Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations
Abstract
1. Introduction
2. Results
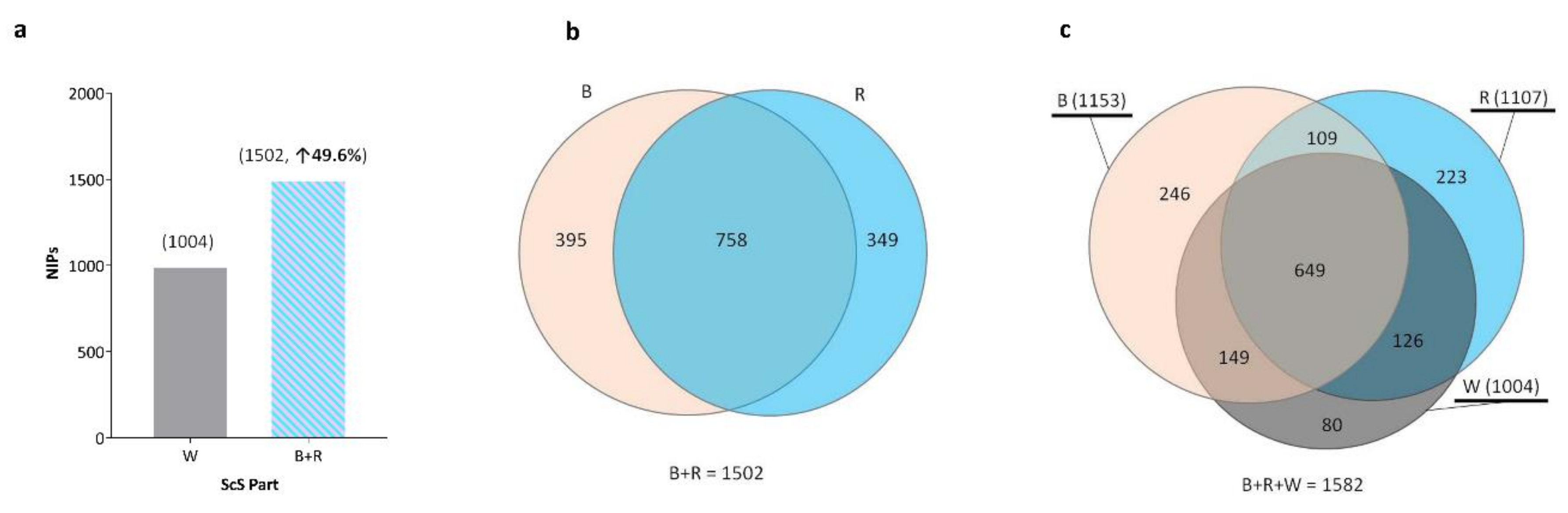
2.1. Number of Identified Proteins (NIPs) and Their Distributions in the Sections of the ScS
2.2. Distribution within ScS Sections and Spectral Counts for the Previously Described Tear Proteins
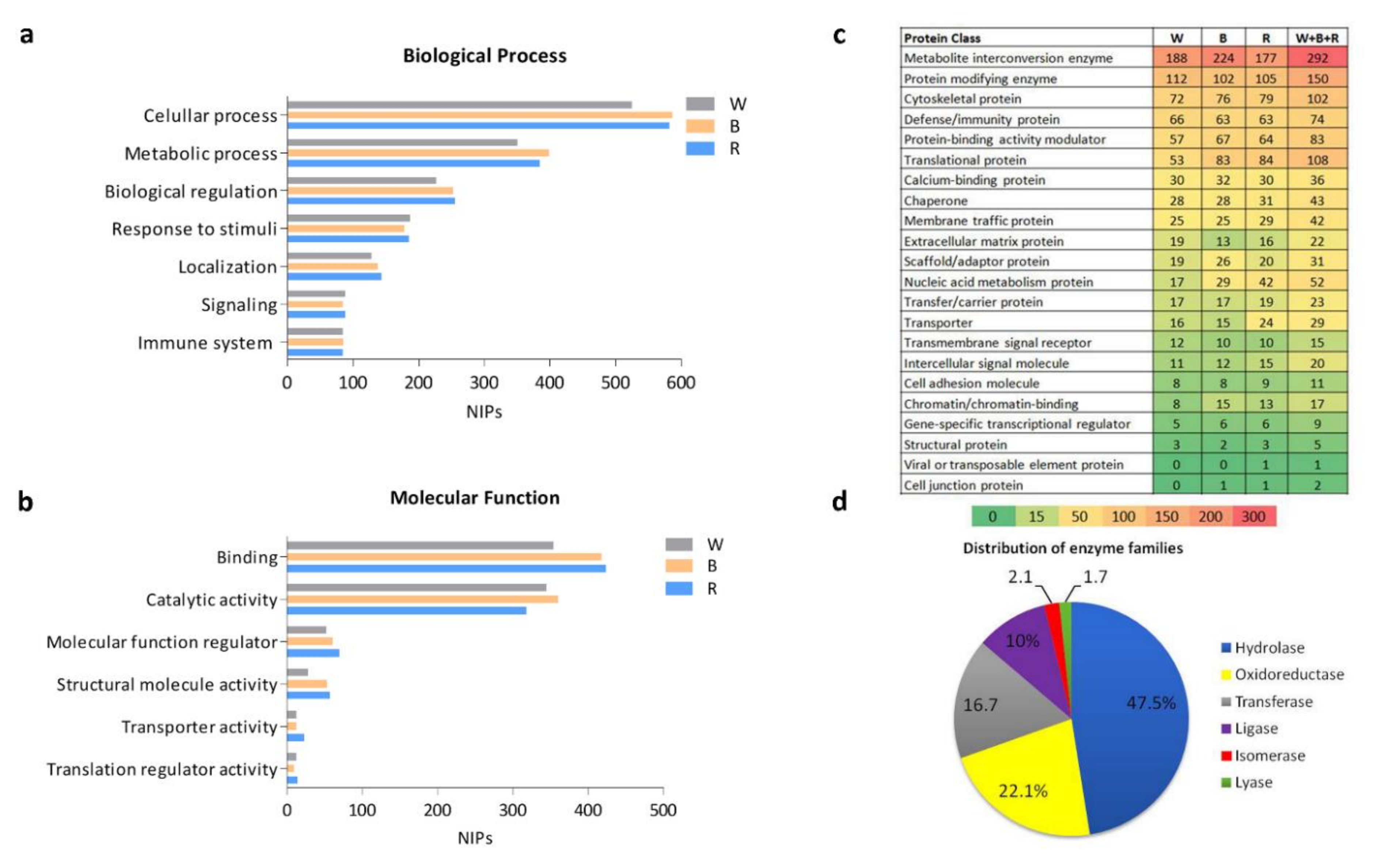
2.3. Functional Annotation Analysis in the Various Sections of the ScS
2.4. Signaling Pathways in the ScS-Extracted Proteome
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Processing
4.2. Sample Preparation for Nano-LC-MS/MS Analysis
4.3. Nano-LC-MS/MS Data Analysis
4.4. Functional Annotation and Signaling Pathway Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willcox, M.D.P.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, D.; Niezgoda, Ł.; Fudalej, E.; Nowak, A.; Maciejewicz, P. Tear Film—Physiology and Disturbances in Various Diseases and Disorders. In Ocular Surface Diseases—Some Current Date on Tear Film Problem and Keratoconic Diagnosis; IntechOpen: Warsaw, Poland, 2021; Volume 32, pp. 137–144. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Cwiklik, L. Tear Film Lipid Layer: A Molecular Level View. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Campos, E.C. Disease Update on Human Tear Proteome. Eur. Ophthalmic Rev. 2013, 07, 36. [Google Scholar] [CrossRef]
- Azkargorta, M.; Soria, J.; Acera, A.; Iloro, I.; Elortza, F. Human Tear Proteomics and Peptidomics in Ophthalmology: Toward the Translation of Proteomic Biomarkers into Clinical Practice. J. Proteom. 2017, 150, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Fraga, J.; Enríquez-de-Salamanca, A.; Calonge, M.; González-García, M.J.; López-Miguel, A.; López-de la Rosa, A.; García-Vázquez, C.; Calder, V.; Stern, M.E.; Fernández, I. Severity, Therapeutic, and Activity Tear Biomarkers in Dry Eye Disease: An Analysis from a Phase III Clinical Trial. Ocul. Surf. 2018, 16, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-De-Salamanca, A.; Bonini, S.; Calonge, M. Molecular and Cellular Biomarkers in Dry Eye Disease and Ocular Allergy. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-T.; Fang, P.-C.; Chao, T.-L.; Chen, A.; Lai, Y.-H.; Huang, Y.-T.; Tseng, C.-Y. Tear Proteomics Approach to Monitoring Sjögren Syndrome or Dry Eye Disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef]
- Pieczyński, J.; Szulc, U.; Harazna, J.; Szulc, A.; Kiewisz, J. Tear Fluid Collection Methods: Review of Current Techniques. Eur. J. Ophthalmol. 2021, 31, 2245–2251. [Google Scholar] [CrossRef]
- Saraygord-Afshari, N.; Naderi-Manesh, H.; Naderi, M. Increasing Proteome Coverage for Gel-Based Human Tear Proteome Maps: Towards a More Comprehensive Profiling. Biomed. Chromatogr. 2015, 29, 1056–1067. [Google Scholar] [CrossRef]
