, S.; Farr, K.; Vega-López, S.; Gu, H. A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox. Metabolites 2021, 11, 552. https://doi.org/10.3390/metabo11080552
Patterson J, Shi X, Bresette W, Eghlimi R, Atlas S, Farr K, Vega-López S, Gu H. A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox. Metabolites. 2021; 11(8):552. https://doi.org/10.3390/metabo11080552
Chicago/Turabian StylePatterson, Jeffrey, Xiaojian Shi, William Bresette, Ryan Eghlimi, Sarah Atlas, Kristin Farr, Sonia Vega-López, and Haiwei Gu. 2021. "A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox" Metabolites 11, no. 8: 552. https://doi.org/10.3390/metabo11080552
APA StylePatterson, J., Shi, X., Bresette, W., Eghlimi, R., Atlas, S., Farr, K., Vega-López, S., & Gu, H. (2021). A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox. Metabolites, 11(8), 552. https://doi.org/10.3390/metabo11080552