A Randomized Controlled Dietary Intervention Improved the Serum Lipid Signature towards a Less Atherogenic Profile in Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. Subjects and Compliance
2.2. Effect of Intervention on Lipid Composite Scores Associated with CVD Risk
2.3. Influence of the Dietary Intervention on Lipid Classes
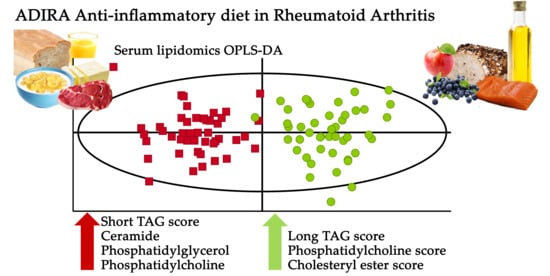
2.4. Influence of Dietary Intervention on Lipid Signature
2.5. Associations between Changes in Known Clinical Markers and Plasma Phospholipid Fatty Acids and Changes in Lipid Signature
2.6. Discriminating Lipid Species
2.7. General Factors Influencing the Lipid Signature
3. Discussion
4. Materials and Methods
4.1. Study Participants and Study Design
4.2. Diets
4.3. Dietary Assessment
4.4. Data Collection and Laboratory Analysis of Descriptives
4.5. Lipidomic Profiling
4.6. Lipid Score Calculation
4.7. Multivariate Statistical Methods
4.8. Univariate Statistical Methods
4.9. Power Calculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moxon, J.V.; Jones, R.E.; Wong, G.; Weir, J.M.; Mellett, N.A.; Kingwell, B.A.; Meikle, P.J.; Golledge, J. Baseline serum phosphatidylcholine plasmalogen concentrations are inversely associated with incident myocardial infarction in patients with mixed peripheral artery disease presentations. Atherosclerosis 2017, 263, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wibetoe, G.; Ikdahl, E.; Rollefstad, S.; Olsen, I.C.; Bergsmark, K.; Kvien, T.K.; Salberg, A.; Soldal, D.M.; Bakland, G.; Lexberg, Å.; et al. Cardiovascular disease risk profiles in inflammatory joint disease entities. Arthritis Res. Ther. 2017, 19, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. Eular recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic, D.; Shi, L.; Calais, F.; Carlsson, F.; Landberg, R.; Hyötyläinen, T.; Frøbert, O. Effects of a lacto-ovo-vegetarian diet on the plasma lipidome and its association with atherosclerotic burden in patients with coronary artery disease-a randomized, open-label, cross-over study. Nutrients 2020, 12, 3586. [Google Scholar] [CrossRef] [PubMed]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population-based finrisk 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundra, P.A.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; Mellett, N.A.; Huynh, K.; et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018, 3, 121326. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; Marz, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond ldl-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Ottosson, F.; Emami Khoonsari, P.; Gerl, M.J.; Simons, K.; Melander, O.; Fernandez, C. A plasma lipid signature predicts incident coronary artery disease. Int. J. Cardiol. 2021, 331, 249–254. [Google Scholar] [CrossRef]
- Hilvo, M.; Wallentin, L.; Ghukasyan Lakic, T.; Held, C.; Kauhanen, D.; Jylhä, A.; Lindbäck, J.; Siegbahn, A.; Granger, C.B.; Koenig, W.; et al. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J. Am. Heart Assoc. 2020, 9, e015258. [Google Scholar] [CrossRef]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur. J. Prev. Cardiol. 2020, 29. [Google Scholar] [CrossRef]