- Brott, N.R.; Ronquillo, Y. Schirmer test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kim, Y.H.; Graham, A.D.; Li, W.; Radke, C.J.; Lin, M.C. Human Lacrimal Production Rate and Wetted Length of Modified Schirmer’s Tear Test Strips. Transl. Vis. Sci. Technol. 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Posa, A.; Bräuer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer Strip vs. Capillary Tube Method: Non-Invasive Methods of Obtaining Proteins from Tear Fluid. Ann. Anat. Anat. Anz. 2013, 195, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Green-Church, K.B.; Nichols, K.K.; Kleinholz, N.M.; Zhang, L.; Nichols, J.J. Investigation of the Human Tear Film Proteome Using Multiple Proteomic Approaches. Mol. Vis. 2008, 14, 456–470. [Google Scholar]
- You, J.; Willcox, M.D.; Madigan, M.C.; Wasinger, V.; Schiller, B.; Walsh, B.J.; Graham, P.H.; Kearsley, J.H.; Li, Y. Tear Fluid Protein Biomarkers. In Advances in Clinical Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 62, pp. 151–196. [Google Scholar] [CrossRef]
- Huang, Z.; Du, C.X.; Pan, X.D. The Use of In-Strip Digestion for Fast Proteomic Analysis on Tear Fluid from Dry Eye Patients. PLoS ONE 2018, 13, e0200702. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-Depth Analysis of the Human Tear Proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Aass, C.; Norheim, I.; Eriksen, E.F.; Thorsby, P.M.; Pepaj, M. Single Unit Filter-Aided Method for Fast Proteomic Analysis of Tear Fluid. Anal. Biochem. 2015, 480, 1–5. [Google Scholar] [CrossRef]
- Kandhavelu, J.; Demonte, N.L.; Namperumalsamy, V.P.; Prajna, L.; Thangavel, C.; Jayapal, J.M.; Kuppamuthu, D. Aspergillus Flavus Induced Alterations in Tear Protein Profile Reveal Pathogen-Induced Host Response to Fungal Infection. J. Proteom. 2017, 152, 13–21. [Google Scholar] [CrossRef]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the Global Protein Content from Healthy Human Tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.A.; Godoy, L.M.F.; Mann, M. Identification of 491 Proteins in the Tear Fluid Proteome Reveals a Large Number of Proteases and Protease Inhibitors. Genome Biol. 2006, 7, R72. [Google Scholar] [CrossRef] [PubMed]
- Nättinen, J.; Aapola, U.; Jylhä, A.; Vaajanen, A.; Uusitalo, H. Comparison of Capillary and Schirmer Strip Tear Fluid Sampling Methods Using SWATH-MS Proteomics Approach. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Wang, Y.; Zheng, X.; Zhang, Y.; Shao, Y. Comparative Analysis of the Tear Protein Profile in Herpes Simplex Virus Type 1 Epithelial Keratitis. BMC Ophthalmol. 2020, 20, 355. [Google Scholar] [CrossRef] [PubMed]
- Ponzini, E.; Ami, D.; Duse, A.; Santambrogio, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Pezzoli, F.; Natalello, A.; Tavazzi, S.; et al. Single-tear proteomics: A feasible approach to precision medicine. Int. J. Mol. Sci. 2021, 22, 10750. [Google Scholar] [CrossRef] [PubMed]
