Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma
Abstract
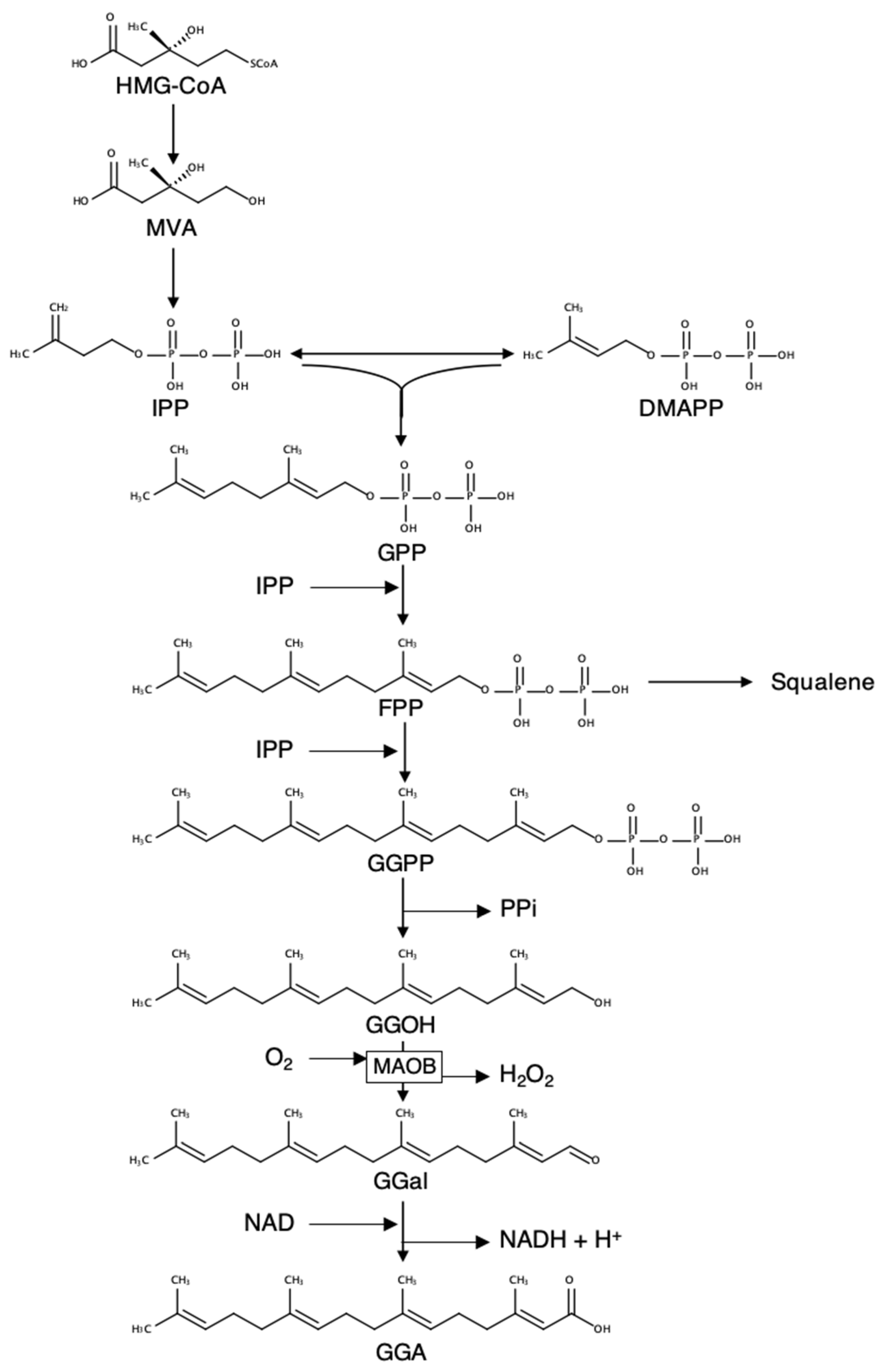
:1. Introduction
2. Results
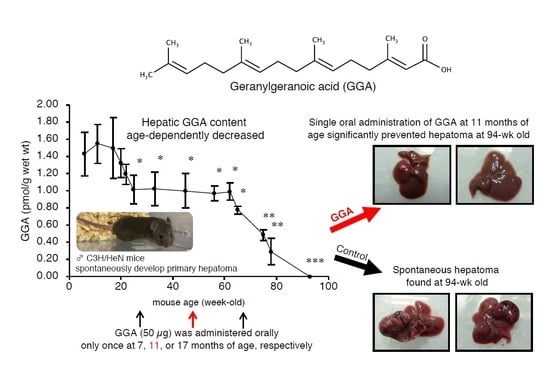
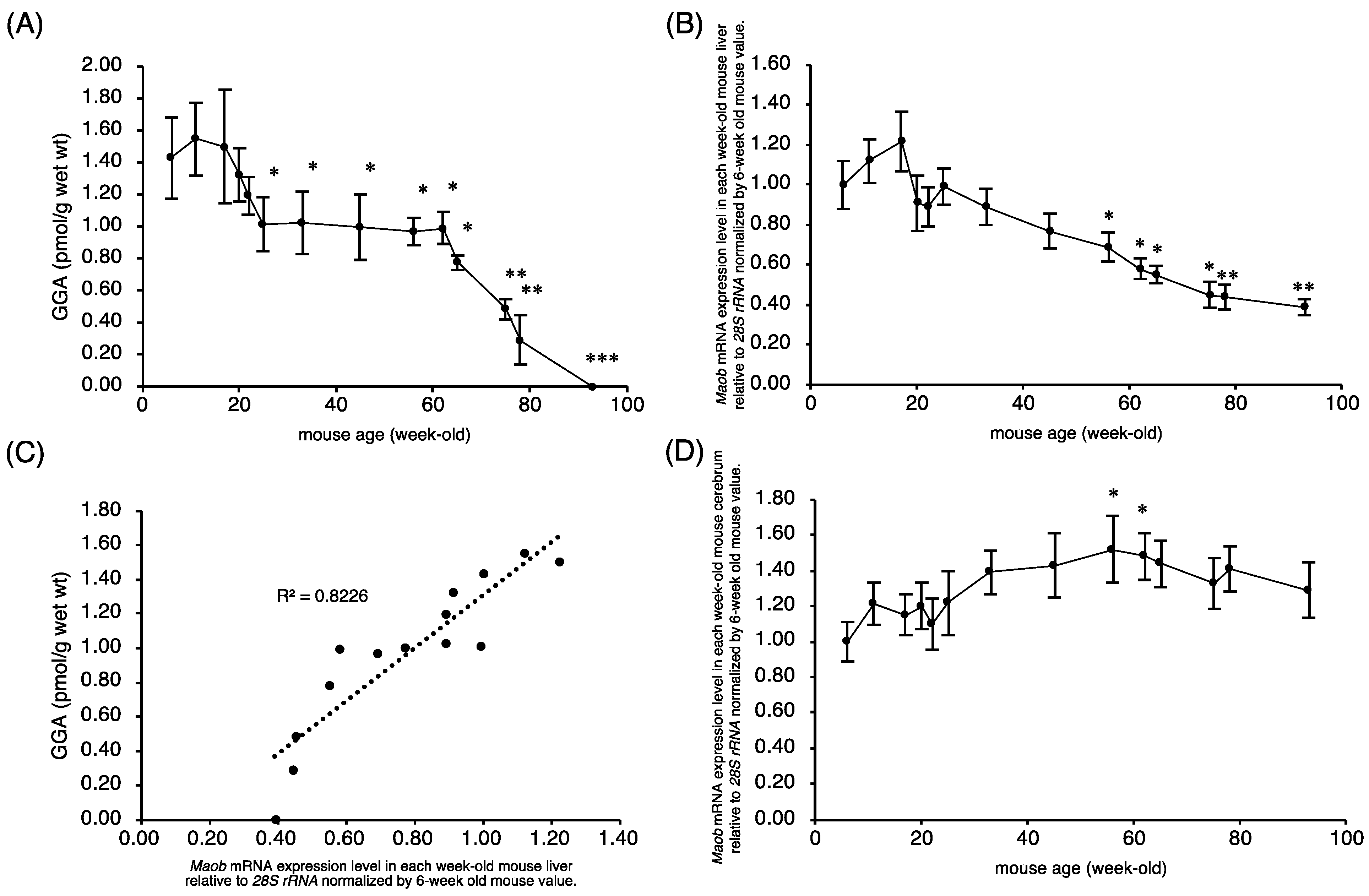
2.1. Endogenous Hepatic GGA Content of C3H/HeN Mice Decreases with Age
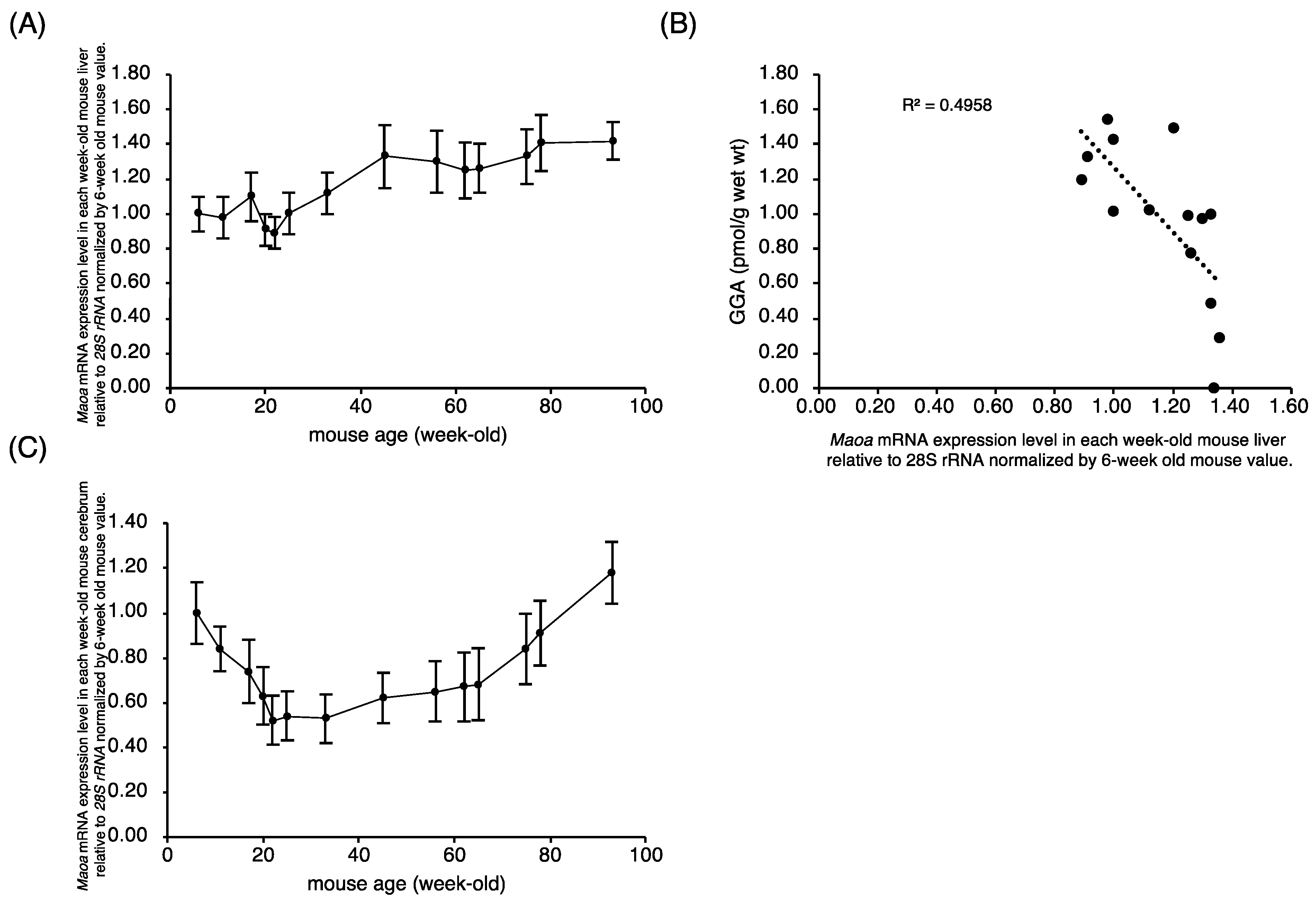
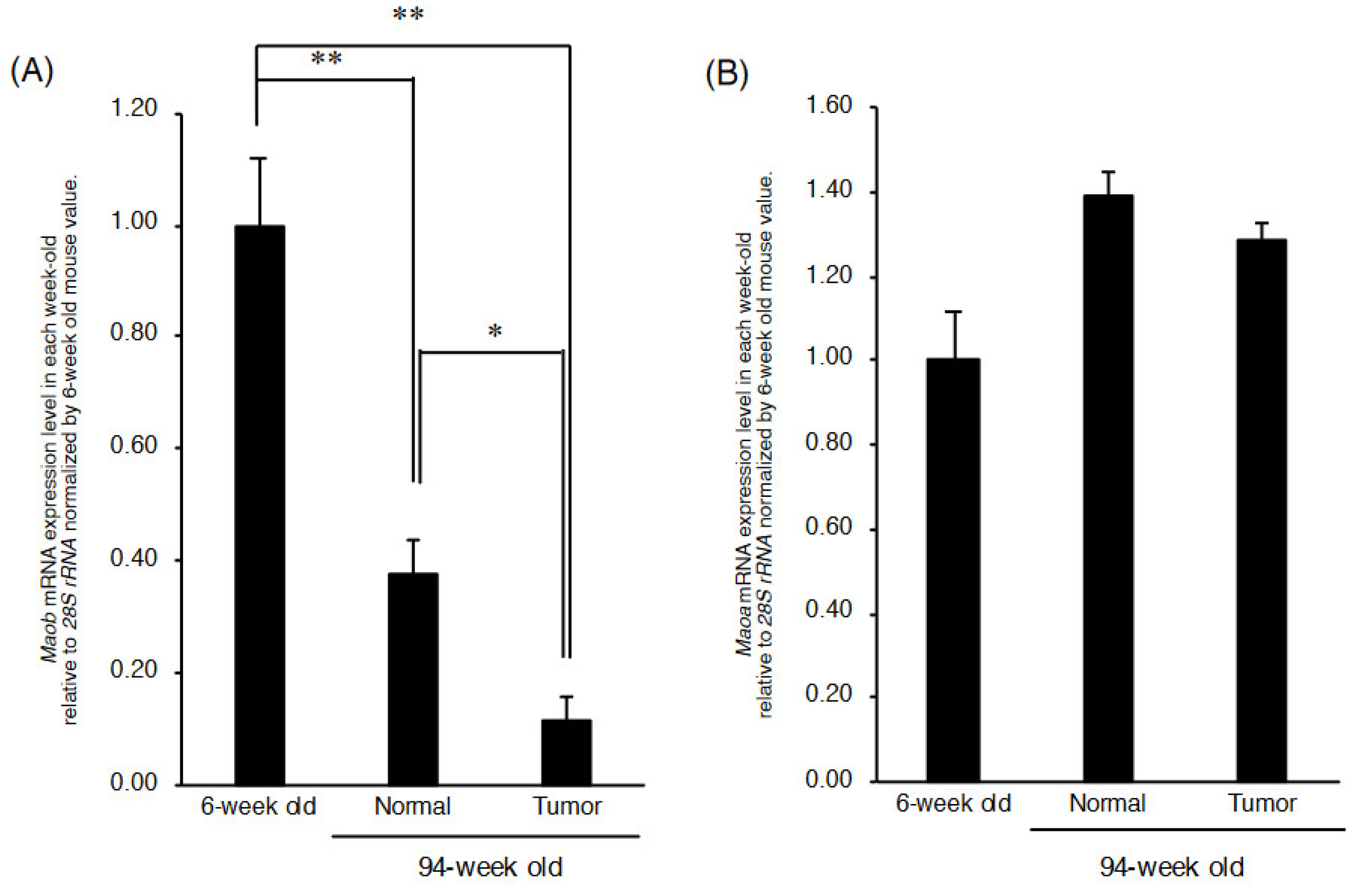
2.2. Maob Expression in C3H/HeN Mouse Liver Decreases with Aging
2.3. Maob mRNA Expression Is Further Reduced in Cancerous Liver Tissue
2.4. A Single Dosing of GGA to 11-Month-Old C3H/HeN Mice Can Efficiently Suppress the Development of Spontaneous Hepatomas
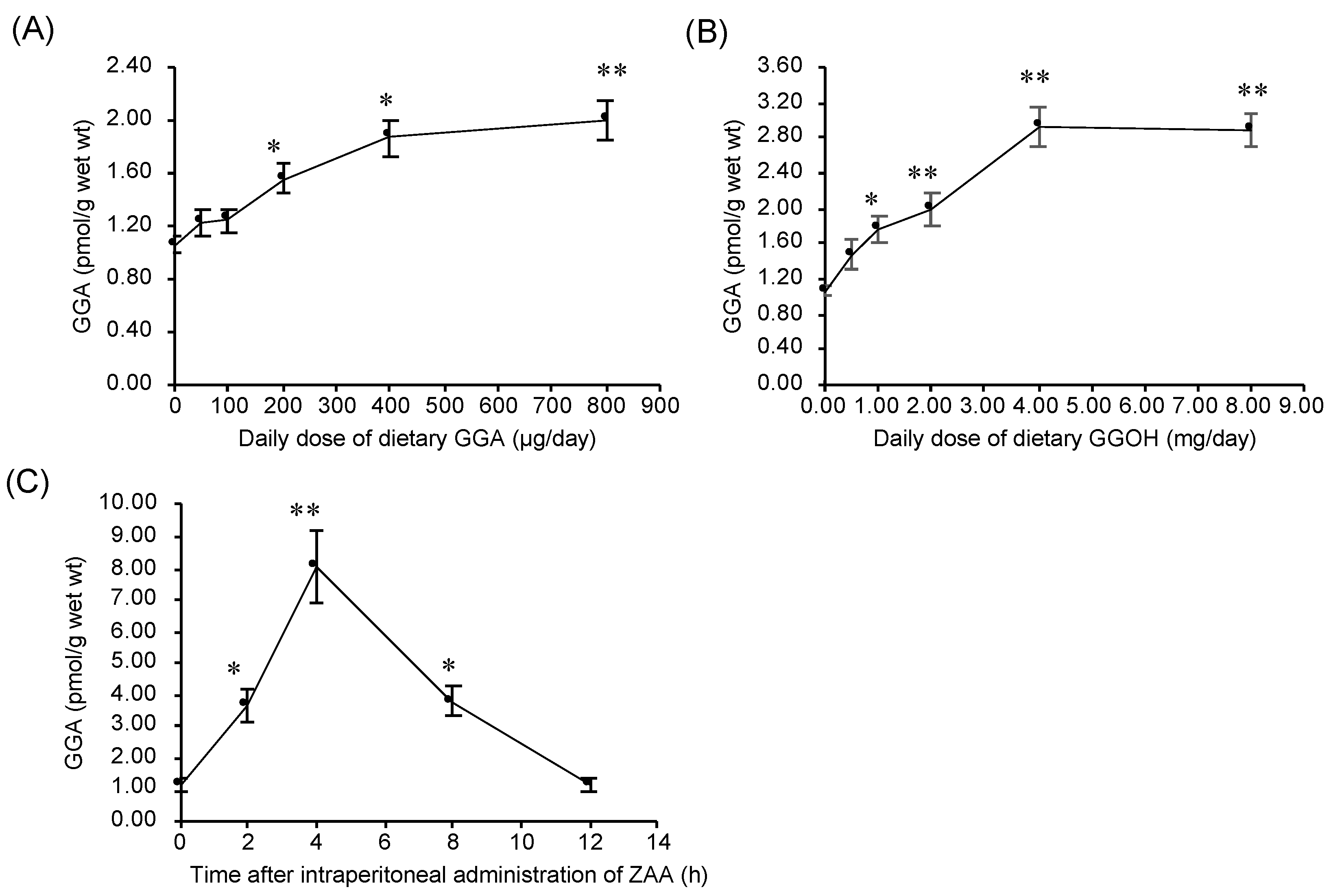
2.5. Dietary Intake of GGA or GGOH Increased Hepatic GGA Level of C3H/HeN Mice
2.6. Intraperitoneal Administration of ZAA Upregulates Hepatic GGA Level of C3H/HeN Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Evaluation of Inhibitory Effects of GGA on the Development of Spontaneous Hepatomas in C3H/HeN Mice
4.4. Preparation of GGA- or GGOH-Containing Diets and Feeding for C3H/HeN Mice
4.5. ZAA Administration to Male C3H/HeN Mice
4.6. RT-qPCR
4.7. Lipid Extraction and Quantitative Measurement of GGA Contents
4.8. Statistical Analysis
4.9. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muto, Y.; Moriwaki, H.; Ninomiya, M.; Adachi, S.; Saito, A.; Takasaki, K.T.; Tanaka, T.; Tsurumi, K.; Okuno, M.; Tomita, E.; et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N. Engl. J. Med. 1996, 334, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Moriwaki, H.; Saito, A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N. Engl. J. Med. 1999, 340, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Izumi, N.; Matsui, O.; Tanaka, K.; Kaneko, S.; Moriwaki, H.; Ikeda, K.; Osaki, Y.; Numata, K.; Nakachi, K.; et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: A randomized double-blind placebo-controlled study. J. Gastroenterol. 2014, 50, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Okita, K.; Izumi, N.; Ikeda, K.; Osaki, Y.; Numata, K.; Ikeda, M.; Kokudo, N.; Imanaka, K.; Nishiguchi, S.; Kondo, S.; et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J. Gastroenterol. 2015, 50, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Shidoji, Y.; Yamada, Y.; Hatakeyama, H.; Moriwaki, H.; Muto, Y. Induction of apoptosis by acyclic retinoid in the human hepatoma derived cell line, HuH-7. Biochem. Biophys. Res. Commun. 1995, 207, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y.; Nakamura, N.; Moriwaki, H.; Muto, Y. Rapid loss in the mitochondrial membrane potential during geranylgeranoic acid-induced apoptosis. Biochem. Biophys. Res. Commun. 1997, 230, 58–63. [Google Scholar] [CrossRef]
