Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges
Abstract
:1. Introduction
2. Perishable Food Ecosystem and Microbiota
2.1. Fungal Spoiler of Foods
2.2. Antifungal Microorganisms in Foods
3. Classification of Antifungal Metabolites Found in Food Habitats
3.1. Organic Acids
3.2. Phenyllactic Acid (PLA)
3.3. Fatty Acids
3.4. Reuterin
3.5. Cyclic Dipeptides (CDP)
3.6. Miscellaneous Antifungal Compounds
4. Mode of Action for Various Metabolites
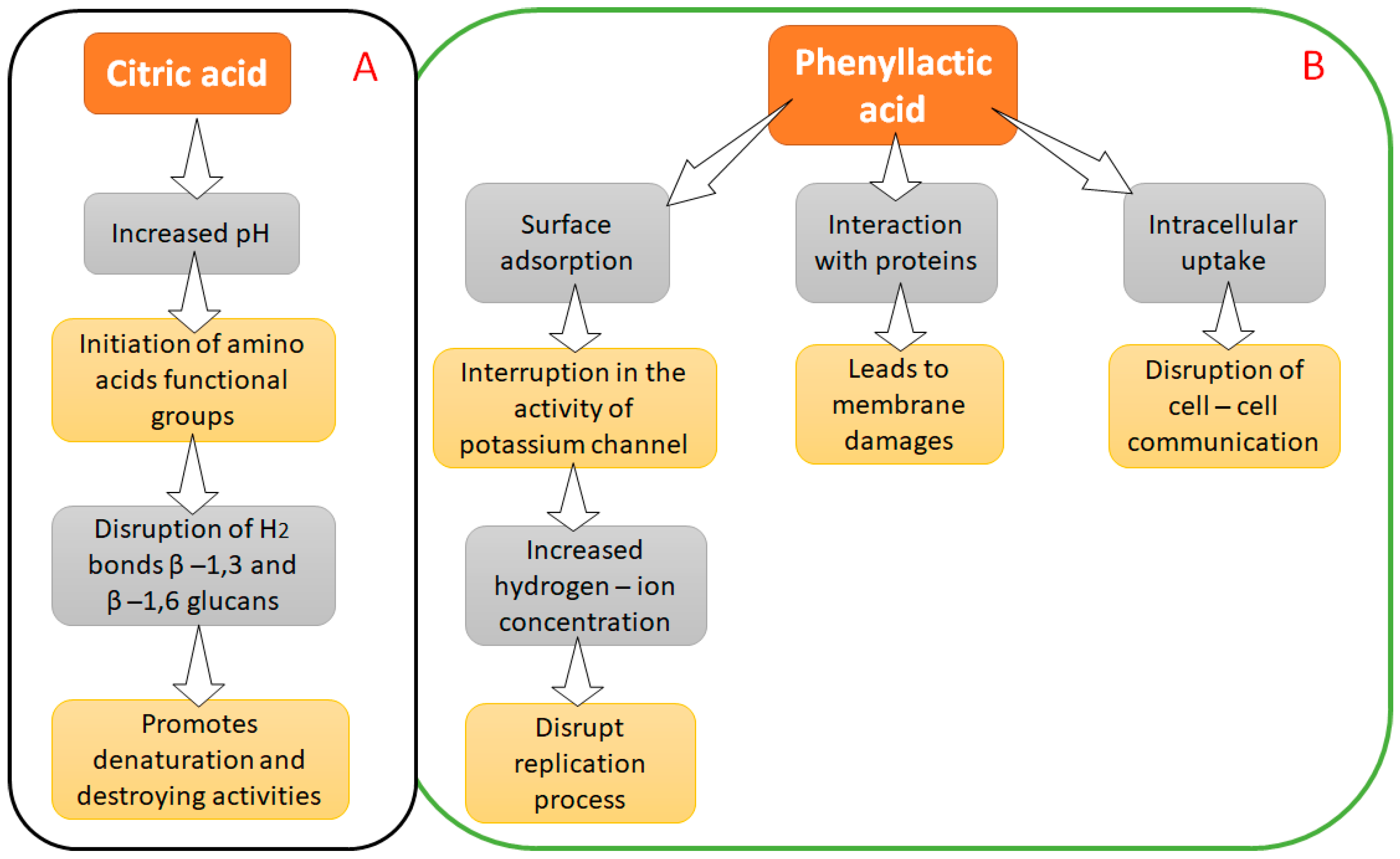
4.1. Citric Acid and Phenyllactic Acid
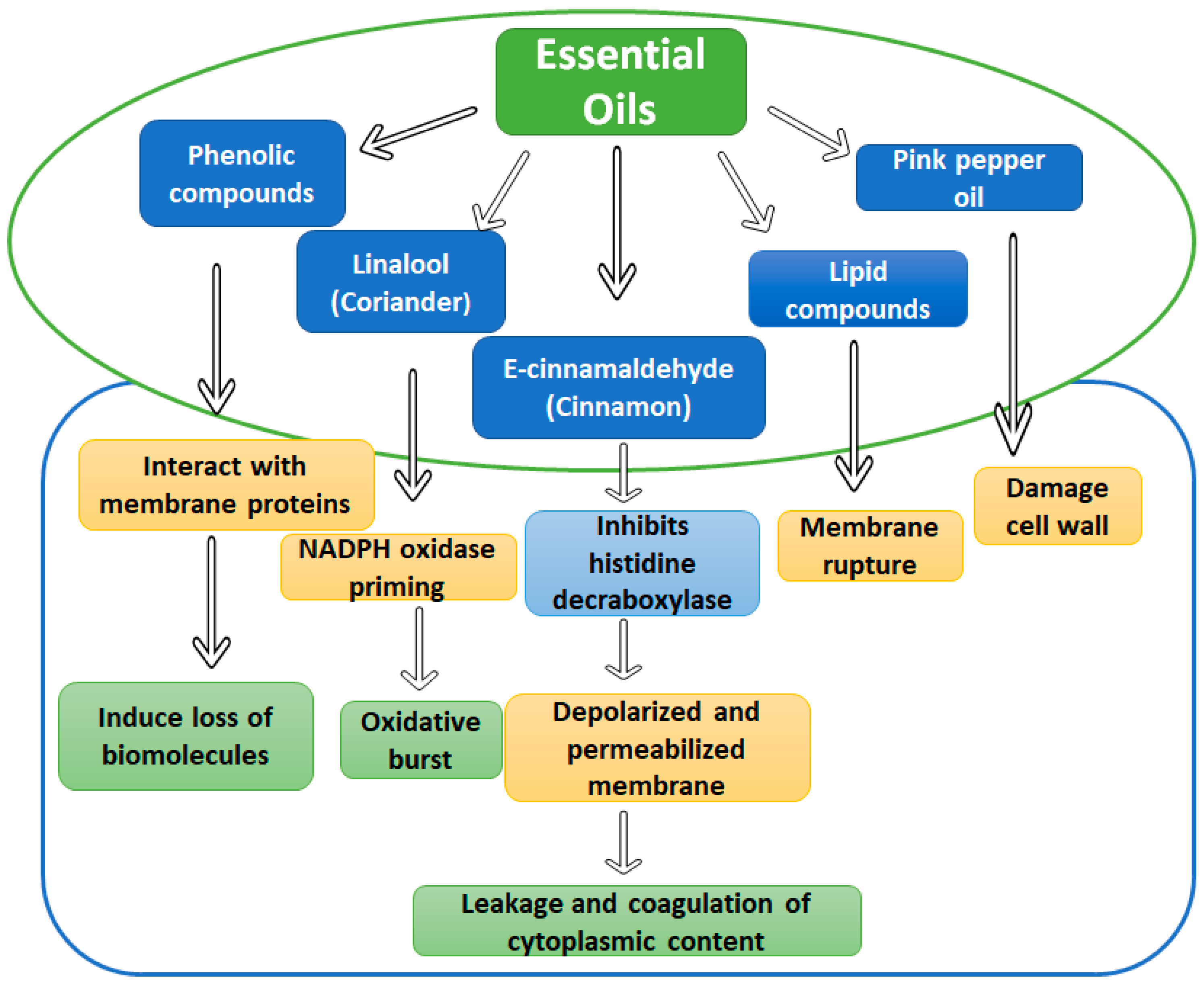
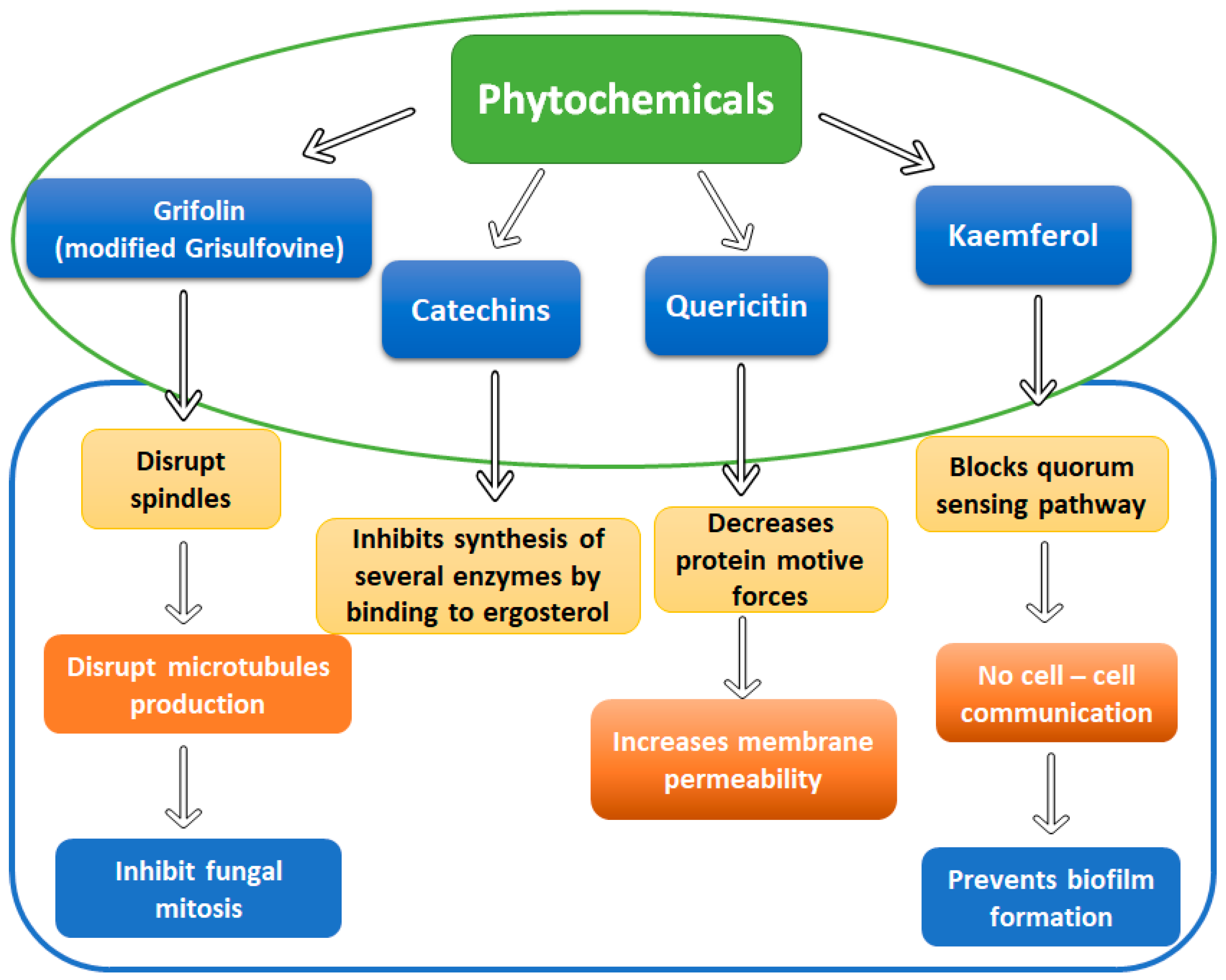
4.2. Essential Oils and Phytochemicals
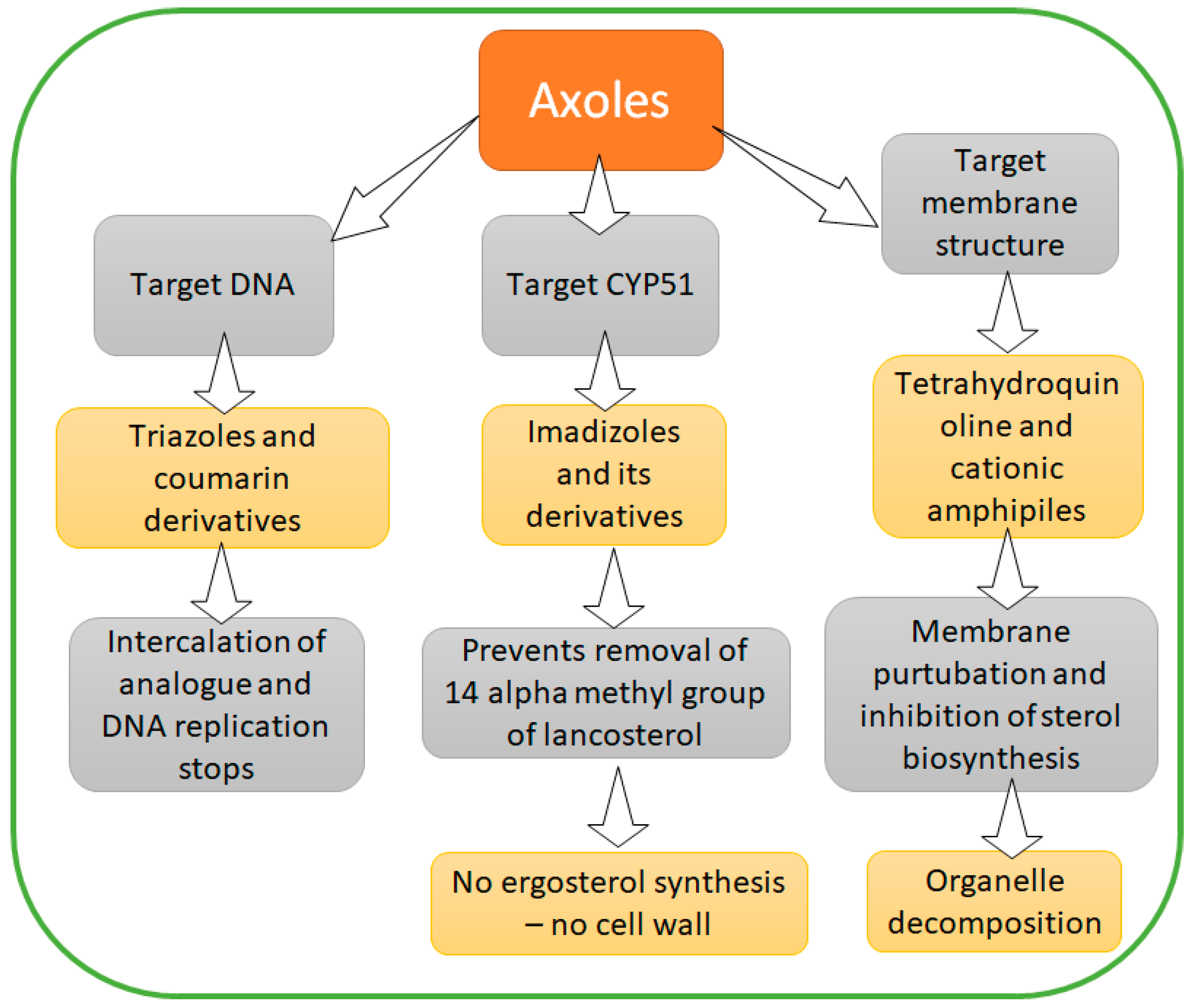
4.3. Azoles
5. Applications Oriented Studies from Laboratory to Pilot Scale
6. Major Challenges and Future Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newbold, K.B. Population Growth. In International Encyclopedia of Geography, 15 Volume Set: People, the Earth, Environment and Technology; Richardson, D., Castree, N., Goodchild, M.F., Kobayashi, A., Liu, W., Marston, R.A., Eds.; Major Reference Works; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 1–6. ISBN 978-0-470-65963-2. [Google Scholar]
- Saltmarsh, M.; Insall, L. Food additives and why they are used. In Essential Guide to Food Additives, 4th ed.; Saltmarsh, M., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2013; pp. 1–13. ISBN 978-1-84973-560-5. [Google Scholar]
- Setälä, H.; McLean, M.A. Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologia 2004, 139, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Filtenborg, O.; Frisvad, J.C.; Thrane, U. Moulds in food spoilage. Int. J. Food Microbiol. 1996, 33, 85–102. [Google Scholar] [CrossRef]
- Nummer, B.A.; Shrestha, S.; Smith, J. V Survival of Salmonella in a high sugar, low water-activity, peanut butter flavored candy fondant. Food Control 2012, 27, 184–187. [Google Scholar] [CrossRef]
- Salaheen, S.; Peng, M.; Biswas, D. Replacement of conventional antimicrobials and preservatives in food production to improve consumer safety and enhance health benefits. In Microbial Food Safety and Preservation Techniques; Rai, V.R., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 311–314. ISBN 9780429168291. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Burek, J.; Nutter, D.W. Environmental implications of perishables storage and retailin. Renew. Sustain. Energy Rev. 2020, 133, 110070. [Google Scholar] [CrossRef]
- Snyder, A.B.; Churey, J.J.; Worobo, R.W. Association of fungal genera from spoiled processed foods with physicochemical food properties and processing conditions. Food Microbiol. 2019, 83, 211–218. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Pennington, J.A.T.; Fisher, R.A. Classification of fruits and vegetables. J. Food Compos. Anal. 2009, 22, S23–S31. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting postharvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Majumdar, A.; Pradhan, N.; Sadasivan, J.; Acharya, A.; Ojha, N.; Babu, S.; Bose, S. Food degradation and foodborne diseases: A microbial approach. In Microbial Contamination and Food Degradation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–148. [Google Scholar]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. food Sci. food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Señorans, F.J.; Ibáñez, E.; Cifuentes, A. New trends in food processing. Crit. Rev. Food Sci. Nutr. 2003, 43, 507–526. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassan, M.H.A.; Alhadrami, H.A.; Hassan, H.M.; Goodfellow, M.; Rateb, M.E. Extreme environments: Microbiology leading to specialized metabolites. J. Appl. Microbiol. 2020, 128, 630–657. [Google Scholar] [CrossRef] [Green Version]