- Zysset-Burri, D.C.; Schlegel, I.; Lincke, J.B.; Jaggi, D.; Keller, I.; Heller, M.; Lagache, S.B.; Wolf, S.; Zinkernagel, M.S. Understanding the interactions between the ocular surface microbiome and the tear proteome. Investig. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.N.; Di, D.; Yuan, Z.C.; Wu, L.; Hu, B. Schirmer Paper Noninvasive Microsampling for Direct Mass Spectrometry Analysis of Human Tears. Anal. Chem. 2020, 92, 6207–6212. [Google Scholar] [CrossRef]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Mäkinen, P.; Beuerman, R.; Pietilä, J.; Vaajanen, A.; Uusitalo, H. Age-Associated Changes in Human Tear Proteome. Clin. Proteom. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W.; Choi, M.C.; Shao, Z.Z.; Xiao, R.L.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of Tear Fluid Biomarkers in Dry Eye Syndrome Using ITRAQ Quantitative Proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef] [PubMed]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Parkkari, M.; Mikhailova, A.; Beuerman, R.W.; Uusitalo, H. Patient Stratification in Clinical Glaucoma Trials Using the Individual Tear Proteome. Sci. Rep. 2018, 8, 12038. [Google Scholar] [CrossRef] [PubMed]
- Grus, F.H.; Podust, V.N.; Bruns, K.; Lackner, K.; Fu, S.; Dalmasso, E.A.; Wirthlin, A.; Pfeiffer, N. SELDI-TOF-MS ProteinChip Array Profiling of Tears from Patients with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 863–876. [Google Scholar] [CrossRef]
- Li, N.; Wang, N.; Zheng, J.; Liu, X.M.; Lever, O.W.; Erickson, P.M.; Li, L. Characterization of Human Tear Proteome Using Multiple Proteomic Analysis Techniques. J. Proteome Res. 2005, 4, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Enríquez-de-Salamanca, A.; Pinto-Fraga, J.; López-Miguel, A.; González-García, M.J.; Stern, M.E.; Calonge, M.; Zhou, L.; et al. Topical Fluorometholone Treatment and Desiccating Stress Change Inflammatory Protein Expression in Tears. Ocul. Surf. 2018, 16, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, L.; Beuerman, R.W.; Zhao, S.Z.; Li, X.R. Association of Tear Proteins with Meibomian Gland Disease and Dry Eye Symptoms. Br. J. Ophthalmol. 2011, 95, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.J.; Bereman, M.S.; Durand, S.; Valaskovic, G.A.; MacCoss, M.J. Effects of Column and Gradient Lengths on Peak Capacity and Peptide Identification in Nanoflow LC-MS/MS of Complex Proteomic Samples. J. Am. Soc. Mass Spectrom. 2013, 24, 148–153. [Google Scholar] [CrossRef]
- Ma, J.Y.W.; Sze, Y.H.; Bian, J.F.; Lam, T.C. Critical Role of Mass Spectrometry Proteomics in Tear Biomarker Discovery for Multifactorial Ocular Diseases (Review). Int. J. Mol. Med. 2021, 47, 83. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, M.; Cuda, G. Nano LC–MS/MS: A robust setup for proteomic analysis. In Methods in Molecular Biology; Toms, S.A., Weil, R.J., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 790, pp. 115–126. [Google Scholar] [CrossRef]
- Macron, C.; Lavigne, R.; Núñez, A.G.; Affolter, M.; Pineau, C.; Dayon, L. Exploration of Human Cerebrospinal Fluid: A Large Proteome Dataset Revealed by Trapped Ion Mobility Time-of-Flight Mass Spectrometry. Data Br. 2020, 31, 105704. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.D.; Koch, S.; Koch, H.; Lubeck, M.; Krause, M.; Goedecke, N.; Decker, J.; Kosinski, T.; Park, M.A.; et al. Online Parallel Accumulation–Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Brunner, A.D.; Frank, M.; Ha, A.; Bludau, I.; Voytik, E.; Kaspar-Schoenefeld, S.; Lubeck, M.; Raether, O.; Bache, N.; et al. DiaPASEF: Parallel Accumulation–Serial Fragmentation Combined with Data-Independent Acquisition. Nat. Methods 2020, 17, 1229–1236. [Google Scholar] [CrossRef]
- Hamada, S.; Pionneau, C.; Parizot, C.; Silvie, O.; Chardonnet, S.; Marinach, C. In-depth Proteomic Analysis of Plasmodium Berghei Sporozoites Using Trapped Ion Mobility Spectrometry with Parallel Accumulation-serial Fragmentation. Proteomics 2021, 21, 2000305. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R. Recent Technical Advances in Proteomics. F1000Research 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Vasilopoulou, C.G.; Sulek, K.; Brunner, A.-D.; Meitei, N.S.; Schweiger-Hufnagel, U.; Meyer, S.W.; Barsch, A.; Mann, M.; Meier, F. Trapped Ion Mobility Spectrometry and PASEF Enable In-Depth Lipidomics from Minimal Sample Amounts. Nat. Commun. 2020, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, X.Y.; Jylha, A.; Aapola, U.; Liu, D.N.; Koh, S.K.; Tian, D.; Quah, J.; Uusitalo, H.; Beuerman, R.W.; et al. Quantitation of 47 Human Tear Proteins Using High Resolution Multiple Reaction Monitoring (HR-MRM) Based-Mass Spectrometry. J. Proteom. 2015, 115, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Denisin, A.K.; Karns, K.; Herr, A.E. Post-Collection Processing of Schirmer Strip-Collected Human Tear Fluid Impacts Protein Content. Analyst 2012, 137, 5088–5096. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, R.; Zhao, P.; Koh, S.K.; Beuerman, R.W.; Ding, C. Proteomic Analysis Revealed the Altered Tear Protein Profile in a Rabbit Model of Sjögren’s Syndrome-Associated Dry Eye. Proteomics 2013, 13, 2469–2481. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Ji, Y.W.; Hwang, H.S.; Oh, J.W.; Kim, H.C.; Lee, H.K.; Kim, K.P. Proteomic Analysis of Human Lacrimal and Tear Fluid in Dry Eye Disease. Sci. Rep. 2017, 7, 13363. [Google Scholar] [CrossRef]
- Erkelens, M.N.; Mebius, R.E. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol. 2017, 38, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Kessal, K.; Rabut, G.; Daull, P.; Garrigue, J.S.; Parsadaniantz, S.M.; Docquier, M.; Baudouin, C.; Brignole-Baudouin, F. Correlation of Clinical Symptoms and Signs with Conjunctival Gene Expression in Primary Sjögren Syndrome Dry Eye Patients. Ocul. Surf. 2019, 17, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN Pathways and Plasmacytoid Dendritic Cell Recruitment in Target Organs of Primary Sjögren’s Syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Murgoci, A.N.; Cardon, T.; Aboulouard, S.; Duhamel, M.; Fournier, I.; Cizkova, D.; Salzet, M. Reference and Ghost Proteins Identification in Rat C6 Glioma Extracellular Vesicles. iScience 2020, 23, 101045. [Google Scholar] [CrossRef] [PubMed]
- Hirai, N.; Kawasaki, S.; Tanioka, H.; Connon, C.J.; Yamasaki, K.; Yokoi, N.; Komuro, A.; Kinoshita, S. Pathological Keratinisation in the Conjunctival Epithelium of Sjögren’s Syndrome. Exp. Eye Res. 2006, 82, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, M.; Zlotnik, A.; Llorente, L.; Hernandez-Molina, G. Markedly High Salivary and Lacrimal CXCL17 Levels in Primary Sjögren’s Syndrome. Jt. Bone Spine 2018, 85, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Ablamowicz, A.F.; Nichols, J.J. Concentrations of MUC16 and MUC5AC Using Three Tear Collection Methods. Mol. Vis. 2017, 23, 529–537. [Google Scholar] [PubMed]
- Gipson, I.K.; Argüeso, P. Role of Mucins in the Function of the Corneal and Conjunctival Epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, F.; Zheng, J.; Yin, J.; Huang, R.; Liu, W.; Lin, Q.; Gao, Y.; Gao, X.; Yu, X.; et al. High Circulating Level of Interleukin-18 in Patients with Primary Sjögren’s Syndrome Is Associated with Disease Activity. Mod. Rheumatol. 2016, 26, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Park, C.S.; You, I.C.; Choi, H.J.; Lee, K.H.; Im, S.K.; Park, H.Y.; Pflugfelder, S.C. Expression of CXCL9, -10, -11, and CXCR3 in the Tear Film and Ocular Surface of Patients with Dry Eye Syndrome. Investig. Ophthalmol. Vis. Sci. 2010, 51, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Sánchez-Guerrero, J.; Betanzos, J.L.; Enriquez, A.B.; Avila-Casado, C.; Llorente, L.; Hernández-Molina, G. Differential Cytokine Expression and Regulatory Cells in Patients with Primary and Secondary Sjögren’s Syndrome. Scand. J. Immunol. 2014, 80, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Barabino, S.; Feraille, L.; Kessal, K.; Docquier, M.; Parsadaniantz, S.M.; Baudouin, C.; Garrigue, J.S. Modulation of Inflammation-Related Genes in the Cornea of a Mouse Model of Dry Eye upon Treatment with Cyclosporine Eye Drops. Curr. Eye Res. 2019, 44, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Marques, P.I.; Matthiesen, R.; Seixas, S. Adaptive Evolution and Divergence of SERPINB3: A Young Duplicate in Great Apes. PLoS ONE 2014, 9, e104935. [Google Scholar] [CrossRef]
- Ciccia, F.; Accardo-Palumbo, A.; Alessandro, R.; Alessandri, C.; Priori, R.; Guggino, G.; Raimondo, S.; Carubbi, F.; Valesini, G.; Giacomelli, R.; et al. Interleukin-36α Axis Is Modulated in Patients with Primary Sjögren’s Syndrome. Clin. Exp. Immunol. 2015, 181, 230–238. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological Functions of Tear Film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Spraggins, J.; Djambazova, K.; Rivera, E.; Migas, L.; Neumann, E.; Fuetterer, A.; Suetering, J.; Goedecke, N.; Ly, A.; Van De Plas, R.; et al. High Performance Molecular Imaging with MALDI Trapped Ion Mobility Time-of-Flight (TimsTOF) Mass Spectrometry. Anal. Chem. 2019, 91, 14552–14560. [Google Scholar] [CrossRef] [PubMed]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of Commonly Used Tear Sampling Methods and Their Relevance in Subsequent Biochemical Analysis. Ann. Clin. Biochem. 2017, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are Antioxidant Enzymes Essential Markers in the Diagnosis and Monitoring of Cancer Patients—A Review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 Proteins in Mouse and Man: From Evolution to Function and Pathology (Including an Update of the Nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical Chemistry of Cytokines—A Review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. Tear Analysis in Ocular Surface Diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, X.; Yuan, X.-R.; Chen, B.; Cai, N.; Zeng, S.; Sun, Y.; Li, H. The Role of IL-36 in the Pathophysiological Processes of Autoimmune Diseases. Front. Pharmacol. 2021, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.H.; Choi, Y.S.; Na, H.G.; Song, S.Y.; Kim, Y.D. Interleukin (IL) 36 Gamma Induces Mucin 5AC, Oligomeric Mucus/Gel-Forming Expression via IL-36 Receptor–Extracellular Signal Regulated Kinase 1 and 2, and P38–Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells in Human Airway Epithelial Cells. Am. J. Rhinol. Allergy 2018, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Song, X.J.; Farley, W.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Apoptosis of Ocular Surface Cells in Experimentally Induced Dry Eye. Investig. Ophthalmol. Vis. Sci. 2003, 44, 124–129. [Google Scholar] [CrossRef] [PubMed]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient Apoptosis Requires Feedback Amplification of Upstream Apoptotic Signals by Effector Caspase-3 or -7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef] [PubMed]
- Cedzyński, M.; Thielens, N.M.; Mollnes, T.E.; Vorup-Jensen, T. Editorial: The Role of Complement in Health and Disease. Front. Immunol. 2019, 10, 1869. [Google Scholar] [CrossRef]
- Willcox, M.D.; Morris, C.A.; Thakur, A.; Sack, R.A.; Wickson, J.; Boey, W. Complement and Complement Regulatory Proteins in Human Tears. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1–8. [Google Scholar]