- Ottestad, I.; Hassani, S.; Borge, G.I.; Kohler, A.; Vogt, G.; Hyötyläinen, T.; Orešič, M.; Brønner, K.W.; Holven, K.B.; Ulven, S.M.; et al. Fish oil supplementation alters the plasma lipidomic profile and increases long-chain pufas of phospholipids and triglycerides in healthy subjects. PLoS ONE 2012, 7, e42550. [Google Scholar] [CrossRef] [Green Version]
- Lankinen, M.; Schwab, U.; Erkkilä, A.; Seppänen-Laakso, T.; Hannila, M.-L.; Mussalo, H.; Lehto, S.; Uusitupa, M.; Gylling, H.; Orešič, M. Fatty fish intake decreases lipids related to inflammation and insulin signaling—A lipidomics approach. PLoS ONE 2009, 4, e5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the predimed trial (prevención con dieta mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadell, A.K.E.; Barebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory diet in rheumatoid arthritis (adira)-a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 esc/eas guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Hulander, E.; Bärebring, L.; Turesson Wadell, A.; Gjertsson, I.; Calder, P.C.; Winkvist, A.; Lindqvist, H.M. Diet intervention improves cardiovascular profile in patients with rheumatoid arthritis: Results from the randomized controlled cross-over trial adira. Nutr. J. 2021, 20, 9. [Google Scholar] [CrossRef]
- Walchuk, C.; Wang, Y.; Suh, M. The impact of epa and dha on ceramide lipotoxicity in the metabolic syndrome. Br. J. Nutr. 2020, 125, 863–875. [Google Scholar] [CrossRef]
- Beyene, H.B.; Hamley, S.; Giles, C.; Huynh, K.; Smith, A.; Cinel, M.; Mellet, N.A.; Morales-Scholz, M.G.; Kloosterman, D.; Howlett, K.F.; et al. Mapping the associations of the plasma lipidome with insulin resistance and response to an oral glucose tolerance test. J. Clin. Endocrinol. Metab. 2020, 105, e1041–e1055. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar]
- Razquin, C.; Liang, L.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Zheng, Y.; Wang, D.D.; Corella, D.; Castaner, O.; Ros, E.; et al. Plasma lipidome patterns associated with cardiovascular risk in the predimed trial: A case-cohort study. Int. J. Cardiol. 2018, 253, 126–132. [Google Scholar] [CrossRef] [PubMed]
- van Riel, P.L. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin. Exp. Rheumatol. 2014, 32 (Suppl. 85), S-65-74. [Google Scholar] [PubMed]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J. Vis. Exp. 2014, 85, e51445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamtani, M.; Kulkarni, H.; Wong, G.; Weir, J.M.; Barlow, C.K.; Dyer, T.D.; Almasy, L.; Mahaney, M.C.; Comuzzie, A.G.; Glahn, D.C.; et al. Lipidomic risk score independently and cost-effectively predicts risk of future type 2 diabetes: Results from diverse cohorts. Lipids Health Dis. 2016, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Braicu, E.I.; Darb-Esfahani, S.; Schmitt, W.D.; Koistinen, K.M.; Heiskanen, L.; Pöhö, P.; Budczies, J.; Kuhberg, M.; Dietel, M.; Frezza, C.; et