- Rouse, S.; Harnett, D.; Vaughan, A.; Sinderen, D. van Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63. [Google Scholar] [CrossRef]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and characterization of an antifungal compound (3-phenyllactic acid) produced by Lactobacillus plantarum strain. Food Bioprocess Technol. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.G.; Maina, H.N.; Katina, K.; Coda, R. Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: Identification of active compounds and impact on shelf-life. Food Control 2020, 118, 107437. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; García, C.; Rondán, J.J.; Núñez, F. Effects of preservative agents on quality attributes of dry-cured fermented sausages. Foods 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Lemaitre, M.; Garric, G.; Harel-Oger, M.; Lê, S.; Mounier, J.; Valence-Bertel, F.; Coton, E.; Thierry, A. Antifungal lactic acid bacteria combinations as biopreservation tool in dairy products. In Proceedings of the 5ième Rencontre Nutrition Alimnetation Métabolisme Santé, Rennes, France, 23 October 2019. [Google Scholar]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. 2013, 58, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Buehler, A.J.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Evaluation of biopreservatives in Greek yogurt to inhibit yeast and mold spoilage and development of a yogurt spoilage predictive model. J. Dairy Sci. 2018, 101, 10759–10774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenninger, S.M.; Lacroix, C.; Truttmann, S.; Jans, C.; Spoerndli, C.; Bigler, L.; Meile, L. Characterization of low-molecular-weight antiyeast metabolites produced by a food-protective Lactobacillus-Propionibacterium coculture. J. Food Prot. 2008, 71, 2481–2487. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal activity of volatile organic compounds produced by Pseudomonas fluorescens ZX and potential biocontrol of blue mold decay on postharvest citrus. Food Control 2021, 120, 107499. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Han, L.R.; Zhang, X.; Feng, J.T. Antifungal activity and action mode of cuminic acid from the seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. niveum (FON) causing Fusarium wilt on watermelon. Molecules 2017, 22, 2053. [Google Scholar] [CrossRef] [Green Version]
- Luz, C.; Carbonell, R.; Quiles, J.M.; Torrijos, R.; de Melo Nazareth, T.; Mañes, J.; Meca, G. Antifungal activity of peracetic acid against toxigenic fungal contaminants of maize and barley at the postharvest stage. LWT 2021, 148, 111754. [Google Scholar] [CrossRef]
- Haddoudi, I.; Cabrefiga, J.; Mora, I.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of Fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 104671. [Google Scholar] [CrossRef]
- Salas, M.L.; Mounier, J.; Maillard, M.-B.; Valence, F.; Coton, E.; Thierry, A. Identification and quantification of natural compounds produced by antifungal bioprotective cultures in dairy products. Food Chem. 2019, 301, 125260. [Google Scholar] [CrossRef]
- Adedokun, E.O.; Rather, I.A.; Bajpai, V.K.; Park, Y.-H. Biocontrol efficacy of Lactobacillus fermentum YML014 against food spoilage moulds using the tomato puree model. Front. Life Sci. 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Reyes-Prieto, M.; Bernáldez, V.; Córdoba, J.J. Identification and control of moulds responsible for black spot spoilage in dry-cured ham. Meat Sci. 2016, 122, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Hwang, H.; Lee, J.-H. Effect of lactic acid bacteria on phenyllactic acid production in kimchi. Food Control 2019, 106, 106701. [Google Scholar] [CrossRef]
- Xu, J.-J.; Sun, J.-Z.; Si, K.-L.; Guo, C.-F. 3-Phenyllactic acid production by Lactobacillus crustorum strains isolated from naturally fermented vegetables. LWT 2021, 111780. [Google Scholar] [CrossRef]
- Guan, J.; Han, C.; Guan, Y.; Zhang, S.; Yun, J.; Yao, S. Optimizational production of phenyllactic acid by a Lactobacillus buchneri strain via uniform design with overlay sampling methodology. Chin. J. Chem. Eng. 2019, 27, 418–425. [Google Scholar] [CrossRef]
- Amiri, S.; Aghamirzaei, M.; Mostashari, P.; Sarbazi, M.; Tizchang, S.; Madahi, H. The impact of biotechnology on dairy industry. In Microbial Biotechnology in Food and Health; Ray, R.C., Ed.; Academic Press: Cambridge, UK, 2021; pp. 53–79. ISBN 9780128198131. [Google Scholar]
- Chaudhari, S.S.; Gokhale, D. V Phenyllactic acid: A potential antimicrobial compound in lactic acid bacteria. J. Bacteriol. Mycol. Open Access 2016, 2, 121–125. [Google Scholar]
- Jung, S.; Woo, C.; Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic potential of Bacillus amyloliquefaciens isolated from Kimchi, a traditional Korean fermented cabbage. Probiotics Antimicrob. Proteins 2021, 13, 1195–1212. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, W.-C.; Gao, Y.-H.; Hsiao, H.-I.; Pan, C.-L. Production of phenyllactic acid from Porphyra residues by Lactic Acid bacterial fermentation. Processes 2021, 9, 678. [Google Scholar] [CrossRef]
- Dung, V.K.; Ngoc, N.N.; Dung, L.S.; Hien, V.T.N. Collection of phenyllactic acid from a strain of Lactobacillus sp. and application in agricultural products preservation. Vietnam. J. Food Control 2021, 4, 22–33. [Google Scholar]
- Pradhan, S.; Ananthanarayan, L.; Prasad, K.; Bhatnagar-Mathur, P. Anti-fungal activity of lactic acid bacterial isolates against aflatoxigenic fungi inoculated on peanut kernels. LWT 2021, 143, 111104. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Zhu, Y.; Zhou, C.; Xiong, Z.; Eweys, A.S.; Zhou, H.; Dong, Y.; Xiao, X. Metabolomics strategy for revealing the components in fermented barley extracts with Lactobacillus plantarum dy-1. Food Res. Int. 2021, 139, 109808. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ju, H.; Wang, Y.; Du, G.; Yan, X.; Cui, Y.; Yuan, Y.; Yue, T. Antifungal activity and mode of action of Lactic acid bacteria isolated from Kefir against Penicillium expansum. Food Control 2021, 108274. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Lopez-Lopez, K.; Gomez-Lopez, E.D.; SERNACOCK, L.; Aguilar, C.N. In-vitro assessment for the control of Fusarium species using a lactic acid bacterium isolated from yellow pitahaya (Selenicereus megalanthus (K. Schum. Ex Vaupel Moran)). J. Integr. Agric. 2021, 20, 159–167. [Google Scholar] [CrossRef]
- Alaoui, K.; Chafik, Z.; Arabi, M.; Abouloifa, H.; Asehraou, A.; Chaoui, J.; Kharmach, E.-Z. In vitro antifungal activity of Lactobacillus against potato Late blight Phytophthora infestans. Mater. Today Proc. 2021, 45, 7725–7733. [Google Scholar] [CrossRef]
- Abouloifa, H.; Gaamouche, S.; Rokni, Y.; Hasnaoui, I.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; D’Hallewin, G.; Ben Salah, R.; et al. Antifungal activity of probiotic Lactobacillus strains isolated from natural fermented green olives and their application as food bio-preservative. Biol. Control 2021, 152, 104450. [Google Scholar] [CrossRef]
- Makki, G.M.; Kozak, S.M.; Jencarelli, K.G.; Alcaine, S.D. Evaluation of the efficacy of commercial protective cultures to inhibit mold and yeast in cottage cheese. J. Dairy Sci. 2021, 104, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental study on the improvement of the antifungal activity of Lactobacillus reuteri R29 through increased production of phenyllactic acid and reuterin. Food Control 2018, 88, 139–148. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Gerez, C.L. Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control 2018, 123, 137–143. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT 2020, 130, 109618. [Google Scholar] [CrossRef]
- Li, X.; Ning, Y.; Liu, D.; Yan, A.; Wang, Z.; Wang, S.; Miao, M.; Zhu, H.; Jia, Y. Metabolic mechanism of phenyllactic acid naturally occurring in Chinese pickles. Food Chem. 2015, 186, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Dallagnol, A.M.; Bustos, A.Y.; Martos, G.I.; de Valdez, G.F.; Gerez, C.L. Antifungal and antimycotoxigenic effect of Lactobacillus plantarum CRL 778 at different water activity values. Rev. Argent. Microbiol. 2019, 51, 164–169. [Google Scholar] [CrossRef]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef]
- Solano, R.J.; Sierra, C.A.; Murillo, M.Á. Antifungal activity of LDPE/lauric acid films against Colletotrichum tamarilloi. Food Packag. Shelf Life 2020, 24, 100495. [Google Scholar] [CrossRef]
- Ouiddir, M.; Bettache, G.; Salas, M.L.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian lactic acid bacteria for use as antifungal bioprotective cultures and application in dairy and bakery products. Food Microbiol. 2019, 82, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.-B.; Jardin, J.; Coton, M.; Salas, M.L.; Coton, E.; Valence, F.; Mounier, J. Antifungal activity of fermented dairy ingredients: Identification of antifungal compounds. Int. J. Food Microbiol. 2020, 322, 108574. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Dacko, A.; Tan, A.K.; Xiang, S.; Curtis, J.M.; Gänzle, M.G. Structure-function relationships of antifungal monohydroxy unsaturated fatty acids (HUFA) of plant and bacterial origin. Food Res. Int. 2020, 134, 109237. [Google Scholar] [CrossRef]
- Souza, J.L.S.; da Silva, A.F.; Carvalho, P.H.A.; Pacheco, B.S.; Pereira, C.M.P.; Lund, R.G. Aliphatic fatty acids and esters: Inhibition of growth and exoenzyme production of Candida, and their cytotoxicity in vitro: Anti-candida effect and cytotoxicity of fatty acids and esters. Arch. Oral Biol. 2014, 59, 880–886. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Dawood, D.H.; Safwat, N.A. Antifungal action and induction of resistance by β-aminobutyric acid against Penicillium digitatum to control green mold in orange fruit. Pestic. Biochem. Physiol. 2021, 171, 104721. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Sahu, M.; Dwivedi, V. Studies on Lactobacillus bacteriocin for production and characterization against some pathogenic and food spoilage bacteria. Int. J. Curr. Res. Acad. Rev. 2021, 9, 1–12. [Google Scholar]
- Vimont, A.; Fernandez, B.; Ahmed, G.; Fortin, H.-P.; Fliss, I. Quantitative antifungal activity of reuterin against food isolates of yeasts and moulds and its potential application in yogurt. Int. J. Food Microbiol. 2019, 289, 182–188. [Google Scholar] [CrossRef] [PubMed]
- El-Ziney, M.G.; van den Tempel, T.; Debevere, J.; Jakobsen, M. Application of Reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation. J. Food Prot. 1999, 62, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef] [Green Version]
- Mishra, K.A.; Choi, J.; Choi, S.-J.; Baek, K.-H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.N.; Nambisan, B.; Mohandas, C. Purification and identification of two antifungal cyclic dipeptides from Bacillus cereus subsp. thuringiensis associated with a rhabditid entomopathogenic nematode especially against Fusarium oxysporum. J. Enzyme Inhib. Med. Chem. 2014, 29, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, M.-K.; Liu, R.; Kim, M.-K.; Moon, D.; Kim, A.H.; Song, S.-H.; Kang, S.-O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef]
- Bayisa, R.A.; Cho, J.Y.; Kim, K.Y. Purification and identification of a new antifungal dipeptide from Bacillus velezensis AR1 culture supernatant. Pest Manag. Sci. 2021, 77, 775–779. [Google Scholar] [CrossRef]
- Adibi, A.; Rees, E.R.; McCarley, S.; Sica, V.P.; Oberlies, N.H. Characterization and isolation of peptide metabolites of an antifungal bacterial isolate identified as Bacillus amyloliquefaciens subspecies plantarum strain FZB42. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Deepa, S.; Sathish, T.; Vinithkumar, N.V.; Limna Mol, V.P.; Kirubagaran, R. Distribution and diversity of macrofoulers in the coastal areas of Port Blair, Andaman and Nicobar Islands. Int. J. Environ. Res. 2015, 9, 1315–1324. [Google Scholar]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Rao Putcha, V.L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, M.; Kapadnis, B. Lactococcus lactis subsp. cremoris of plant origin produces antifungal Cyclo-(Leu-Pro) and tetradecanoic acid. Indian J. Microbiol. 2021, 61, 74–80. [Google Scholar] [CrossRef]
- Tanaka, E.; Hosoe, T.; Degawa, Y.; Kolařík, M. Revision of the genus Aciculosporium (Clavicipitaceae) with a description of a new species on wavyleaf basketgrass, and proline-containing cyclic dipeptide production by A. take. Mycoscience 2021, MYC527. [Google Scholar] [CrossRef]
- Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and antibiofilm activities of the fungal metabolites isolated from the marine endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, J.; Li, X.; Quan, C. Effect of the environmental factors on diketopiperazine cyclo (Pro-Phe) production and antifungal activity of Bacillus amyloliquefaciens Q-426. Biologia 2021, 76, 1789–1795. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Wu, W.; Li, H.; An, Z.; Zhou, F. C7-prenylation of tryptophan-containing cyclic dipeptides by 7-dimethylallyl tryptophan synthase significantly increases the anticancer and antimicrobial activities. Molecules 2020, 25, 3676. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Sadeghi, A.; Mortazavi, S.A. The use of cyclic dipeptide producing LAB with potent anti-aflatoxigenic capability to improve techno-functional properties of clean-label bread. Ann. Microbiol. 2020, 70, 24. [Google Scholar] [CrossRef]
- Qiu, S.; Avula, B.; Guan, S.; Ravu, R.R.; Wang, M.; Zhao, J.; Khan, I.A.; Hinchee, M.; Li, X.-C. Identification of fusaricidins from the antifungal microbial strain Paenibacillus sp. MS2379 using ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1586, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-K.; Liu, R.; Kang, S.-O. Antimicrobial activity of cyclic dipeptides produced by Lactobacillus plantarum LBP-K10 against multidrug-resistant bacteria, pathogenic fungi, and influenza A virus. Food Control 2018, 85, 223–234. [Google Scholar] [CrossRef]
- Borthwick, A.D.; Da Costa, N.C. 2,5-diketopiperazines in food and beverages: Taste and bioactivity. Crit. Rev. Food Sci. Nutr. 2017, 57, 718–742. [Google Scholar] [CrossRef]
- Kumar, N.; Mohandas, C.; Nambisan, B.; Kumar, D.R.S.; Lankalapalli, R.S. Isolation of proline-based cyclic dipeptides from Bacillus sp. N strain associated with rhabitid entomopathogenic nematode and its antimicrobial properties. World J. Microbiol. Biotechnol. 2013, 29, 355–364. [Google Scholar] [CrossRef]
- Dover, S.E.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Natural antimicrobials and their role in vaginal health: A short review. Int. J. Probiotics Prebiotics 2008, 3, 219. [Google Scholar] [PubMed]
- Ahmad, H.; Matsubara, Y. Suppression of anthracnose in strawberry using water extracts of lamiaceae herbs and identification of antifungal metabolites. Hortic. J. 2020, 89, 359–366. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, W.; Xu, S.X.; Magarvey, N.A.; McCormick, J.K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. USA 2011, 108, 3360–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhardt, T.; Albano, H.; Kiskó, G.; Mohácsi-Farkas, C.; Teixeira, P. Antilisterial activity of bacteriocinogenic Pediococcus acidilactici HA6111-2 and Lactobacillus plantarum ESB 202 grown under pH and osmotic stress conditions. Food Microbiol. 2015, 48, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P. V Phenyllactic Acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Kadyan, S.; Pradhan, D. Antifungal Lactic Acid Bacteria (LAB): Potential use in food systems. In Novel Strategies to Improve Shelf-Life and Quality of Foods; Mishra, S.K., Goyal, M.R., Eds.; Apple Academic Press: New York, NY, USA, 2020; pp. 73–94. ISBN 100301027X. [Google Scholar]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.-Y.; Li, Z.-H.; Ding, Z.-H.; Dong, Z.-J.; Li, G.-T.; Li, Y.; Liu, J.-K. Meroterpenoid pigments from the basidiomycete Albatrellus ovinus. J. Nat. Prod. 2013, 76, 79–84. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, S.; Sharma, M.; Kumar, N.; Pruthi, V.; Poluri, K.M. Effectiveness of phytoactive molecules on transcriptional expression, biofilm matrix, and cell wall components of Candida glabrata and its clinical isolates. ACS Omega 2018, 3, 12201–12214. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Inhibition of quorum-sensing: A new paradigm in controlling bacterial virulence and biofilm formation. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Singapore, 2018; pp. 3–21. [Google Scholar]
- Tjia, J.A. Journey into C. albicans Biofilms: Proteomic and Functional Genomic Approaches to Uncovering Mechanisms of Adherence; University of Toronto: Toronto, ON, Canada, 2016. [Google Scholar]
- Krishnamoorthy, R.; Gassem, M.A.; Athinarayanan, J.; Periyasamy, V.S.; Prasad, S.; Alshatwi, A.A. Antifungal activity of nanoemulsion from Cleome viscosa essential oil against foodborne pathogenic Candida albicans. Saudi J. Biol. Sci. 2021, 28, 286–293. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Ali, E.M.; Alkuwayti, M.A.; Aldayel, M.F.; Abdallah, B.M. Coumarin derivative, 5′-hydroxy-auraptene, extracted from Lotus lalambensis, displays antifungal and anti-aflatoxigenic activities against Aspergillus flavus. J. King Saud Univ.-Sci. 2021, 33, 101216. [Google Scholar] [CrossRef]
- Reyes, D.C.; Annis, S.L.; Rivera, S.A.; Leon-Tinoco, A.Y.; Wu, C.; Perkins, L.B.; Perry, J.J.; Ma, Z.X.; Knight, C.W.; Castillo, M.S.; et al. In vitro screening of technical lignins to determine their potential as hay preservatives. J. Dairy Sci. 2020, 103, 6114–6134. [Google Scholar] [CrossRef]
- Dalhoff, A.A.H.; Levy, S.B. Does use of the polyene natamycin as a food preservative jeopardise the clinical efficacy of amphotericin B? A word of concern. Int. J. Antimicrob. Agents 2015, 45, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. J. Dairy Sci. 2018, 101, 3859–3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebbia, S.; Lamberti, C.; Lo Bianco, G.; Cirrincione, S.; Laroute, V.; Cocaign-Bousquet, M.; Cavallarin, L.; Giuffrida, M.G.; Pessione, E. Antimicrobial potential of food Lactic Acid Bacteria: Bioactive peptide decrypting from caseins and bacteriocin production. Microorganisms 2021, 9, 65. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A. Novel antifungal bacteriocin from Lactobacillus paracasei KC39 with anti-mycotoxigenic properties. Biosci. Res. 2018, 15, 4171–4183. [Google Scholar]
- Ahmad Rather, I.; Seo, B.J.; Rejish Kumar, V.J.; Choi, U.; Choi, K.; Lim, J.H.; Park, Y. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML 007 and its application as a food preservative. Lett. Appl. Microbiol. 2013, 57, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.H.; Yang, E.J.; Woo, E.R.; Chang, H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Badr, A.N.; Abdel-Fatah, S.M.; Sree, Y.H.A.; Amra, H.A. Mycotoxigenic fungi and mycotoxins in Egyptian barley under climate changes. Res. J. Environ. Toxicol. 2017, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Badr, A.N.; Nada, F.; Shehata, M.G.; Amra, H.A. Anti-mycotic and anti-mycotoxigenic properties of Egyptian dill. J. Appl. Sci. 2017, 17, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Manso, S.; Becerril, R.; Nerín, C.; Gómez-Lus, R. Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Solano, A.C.V.; de Rojas Gante, C. Two different processes to obtain antimicrobial packaging containing natural oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef] [Green Version]
- Becerril, R.; Manso, S.; Nerin, C.; Gómez-Lus, R. Antimicrobial activity of Lauroyl Arginate Ethyl (LAE), against selected foodborne bacteria. Food Control 2013, 32, 404–408. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Obregozo, M.D.; de Valdez, G.F. A ready-to-use antifungal starter culture improves the shelf life of packaged bread. J. Food Prot. 2010, 73, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, Á.; Muñoz, E.; López, M.C.; Cordero, M.; Martínez, J.P.; Viñas, M. Transcriptomics as a tool to discover new antibacterial targets. Biotechnol. Lett. 2017, 39, 819–828. [Google Scholar] [CrossRef] [PubMed]

| Source of Organic Acid | Organic Acid | Microbial Target | Primary Food Products | References |
|---|---|---|---|---|
| Lactobacillus brevis AM7 | Lactic acid, Acetic acid | Penicillium roqueforti P1, Eurotium herbariorum CBS 117336 and Penicillium albocoremium CBS 109582 | Bread | [22] |
| Lactobacillus paracasei subsp. paracasei SM20, Propionibacterium jensenii SM11 | Propionic acid, 2-pyrrolidone-5-carboxylic acid, Acetic acid, Lactic acid, 3-phenyllactic acid, Hydroxyphenyllactic acid, Succinic acid | Spoilage yeasts and molds | Yoghurt and cheese surfaces | [28] |
| Pseudomonas fluorescens ZX | Butyric acid, Acetic acid, Isobutyric acid, 2-Methylbutyric acid, 3-Methylbutyric acid | Penicillium italicum | Citrus fruits | [29] |
| Seeds of Cuminum cyminum L. | Cuminic acid | Fusarium oxysporum f. sp. niveum | Horticultural crops, watermelon | [30] |
| Acetylation reaction of CH3COOH with H2O2 | Peracetic acid | Aspergillus flavus, Penicillium verrucosum | Maize and barley grain | [31] |
| Bacillus cereus, B. subtilis B. mojavensis, B. velezensis | Indoleacetic acid | Fusarium equiseti | Vicia faba | [32] |
| Lactobacillus plantarum IS10, L. brevis NCDC 02, L. paracasei M3 | 5-Oxopyrrolidine-2-carboxylic acid, 3-(4-Hydroxyphenyl) propanoic acid, 3-Phenylpropanoic acid, Hydroxyphenyllactic acid, Dodecanoic acid | Mucor, Penicillium | Yoghurt, cheese, sour cream | [33] |
| Lactobacillus fermentum YML014 | Lactic acid, Acetic acid | Aspergillus niger, A. flavus, Candida albicans, Penicillium expansum, Zygosaccharomyces rouxii | Fermented vegetables | [34] |
| Penicillium chrysogenum CECT 20922 | Hexanoic acid Octanoic acid | Cladosporium cladosporioides, C. herbarum, C. oxysporum | Meat, dry-cured fermented sausages | [23,35] |
| Source of PLA | Microbial Target | Primary Food Products | References |
|---|---|---|---|
| Latobacillus crustorum | Broad range of bacterial and fungal species | Naturally fermented Chinese vegetable | [37] |
| Lactobacillus brevis, L. plantarum, L. sakei, Leuconostoc lactis, Leuconostoc mesenteroide, Pediococcus pentosaceus, | Aspergillus, Penicillin spp., Candida, Rhodotorula spp. | Kimchi (a fermented vegetable food product in Korea) | [41] |
| Lactobacillus plantarum KP3 L. plantarum KP4 | Gram-positive/negative bacteria and some fungal species | Porphyra residues | [42] |
| Lactobacillus sp. MX3.2 | Aspergillus niger, A. flavus, A. oryzae, E. coli, Salmonella enterica, Shigela flexneri | Mango and chilli | [43] |
| Lactobacillus plantarum argentoratensis, Enterococcus faecium | Aflatoxigenic fungi | Agricultural commodities | [44] |
| Lactobacillus buchneri GBS3 | Aspergillus, Penicillium, Fusarium species | Traditional Chinese pickles | [38] |
| Lactobacillus plantarum dy-1 | Broad spectrum of fungi | Fermented barley extracts | [45] |
| Lactobacillus kefiri M4, Pediococcus acidilactici MRS-7 | Penicillium expansum | Fruits | [46] |
| Lactobacillus plantarum | Fusarium oxysporum, F. fujikuroi | Crops | [47] |
| Lactobacillus brevis, L. plantarum | Phytophthora infestans | Fermented green olives | [48] |
| Lactobacillus brevis, L. plantarum, L. pentosus | Fungi (Candida pelliculosa and Penicillium digitatum) Molds (Penicillium sp., Aspergillus niger, Rhizopus sp., Fusarium oxysporum), Yeasts (Candida pelliculosa and Rhodotorula sp.), | Fermented green olives | [49] |
| Lacticaseibacillus spp. and Lactiplantibacillus spp. | Debaryomyces hansenii, Torulaspora delbrueckii, Meyerozyma guilliermondii | Cottage cheese | [50] |
| Lactobacillus reuteri R29 | Fusarium culmorum | Bread system | [51] |
| Pediococcus acidilactici CRL 1753 | Aspergillus niger CH2, Candida tropicalis CH6, Penicillium roqueforti CH4, Metschnikowia pulcherrima CH7 | Bread | [52] |
| Lactobacillus plantarum TR7, L. plantarum TR71 | Penicillium expansum, Aspergillus flavus | Tomato | [53] |
| Lactobacillus plantarum | Aspergillus fumigatus, Penicillium roqueforti | Chinese pickles | [54] |
| Lactobacillus hammesii | Aspergillus niger, Penicillium roqueforti | Wheat bread | [55] |
| Lactobacillus plantarum CRL 778 | Aspergillus niger | Fermented foods | [56] |
| Lactobacillus fermentum, L. plantarum | Aspergillus and Penicillium genera | Fermented and dried cocoa beans | [57] |
| Sources of CDP | Identified CDP | Microbial Target | References |
|---|---|---|---|
| Lactococcus lactis subsp. cremoris | cyclo(Leu-Pro) | Fungal Species | [77] |
| Aciculosporium take | cyclo(L-pro-L-Phe), cyclo L-pro-L-Leu), cyclo(L-pro-L-Ile) | Fungal species | [78] |
| Epicoccum nigrum M13 | cyclo(L-Pro-L-Ile), cyclo(L-Pro-L-Val), cyclo(L-Pro-L-Tyr), cyclo(L-Pro-L-Phe), | Fungal and bacterial species | [79] |
| Bacillus amyloliquefaciens Q-426 | cyclo(L-Pro-L-Phe), cyclo(L-Pro-D-Phe), cyclo(D-Pro-D-Phe), cyclo(D-Phe-L-Pro) | Broad range of fungi | [80] |
| Bacillus amyloliquefaciens subsp. plantarum strain FZB42 | cis-cyclo(L-Pro-L-Ile), cis-cyclo(L-Pro-L-Leu), cis-cyclo(L-Pro-L-Phe), cis-cyclo(L-Pro-L-Pro), cis-cyclo(L-Pro-L-Val), | Filamentous fungi | [74] |
| Bacillus velezensis AR1 | 5-N-tyrosinylornithine | Monilinia fructicola and Colletotricum goeosporioides | [73] |
| Prenylation of tryptophan with cyclic dipeptides at C7 position by 7-Dimethylallyl | cyclo(L-Trp-Gly), cyclo(L-Trp-L-Ala), cyclo(L-Trp-L-Phe), cyclo(L-Trp-L-Leu), cyclo(L-Trp-L-Pro), cyclo(L-Trp-L-Trp), cyclo(L-Trp-L-Tyr) | Aspergillus flavus, Candida albicans, Fusarium oxysporum, Alternaria brassicae, Rhizoctonia solani, Penicillium expansum | [81] |
| Pediococcus pentosaceus | Hexahydro-7-hydroxy-phenylmethyl | Aspergillus niger | [82] |
| Lactobacillus rhamnosus | 9-amino acid peptide (a derivative of αs2-casein) | Mucor racemosus, Rhodotorula mucilaginosa | [60] |
| Paenibacillus sp. MS2379 | Fusaricidins along with amino acid residues of γ-aminobutyric acid and serine | Broad array of fungal pathogens | [83] |
| Lactobacillus plantarum LBPK10 | cyclo(Val-Pro), cyclo(Tyr-Pro), cyclo(Ser-Pro), | Broad range of fungal species | [84] |
| Lactobacillus plantarum LBPK10 | cis-cyclo(L-Leu-L-Hyp), cyclo(Phe-Pro), cyclo(Leu-Pro) | Bacterial, virus and fungal pathogen. | [84] |
| Lactobacillus casei AST18, L. plantarum AF1, L. Plantarum MiLAB 393, | 2,6-diketopiperazines and their derivatives, 2,5-diketopiperazines, 2,3-diketopiperazines | Fungi and Gram-positive/negative bacteria | [85] |
| Pediococcus pentosaceus | Non-pediocin-like peptides | Fusarium graminearum | [76] |
| Bacillus cereus subsp. thuringiensis | cyclo(D-Pro-L-Met) cyclo(D-Pro-D-Tyr) | Rhizoctonia solani, Fusarium oxysporum, Penicillium expansum | [71] |
| Lactobacillus plantarum | cyclo(Tyr-Pro), cis-cyclo(L-Leu-L-Pro), cis-cyclo(L-Val-L-Pro), cis-cyclo(L-Phe-L-Pro) | Broad spectrum of fungi, bacteria, and virus | [72] |
| Bacillus spp. | cyclo(L-Pro-L-Tyr), cyclo(D-Pro-L-Leu), cyclo(L-Pro-L-Met), cyclo(D-Pro-L-Phe), cyclo(L-Pro-D-Tyr), cyclo(L-Pro-L-Phe) | Rhizoctonia solani, Aspergillus flavus, Candidaalbicans, Penicillium expansum, Fusarium oxysporum | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, B.; Mishra, A.K.; Kumar, S.; Mandal, S.K.; NSV, L.; Kumar, V.; Baek, K.-H.; Mohanta, Y.K. Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges. Metabolites 2022, 12, 12. https://doi.org/10.3390/metabo12010012
Mishra B, Mishra AK, Kumar S, Mandal SK, NSV L, Kumar V, Baek K-H, Mohanta YK. Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges. Metabolites. 2022; 12(1):12. https://doi.org/10.3390/metabo12010012
Chicago/Turabian StyleMishra, Bishwambhar, Awdhesh Kumar Mishra, Sanjay Kumar, Sanjeeb Kumar Mandal, Lakshmayya NSV, Vijay Kumar, Kwang-Hyun Baek, and Yugal Kishore Mohanta. 2022. "Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges" Metabolites 12, no. 1: 12. https://doi.org/10.3390/metabo12010012