- Okamoto, K.; Sakimoto, Y.; Imai, K.; Senoo, H.; Shidoji, Y. Induction of an incomplete autophagic response by cancer-preventive geranylgeranoic acid (GGA) in a human hepatoma-derived cell line. Biochem. J. 2011, 440, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Iwao, C.; Shidoji, Y. Polyunsaturated branched-chain fatty acid geranylgeranoic acid induces unfolded protein response in human hepatoma cells. PLoS ONE 2015, 10, e132761. [Google Scholar] [CrossRef]
- Yabuta, S.; Shidoji, Y. TLR4-mediated pyroptosis in human hepatoma-derived HuH-7 cells induced by a branched-chain polyunsaturated fatty acid, geranylgeranoic acid. Biosci. Rep. 2020, 40, BSR20194118. [Google Scholar] [CrossRef] [Green Version]
- Shidoji, Y.; Ogawa, H. Natural occurrence of cancer-preventive geranylgeranoic acid in medicinal herbs. J. Lipid Res. 2004, 45, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Fliesler, S.J.; Schroepfer, G.J. Metabolism of mevalonic acid in cell-free homogenates of bovine retinas. Formation of novel isoprenoid acids. J. Biol. Chem. 1983, 258, 15062–15070. [Google Scholar] [CrossRef]
- Foster, J.M.; Pennock, J.F.; Marshall, I.; Rees, H.H. Biosynthesis of isoprenoid compounds in Schistosoma mansoni. Mol. Biochem. Parasitol. 1993, 61, 275–284. [Google Scholar] [CrossRef]
- Shidoji, Y.; Tabata, Y. Unequivocal evidence for endogenous geranylgeranoic acid biosynthesized from mevalonate in mammalian cells. J. Lipid Res. 2019, 60, 579–593. [Google Scholar] [CrossRef] [Green Version]
- Tabata, Y.; Shidoji, Y. Hepatic monoamine oxidase B is involved in endogenous geranylgeranoic acid synthesis in mammalian liver cells. J. Lipid Res. 2020, 61, 778–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicotra, A.; Pierucci, F.; Parvez, H.; Senatori, O. Monoamine oxidase expression during development and aging. Neurotoxicology 2004, 25, 155–165. [Google Scholar] [CrossRef]
- Saura, J.; Richards, J.G.; Mahy, N. Differential age-related changes of mao-a and mao-b in mouse brain and pe peripheral organs. Neurobiol. Aging 1994, 15, 399–408. [Google Scholar] [CrossRef]
- Becker, F.F. Inhibition of spontaneous hepatocarcinogenesis in C3H/HeN mice by edi pro A, an isolated soy protein. Carcinogenesis 1981, 2, 1213–1214. [Google Scholar] [CrossRef]
- Becker, F.F. Inhibition of spontaneous hepatocarcinogenesis in C3H/HeN mice by transplanted hepatocellular carcinomas. Cancer Res. 1981, 41, 3320–3323. [Google Scholar]
- Fox, J.G.; Feng, Y.; Theve, E.J.; Raczynski, A.R.; Fiala, J.L.A.; Doernte, A.L.; Williams, M.; McFaline, J.L.; Essigmann, J.M.; Schauer, D.B.; et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010, 59, 88–97. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hara, Y.; Imanaka, M.; Wanibuchi, H.; Tanaka, K.; Ishikawa, T.; Mori, S.; Fukusato, T. No inhibitory effects of (-)-epigallocatechin gallate and lycopene on spontaneous hepatotumorigenesis in C3H/HeN mice. Fukushima J. Med. Sci. 2010, 56, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitake, M.; Shidoji, Y. Geranylgeraniol oxidase activity involved in oxidative formation of geranylgeranoic acid in human hepatoma cells. Biomed. Res. 2012, 33, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer. 2018, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Tewari, K.K. Structural and functional changes in Novikoff hepatoma mitochondria. Cancer Res. 1973, 33, 1645–1653. [Google Scholar]
- Pedersen, P.L.; Greenawalt, J.W.; Chan, T.L.; Morris, H.P. A comparison of some ultrastructural and biochemical properties of mitochondria from Morris hepatomas 9618A, 7800, and 3924A. Cancer Res. 1970, 30, 2620–2626. [Google Scholar] [PubMed]
- Shidoji, Y. Autophagy—Cancer, Other Pathologies, Inflammation, Immunity, Infection and Aging, 1st ed.; Elsevier Inc.: New York, NY, USA, 2014; pp. 177–183. [Google Scholar]
- Iishi, H.; Tatsuta, M.; Baba, M.; Taniguchi, H. Monoamine oxidase B inhibitor enhances experimental carcinogenesis in rat colon induced by azoxymethane. Cancer Lett. 1994, 76, 177–183. [Google Scholar] [CrossRef]
- Watanabe, M.; Omori, M. Studies on the inhibitory effects of a single dosing of acyclic retinoid (polyprenoic acid) on the development of spontaneous hepatoma in mice, with special reference to histologic findings. Acta Sch. Med. Univ. Gifu 1997, 45, 110–120. [Google Scholar]
- Omori, M.; Watanabe, M.; Shidoji, Y.; Moriwaki, H.; Muto, Y. Inhibitory effects of a timely dosing of acyclic retinoid on the development of spontaneous hepatomas in C3H/HeNCrj mice. Recent Adv. Gastroenterol. Carcinog. I 1996, 1, 523–526. [Google Scholar]
- Mitake, M.; Ogawa, H.; Uebaba, K.; Shidoji, Y. Increase in plasma concentrations of geranylgeranoic Acid after turmeric tablet intake by healthy volunteers. J. Clin. Biochem. Nutr. 2010, 46, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Tabata, Y.; Uematsu, S.; Shidoji, Y. Supplementation with geranylgeranoic acid during mating, pregnancy and lactation improves reproduction index in C3H/HeN mice. J. Pet Anim. Nutr. 2020, 23, 1–8. [Google Scholar] [CrossRef]
- Muraguchi, T.; Okamoto, K.; Mitake, M.; Ogawa, H.; Shidoji, Y. Polished rice as natural sources of cancer-preventing geranylgeranoic acid. J. Clin. Biochem. Nutr. 2011, 49, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moura Espíndola, R.; Mazzantini, R.P.; Ong, T.P.; de Conti, A.; Heidor, R.; Moreno, F.S. Geranylgeraniol and beta-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-kappaB activation. Carcinogenesis 2005, 26, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabata, Y.; Omori, M.; Shidoji, Y. Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma. Metabolites 2021, 11, 634. https://doi.org/10.3390/metabo11090634
Tabata Y, Omori M, Shidoji Y. Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma. Metabolites. 2021; 11(9):634. https://doi.org/10.3390/metabo11090634
Chicago/Turabian StyleTabata, Yuki, Masahide Omori, and Yoshihiro Shidoji. 2021. "Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma" Metabolites 11, no. 9: 634. https://doi.org/10.3390/metabo11090634
APA StyleTabata, Y., Omori, M., & Shidoji, Y. (2021). Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma. Metabolites, 11(9), 634. https://doi.org/10.3390/metabo11090634