al. High-grade ovarian serous carcinoma patients exhibit profound alterations in lipid metabolism. Oncotarget 2017, 8, 102912–102922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilvo, M.; Jylhä, A.; Lääperi, M.; Jousilahti, P.; Laaksonen, R. Absolute and relative risk prediction in cardiovascular primary prevention with a modified score chart incorporating ceramide-phospholipid risk score and diabetes mellitus. Eur. Heart J. Open 2021, oeab010. [Google Scholar] [CrossRef]




| Baseline Characteristics | Median (IQR) c |
|---|---|
| Sex F/M (n) | 36/10 |
| Age (year) | 63 (53, 71) |
| Body mass index (kg/m2) | 26.5 (24.0, 31.6) |
| Weight (kg) | 77.2 (66.8, 84.8) |
| Fat mass a (kg) | 27.8 (19.5, 32.3) |
| Lean mass a (kg) | 48.2 (42.8, 52.3) |
| SBP (mmHg) | 135 (125, 140) |
| DBP (mmHg) | 80 (72, 89) |
| Serum TC (mmol/L) | 5.5 (4.6, 6.0) |
| Serum LDL (mmol/L) | 3.35 (2.78, 4.23) |
| Serum HDL (mmol/L) | 1.60 (1.30, 2.03) |
| Serum triacylglycerides (mmol/L) | 1.10 (0.84, 1.53) |
| DAS28 b | 3.70 (3.06, 4.65) |
| DAS28-CRP b | 3.47 (2.85, 4.14) |
| Medications used at baseline | n (%) |
| Cardiovascular agents | 22 (48) |
| Vasodilator | 16 (35) |
| Statins | 7 (15) |
| Betablocker | 7 (15) |
| Anticoagulants | 4 (9) |
| Diuretics | 4 (9) |
| bDMARD | 17 (37) |
| csDMARD | 34 (74) |
| Intervention Pre Median (IQR) a (n = 46) | Intervention Post Median (IQR) a (n = 45) | P Intervention Pre vs. Post b (n = 45) | Control Pre Median (IQR) a (n = 47) | Control Post Median (IQR) a (n = 45) | P Control Pre vs. Post b (n = 44) | P I vs. C d (n = 46) | P I vs. C e (n = 28) | |
|---|---|---|---|---|---|---|---|---|
| CERT2 | 6.0 (4.0, 8.0) | 6.0 (4.0, 8.0) | 0.493 | 6.0 (4.0, 8.0) | 7.0 (4.0, 8.5) | 0.041 | 0.245 f | 0.404 f |
| CERT2 components: | ||||||||
| Cer(d18:1/24:1)/ Cer(d18:1/24:0) | 0.56 (0.49, 0.61) | 0.58 (0.47, 0.64) | 0.329 | 0.54 (0.49, 0.61) | 0.35 (0.29, 0.41) | 0.082 | 0.135 | 0.182 |
| Cer(d18:1/16:0)/ PC 16:0/22:5 | 0.0079 (0.0064, 0.0096) | 0.0079 (0.0070, 0.010) | 0.018 | 0.0082 (0.0066, 0.0098) | 0.0087 (0.0065, 0.0097) | 0.327 | 0.376 | 0.271 |
| Cer(d18:1/18:0)/ PC 14:0/22:6 | 0.11 (0.084, 0.18) | 0.092 (0.067, 0.14) | 0.257 | 0.14 (0.089, 0.19) | 0.12 (0.11, 0.16) | 0.455 | 0.002 | 0.005 |
| PC 16:0/16:0 c | 22 (19, 23) | 21 (19, 24) | 0.986 | 22 (19, 26) | 22 (19, 25) | 0.212 | 0.079 | 0.088 |
| Included lipids: | ||||||||
| Cer(d18:1/16:0) c | 0.35 (0.29, 0.39) | 0.34 (0.29, 0.39) | 0.103 | 0.35 (0.30, 0.42) | 0.38 (0.30, 0.42) | 0.007 | 0.000 | 0.006 |
| Cer(d18:1/18:0) c | 0.11 (0.081, 0.14) | 0.10(0.075, 0.13) | 0.291 | 0.11 (0.080, 0.14) | 0.12 (0.088, 0.14) | 0.033 | 0.011 | 0.010 |
| Cer(d18:1/24:0) c | 3.2 (2.8. 3.5) | 3.0 (2.6. 3.7) | 0.110 | 3.1 (2.8. 3.6) | 3.3 (2.8. 3.8) | 0.054 | 0.005 | 0.002 |
| Cer(d18:1/24:1) c | 1.7 (1.4, 2.0) | 1.7 (1.3, 2.0) | 0.243 | 1.7 (1.5, 2.0) | 1.7 (1.4, 2.0) | 0.935 | 0.272 | 0.301 |
| PC 14:0/22:6 c | 0.92 (0.65, 1.1) | 1.0 (0.80, 1.3) | 0.013 | 0.88 (0.61, 1.1) | 0.93 (0.70, 1.1) | 0.825 | 0.020 | 0.080 |
| PC 16:0/22:5 c | 43 (38, 49) | 40 (35, 44) | 0.000 | 43 (38, 46) | 45 (37, 50) | 0.037 | 0.000 | 0.001 |
| Model | Scaling | Nr of Lv a | N | R2X (cum) b | R2Y (cum) c | Q2 (cum) d | CV-ANOVA e (p-Value) | Permutation Test (Q2) f | Correct Classified (%C/%I) g |
|---|---|---|---|---|---|---|---|---|---|
| PCA baseline | UV | 7 | 43 | 0.626 | 0.319 | ||||
| OPLS baseline h | UV | 1 + 1 + 0 | 43 | 0.235 | 0.709 | 0.373 | 0.0011 | ||
| OPLS dietary intake i | UV | 1 + 0 + 0 | 174 l | 0.130 | 0.190 | 0.125 | <0.0001 all year | ||
| OPLS clinical markers j | UV | 3 + 0 + 0 | 90 | 0.325 | 0.587 | 0.461 | <0.000001 all year | ||
| OPLS fatty acids k | UV | 6 + 2 + 0 | 90 | 0.521 | 0.794 | 0.659 | <0.00001 all year | ||
| OPLS-EP | UVN | 1 + 1 + 0 | 43 | 0.317 | 0.825 | 0.635 | |||
| OPLS-DA | UV | 1 + 2 + 0 | 90 | 0.267 | 0.822 | 0.504 | 5.55 × 10−11 | −0.439 | 100/98 |
| OPLS-EP S m | UVN | 1 + 2 + 0 | 28 | 0.408 | 0.961 | 0.697 | |||
| OPLS-DA S m | UV | 1 + 1 + 0 | 56 | 0.238 | 0.731 | 0.471 | 1.14 × 10−6 | −0.429 | 86/91 |
| Lipid Species | Models | Post I vs. Post C | P Intervention Pre vs. Post a (n = 45) | P Control Pre vs. Post a (n = 44) | P Intervention vs. Control b (n = 46) |
|---|---|---|---|---|---|
| PC 35:1_sn2 | OPLS-DA | ↓ | 0.005 | 0.000 | 0.000 |
| PC(O) 34:0 | OPLS-DA S | ↓ | 0.000 | 0.001 | 0.000 |
| PC(O) 38:3 | OPLS-DA, OPLS-DA S | ↓ | 0.000 | 0.000 | 0.000 |
| PC(O) 38:4b | OPLS-DA | ↓ | 0.000 | 0.007 | 0.000 |
| PC(O) 38:4c | OPLS-DA, OPLS-DA S | ↓ | 0.000 | 0.046 | 0.000 |
| PC(O) 40:4 | OPLS-DA, OPLS-DA S, OPLS-EP | ↓ | 0.000 | 0.002 | 0.000 |
| PC(P) 38:3 | OPLS-DA, OPLS-DA S | ↓ | 0.000 | 0.041 | 0.000 |
| PC(P) 40:3 | OPLS-DA | ↓ | 0.000 | 0.017 | 0.000 |
| PC(P) 40:6 | OPLS-DA, OPLS-DA S | ↑ | 0.000 | 0.000 | 0.000 |
| Glc/GalCer(d18:1/26:1) | OPLS-DA | ↑ | 0.000 | 0.004 | 0.000 |
| SM 44:3 | OPLS-DA | ↑ | 0.000 | 0.002 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindqvist, H.M.; Bärebring, L.; Gjertsson, I.; Jylhä, A.; Laaksonen, R.; Winkvist, A.; Hilvo, M. A Randomized Controlled Dietary Intervention Improved the Serum Lipid Signature towards a Less Atherogenic Profile in Patients with Rheumatoid Arthritis. Metabolites 2021, 11, 632. https://doi.org/10.3390/metabo11090632
Lindqvist HM, Bärebring L, Gjertsson I, Jylhä A, Laaksonen R, Winkvist A, Hilvo M. A Randomized Controlled Dietary Intervention Improved the Serum Lipid Signature towards a Less Atherogenic Profile in Patients with Rheumatoid Arthritis. Metabolites. 2021; 11(9):632. https://doi.org/10.3390/metabo11090632
Chicago/Turabian StyleLindqvist, Helen M., Linnea Bärebring, Inger Gjertsson, Antti Jylhä, Reijo Laaksonen, Anna Winkvist, and Mika Hilvo. 2021. "A Randomized Controlled Dietary Intervention Improved the Serum Lipid Signature towards a Less Atherogenic Profile in Patients with Rheumatoid Arthritis" Metabolites 11, no. 9: 632. https://doi.org/10.3390/metabo11090632