- Kessal, K.; Liang, H.; Rabut, G.; Daull, P.; Garrigue, J.S.; Docquier, M.; Parsadaniantz, S.M.; Baudouin, C.; Brignole-Baudouin, F. Conjunctival Inflammatory Gene Expression Profiling in Dry Eye Disease: Correlations with HLA-DRA and HLA-DRB1. Front. Immunol. 2018, 9, 2271. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry Eye as a Mucosal Autoimmune Disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chauhan, S.K.; Saban, D.R.; Sadrai, Z.; Okanobo, A.; Dana, R. Interferon-γ-Secreting NK Cells Promote Induction of Dry Eye Disease. J. Leukoc. Biol. 2011, 89, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signaling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Chotikavanich, S.; de Paiva, C.S.; Li, D.Q.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and Activity of Matrix Metalloproteinase-9 on the Ocular Surface Increase in Dysfunctional Tear Syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Chen, Z.; Song, X.J.; Luo, L.; Pflugfelder, S.C. Stimulation of Matrix Metalloproteinases by Hyperosmolarity via a JNK Pathway in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; von Lindenfels, V.; Garbe, A.; Kampik, A. Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay. Ophthalmology 2016, 123, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Mauris, J.; Argüeso, P. The Ocular Surface Epithelial Barrier and Other Mechanisms of Mucosal Protection. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 563–568. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Magtanong, L.; Ko, P.J.; Dixon, S.J. Emerging Roles for Lipids in Non-Apoptotic Cell Death. Cell Death Differ. 2016, 23, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in Apoptosis: An Overview and Current Perspectives. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Magny, R.; Kessal, K.; Regazzetti, A.; Ben Yedder, A.; Baudouin, C.; Mélik Parsadaniantz, S.; Brignole-Baudouin, F.; Laprévote, O.; Auzeil, N. Lipidomic Analysis of Epithelial Corneal Cells Following Hyperosmolarity and Benzalkonium Chloride Exposure: New Insights in Dry Eye Disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158728. [Google Scholar] [CrossRef]
- Magny, R.; Regazzetti, A.; Kessal, K.; Genta-Jouve, G.; Baudouin, C.; Mélik-Parsadaniantz, S.; Brignole-Baudouin, F.; Laprévote, O.; Auzeil, N. Lipid Annotation by Combination of UHPLC-HRMS (Ms), Molecular Networking, and Retention Time Prediction: Application to a Lipidomic Study of in Vitro Models of Dry Eye Disease. Metabolites 2020, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Birts, C.N.; Barton, C.H.; Wilton, D.C. Catalytic and Non-Catalytic Functions of Human IIA Phospholipase A2. Trends Biochem. Sci. 2010, 35, 28–35. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, H.D.; Hwang, S.-I.; Wu, L.; Han, K.D. Role of Spectral Counting in Quantitative Proteomics. (Report) Pub: Expert. Expert Rev. Proteom. 2010, 7, 39–53. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-Scale Gene Function Analysis with the Panther Classification System. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Tope, G.; Gillespie, M.; Vastrik, I.; D’Eustachio, P.; Schmidt, E.; de Bono, B.; Jassal, B.; