Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities
Abstract
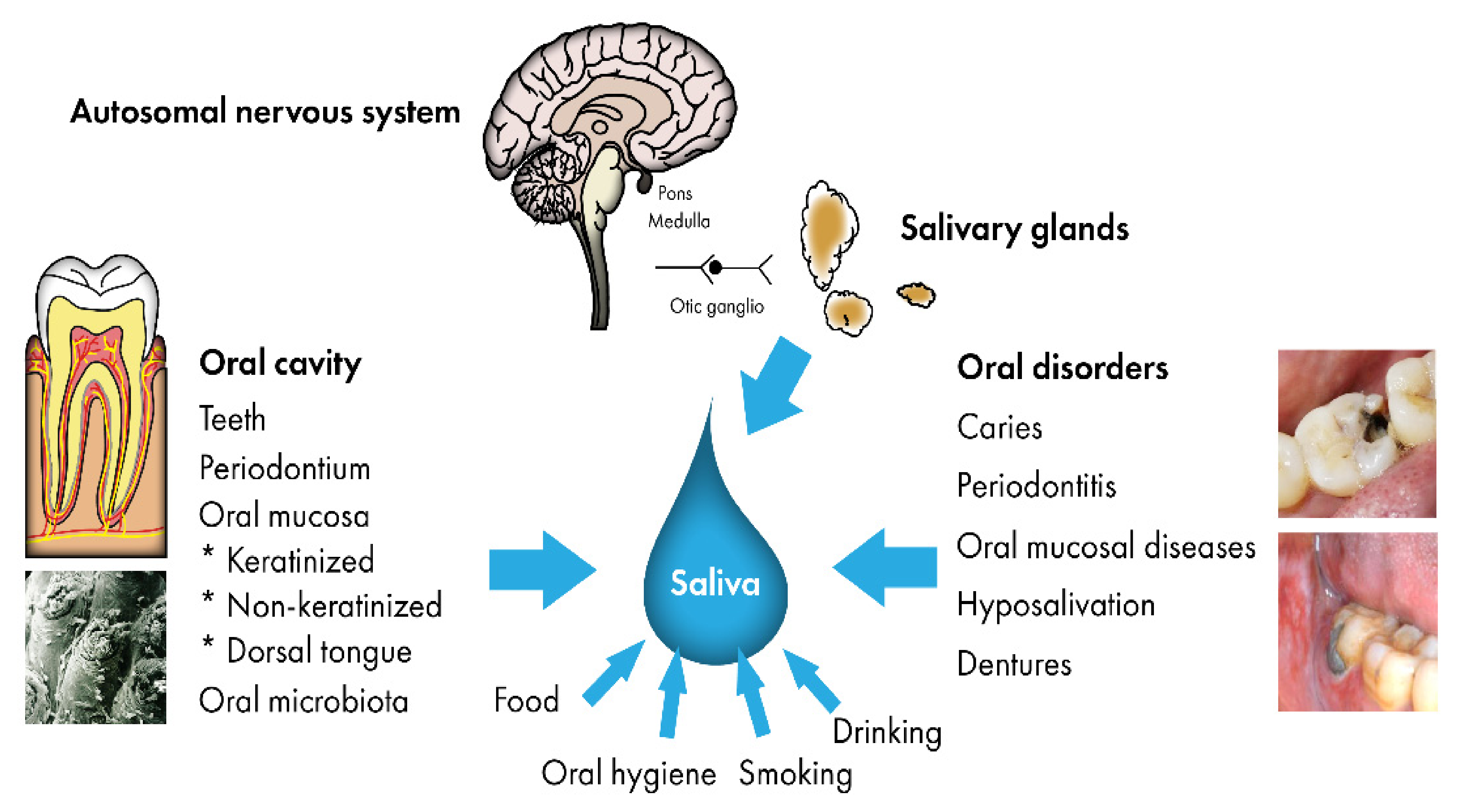
:1. Introduction
2. Saliva in the Oral Defense
3. Salivary Metabolomics in Oral Diseases
4. Salivary Metabolites and Systemic Diseases
5. Saliva Collection
6. Methods to Study Salivary Metabolites
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment: Validity and Validation. Environmental Health Criteria 222. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 16 April 2021).
- Bardow, A.; Lynge Pedersen, A.M.; Nauntofte, B. Saliva. In Clinical Oral Physiology, 1st ed.; Miles, T.S., Nauntofte, B., Svensson, P., Eds.; Quintessence Publishing Co. Ltd.: Copenhagen, Denmark, 2004; pp. 17–51. [Google Scholar]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. Basic Clin. 2007, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hanning, C.; Hanning, M.; Kensche, A.; Carpenter, G. The mucosal pellicle- An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, L.; Altini, M.; Khammissa, R.A.G.; Chandran, R.; Bouckaert, M.; Lemmer, J. Oral mucosal immunity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 576–583. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, 3–12. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Ann. Rev. Pathol. Mech. Dis. 2012, 7, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Cacciatore, S.; Graziano, A.; Tenori, L. Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics 2012, 8, 465–474. [Google Scholar] [CrossRef]
- Barnes, V.M.; Ciancio, S.G.; Shibly, O.; Xu, T.; Devizio, W.; Trivedi, H.M.; Guo, L.; Jönsson, T.J. Metabolomics reveals elevated macromolecular degradation in periodontal disease. J. Dent. Res. 2011, 90, 1293–1297. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Morelli, T.; Moss, K.; Barros, S.P.; Ward, M.; Jenkins, W.; Aspiras, M.B.; Offenbacher, S. Association of synergistetes and cyclodipeptides with periodontitis. J. Dent. Res. 2015, 94, 1425–1431. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Bamba, T.; Fukusaki, E.; Amano, A. Prediction of periodontal inflammation via metabolic profiling of saliva. J. Dent. Res. 2016, 95, 1381–1386. [Google Scholar] [CrossRef]
- Rzeznik, M.; Triba, M.N.; Levy, P.; Jungo, S.; Botosoa, E.; Duchemann, B.; Le Moyec, L.; Bernaudin, J.F.; Savarin, P.; Guez, D. Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1H nuclear magnetic resonance (NMR) spectroscopy. PLoS ONE 2017, 12, e0182767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Tenori, L.; Cacciatore, S.; Aimetti, M. Analysis of salivary phenotypes of generalized aggressive and chronic periodontitis through nuclear magnetic resonance-based metabolomics. J. Periodontol. 2018, 89, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.P.; Saxena, M.; Saimbi, C.S.; Siddiqui, M.H.; Roy, R. Post-periodontal surgery propounds early repair salivary biomarkers by 1 H NMR based metabolomics. Metabolomics 2019, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, C.; Pitchika, V.; Pink, C.; Samietz, S.; Kastenmüller, G.; Artati, A.; Suhre, K.; Adamski, J.; Nauck, M.; Völzke, H.; et al. The saliva metabolome in association to oral health status. J. Dent. Res. 2019, 98, 642–651. [Google Scholar] [CrossRef]
- Citterio, F.; Romano, F.; Meoni, G.; Iaderosa, G.; Grossi, S.; Sobrero, A.; Dego, F.; Corana, M.; Berta, C.N.; Tenori, L.; et al. Changes in the salivary metabolic profile of generalized periodontitis patients after non-surgical periodontal therapy: A metabolomic analysis using nuclear magnetic resonance spectroscopy. J. Clin. Med. 2020, 9, 3977. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J.; Song, Y.; Lee, A.H.; Kim, S.; Chung, J. Metabolic phenotyping of saliva to identify possible biomarkers of periodontitis using proton nuclear magnetic resonance. J. Clin. Periodontol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pietiäinen, M.; Liljestrand, J.M.; Kopra, E.; Pussinen, P.J. Mediators between oral dysbiosis and cardiovascular diseases. Eur. J. Oral Sci. 2018, 126, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; Angeli, R.; Muniz, A.M.S.; Gonsalves, E.; Santos, R.; Nadal, J.; Almeida, F.C.L.; Valente, A.P.; Souza, I.P.R. Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics 2013, 9, 657–666. [Google Scholar] [CrossRef]
- Pereira, J.L.; Duarte, D.; Carneiro, T.J.; Ferreira, S.; Cunha, B.; Soares, D.; Costa, A.L.; Gil, A.M. Saliva NMR metabolomics: Analytical issues in pediatric oral health research. Oral Dis. 2019, 25, 1545–1554. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci Rep. 2014, 4, 6802. [Google Scholar] [CrossRef]
- Lohavanichbutr, P.; Zhang, Y.; Wang, P.; Gu, H.; Nagana Gowda, G.A.; Djukovic, D.; Buas, M.F.; Raftery, D.; Chen, C. Salivary metabolite profiling distinguishes patients with oral cavity squamous cell carcinoma from normal controls. PLoS ONE 2018, 13, e0204249. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, S.; Wong, D.T.W.; Sugimoto, M.; Gleber-Netto, F.O.; Li, F.; Tu, M.; Zhang, Y.; Akin, D.; Iino, M. Identification of salivary metabolites for oral squamous cell carcinoma and oral epithelial dysplasia screening from persistent suspicious oral mucosal lesions. Clin. Oral Investig. 2019, 23, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral Dis. 2020, 26, 35–42. [Google Scholar] [CrossRef]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Uezono, Y.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Sugimoto, M.; Ushijima, T. Metabolomic profiling reveals salivary hypotaurine as a potential early detection marker for medication-related osteonecrosis of the jaw. PLoS ONE 2019, 14, e0220712. [Google Scholar] [CrossRef] [Green Version]
- Washio, J.; Takahashi, N. Metabolomic studies of oral biofilm, oral cancer, and beyond. Int. J. Mol. Sci. 2016, 17, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toan, N.K.; Ahn, S.G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, L.; Zhou, H.; Lee, J.M.; Garon, E.B.; Wong, D.T.W. Proteomic analysis of human saliva from lung cancer patients using two-dimensional difference gel electrophoresis and mass spectrometry. Mol. Cell. Proteom. 2012, 11, M111.012112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taware, R.; Taunk, K.; Pereira, J.A.M.; Shirolkar, A.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. Volatilomic insight of head and neck cancer via the effects observed on saliva metabolites. Sci. Rep. 2018, 8, 17725. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, J.J.W.; Singh, S.P.; Akhi, R.; Salo, T.; Lappalainen, R.; González-Arriagada, W.A.; Lopes, M.A.; Kullaa, A.M.; Myllymaa, S. Potential role of nuclear magnetic resonance spectroscopy to identify salivary metabolite alterations in patients with head and neck cancer. Oncol. Lett. 2018, 16, 6795–6800. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, M.; Palisi, A.; Rossi, G.; Stillitano, I.; Faiella, F.; Montoro, P.; Rodriquez, M.; Palladino, R.; D’Ursi, A.M.; Romano, R. Saliva of patients affected by salivary gland tumour: An NMR metabolomics analysis. J. Pharm. Biomed. Anal. 2018, 160, 436–442. [Google Scholar] [CrossRef]
- Murata, T.; Yanagisawa, T.; Kurihara, T.; Kaneko, M.; Ota, S.; Enomoto, A.; Tomita, M.; Sugimoto, M.; Sunamura, M.; Hayashida, T.; et al. Salivary metabolomics with alternative decision tree-based machine learning methods for breast cancer discrimination. Breast Cancer Res Treat. 2019, 177, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Xavier Assad, D.; Acevedo, A.C.; Cançado Porto Mascarenhas, E.; Costa Normando, A.G.; Pichon, V.; Chardin, H.; Neves Silva Guerra, E.; Combes, A. Using an untargeted metabolomics approach to identify salivary metabolites in women with breast cancer. Metabolites 2020, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, J.J.W.; Herrala, M.; Soininen, P.; Lappalainen, R.; Tjäderhane, L.; Seitsalo, H.; Niemelä, R.; Salo, T.; Kullaa, A.M.; Myllymaa, S. Metabolic Profiling of Saliva in Patients with Primary Sjögren’s syndrome. Metabolomics 2013, 3, 128. [Google Scholar]
- Kageyama, G.; Saegusa, J.; Irino, Y.; Tanaka, S.; Tsuda, K.; Takahashi, S.; Sendo, S.; Morinobu, A. Metabolomics analysis of saliva from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 182, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Herrala, M.; Mikkonen, J.J.W.; Pesonen, P.; Lappalainen, R.; Tjäderhane, L.; Niemelä, R.K.; Seitsalo, H.; Salo, T.; Myllymaa, S.; Kullaa, A.M. Metabolomics analysis of saliva from patients with primary Sjögren’s syndrome. J. Oral Dis. 2020, 63, 22–26. [Google Scholar] [CrossRef]
- Barnes, V.M.; Kennedy, A.D.; Panagakos, F.; Devizio, W.; Trivedi, H.M.; Jönsson, T.; Guo, L.; Cervi, S.; Scannapieco, F.A. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE 2014, 9, e105181. [Google Scholar] [CrossRef]
- De Oliveira, L.R.; Martins, C.; Fidalgo, T.K.; Freitas-Fernandes, L.B.; de Oliveira Torres, R.; Soares, A.L.; Almeida, F.C.; Valente, A.P.; de Souza, I.P. Salivary Metabolite Fingerprint of Type 1 Diabetes in Young Children. J. Proteome Res. 2016, 15, 2491–2499. [Google Scholar] [CrossRef]
- Figueira, J.; Jonsson, P.; Adolfsson, A.N.; Adolfsson, R.; Nyberg, L.; Öhman, A. NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Mol. Biosyst. 2016, 12, 2562–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symons, F.J.; ElGhazi, I.; Reilly, B.G.; Barney, C.C.; Hanson, L.; Panoskaltsis-Mortari, A.; Armitage, I.M.; Wilcox, G.L. Can biomarkers differentiate pain and no pain subgroups of nonverbal children with cerebral palsy? A preliminary investigation based on noninvasive saliva sampling. Pain Med. 2015, 16, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Carro, E.; Bartolomé, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Calero, M.; Rabano, A.; Cantero, J.L.; Orive, G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimers Dement 2017, 8, 131–138. [Google Scholar] [CrossRef]
- Yilmaz, A.; Geddes, T.; Han, B.; Bahado-Singh, R.O.; Wilson, G.D.; Imam, K.; Maddens, M.; Graham, S.F. Diagnostic biomarkers of Alzheimer’s disease as identified in saliva using 1H NMR-based metabolomics. J. Alzheimers Dis. 2017, 58, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, V.; Kumaran, S.S.; Dwivedi, S.N.; Srivastava, A.; Jagannathan, N.R. Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls. Neurol. Sci. 2020, 41, 1201–1210. [Google Scholar] [CrossRef]
- Belstrøm, D.; Holmstrup, P.; Bardow, A.; Kokaras, A.; Fiehn, N.E.; Paster, B.J. Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. J. Oral Microbiol. 2016, 8, 30112. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Pina, G.M.S.; Mota Carvalho, R.; Silva, B.S.F.; Almeida, F.T. Prevalence of hyposalivation in older people: A systematic review and meta-analysis. Gerodontology 2020, 37, 317–331. [Google Scholar] [CrossRef]
- Mikkonen, J.J.W. Infrared and Nuclear Magnetic Resonance Spectroscopic Methods for Salivary Analysis. Ph.D. Thesis, University of Eastern Finland, Kuopio, Finland, 2019; p. 341. [Google Scholar]
- Gardner, A.; Parkes, H.G.; Carpenter, G.H.; So, P.W. Developing and standardizing a protocol for quantitative proton nuclear magnetic resonance (1H NMR) spectroscopy of saliva. J. Proteome Res. 2018, 17, 1521–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, D.; Castro, B.; Pereira, J.L.; Marques, J.F.; Costa, A.L.; Gil, A.M. Evaluation of saliva stability for NMR metabolomics: Collection and handling protocols. Metabolites 2020, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, J.J.W.; Raittila, J.; Rieppo, L.; Lappalainen, R.; Kullaa, A.M.; Myllymaa, S. Fourier transform infrared spectroscopy and photoacoustic spectroscopy for saliva analysis. Appl. Spectrosc. 2016, 70, 1502–1510. [Google Scholar] [CrossRef]
- Hurskainen, M.; Sarin, J.K.; Myllymaa, S.; González-Arriagada, G.A.; Kullaa, A.; Lappalainen, R. Feasibility of near-infrared spectroscopy for identification of L-fucose and L-proline—Towards detecting biomarkers from saliva. Metabolites 2021. under review. [Google Scholar]
- Trezzi, J.P.; Jäger, C.; Galozzi, S.; Barkovits, K.; Marcus, K.; Mollenhauer, B.; Hiller, K. Metabolic profiling of body fluids and multivariate data analysis. MethodsX 2017, 4, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Blekherman, G.; Laubenbacher, R.; Cortes, D.F.; Mendes, P.; Torti, F.M.; Akman, S.; Torti, S.V.; Shulaev, V. Bioinformatics tools for cancer metabolomics. Metabolomics 2011, 7, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, K.; Ota, S.; Kaneko, M.; Hirayama, A.; Enomoto, M.; Katumata, K.; Sugimoto, M.; Soga, T. High-throughput screening of salivary polyamine markers for discrimination of colorectal cancer by multisegment injection capillary electrophoresis tandem mass spectrometry. J. Chromatogr. A 2021, 1652, 462355. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, 388–396. [Google Scholar] [CrossRef]
- Andörfer, L.; Holtfreter, B.; Weiss, S.; Matthes, R.; Pitchika, V.; Schmidt, C.O.; Samietz, S.; Kastenmüller, G.; Nauck, M.; Völker, U.; et al. Salivary metabolites associated with a 5-year tooth loss identified in a population-based setting. BMC Med. 2021, 14, 161. [Google Scholar] [CrossRef]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The oral microbiota is modified by systemic diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.D.; Malcangi, G.; Pham, V.H.; Inchingolo, A.M.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Costa Dos Santos Junior, G.; Pereira, C.M.; Kelly da Silva Fidalgo, T.; Valente, A.P. Saliva NMR-based metabolomics in the war against COVID-19. Anal. Chem. 2020, 92, 15688–15692. [Google Scholar] [CrossRef] [PubMed]

| Author, Year [Ref.] | Oral Diseases | Type of Saliva | Method |
|---|---|---|---|
| Aimetti et al. 2012 [9] | Periodontitis | WS | NMR |
| Barnes et al. 2014 [10] | Periodontitis and diabetes | NM | GC/MS and LC/MS |
| Marchesan et al. 2015 [11] | Periodontal diseases (dysbiosis) | WS | GC/MS and LC/MS |
| Kuboniwa et al. 2016 [12] | Periodontal inflammation | USWS | GC/MS |
| Rzeznik et al. 2017 [13] | Periodontitis | SWS | NMR |
| Romano et al. 2018 [14] | Periodontitis | USWS | NMR |
| Singh et al. 2019 [15] | Chronic periodontitis | WS | NMR |
| Liebsch et al. 2019 [16] | Periodontitis | SWS | UHPLC-MS/MS |
| Citterio et. al. 2020 [17] | Periodontitis after therapy | USWS | NMR |
| Kim et al. 2021 [18] | Periodontitis | SWS | NMR |
| Fidalgo et al. 2013 [20] | Children’s caries | USWS | NMR |
| Pereira et al. 2019 [21] | Children’s caries | USWS, SWS | NMR |
| Sugimoto et al. 2010 [22] | OSCC | WS | CE-TOF-MS |
| Wei et al. 2011 [23] | OSCC/leukoplakia | NM | LC-TOF-MS |
| Wang et al. 2014 [24] | OSCC | USWS | CE-MS |
| Lohavanichbutr et al. 2018 [25] | OSCC | NM | NMR, LC-MS |
| Ishikawa et al. 2019 [26] | OSCC (OED, PSOML) | USWS | CE-MS |
| Ishikawa et al. 2020 [27] | Lichen planus/OSCC | USWS | CE-MS |
| Yatsuoka et al. 2019 [28] | MRONJ | WS | CE-MS |
| Author, Year [Ref.] | Systemic Diseases | Type of Saliva | Method |
|---|---|---|---|
| Sugimoto et al. 2010 [22] | Breast and pancreatic cancer (OSCC) | WS | CE-TOF-MS |
| Xiao et al. 2012 [31] | Lung cancer | USWS | 2D-DIGE-MS |
| Taware et al. 2018 [32] | HNC | USWS | GC/MS |
| Mikkonen et al. 2018 [33] | HNC | SWS | NMR |
| Grimaldi et al. 2018 [34] | Salivary gland (parotid) tumors | NM | NMR |
| Murata et al. 2019 [35] | Breast cancer | USWS | CE-TOF-MS |
| Xavier Assad et al. 2020 [36] | Breast cancer | NM | LC/MS |
| Mikkonen et al. 2013 [37] | Sjögren’s syndrome | SWS | NMR |
| Kageyama et al. 2015 [38] | Sjögren’s syndrome | USWS | GS/MS |
| Herrala et al. 2020 [39] | Sjögren’s syndrome | SWS | NMR |
| Barnes et al. 2014 [40] | Diabetes and periodontitis | NM | GC/MS and LC/MS |
| de Oliveira et al. 2016 [41] | Diabetes | WS | NMR |
| Figuera et al. 2016 [42] | Dementia | Collected at home | NMR |
| Symons et al. 2015 [43] | Cerebral palsy | USWS | NMR |
| Carro et al. 2017 [44] | AD and MCI | USWS | MALDI-TOF/MS |
| Yilmaz et al. 2017 [45] | AD and MCI | WS | NMR |
| Kumari et al. 2020 [46] | PD | USWS | NMR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. https://doi.org/10.3390/metabo11090587
Hyvärinen E, Savolainen M, Mikkonen JJW, Kullaa AM. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites. 2021; 11(9):587. https://doi.org/10.3390/metabo11090587
Chicago/Turabian StyleHyvärinen, Eelis, Minttu Savolainen, Jopi J. W. Mikkonen, and Arja M. Kullaa. 2021. "Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities" Metabolites 11, no. 9: 587. https://doi.org/10.3390/metabo11090587






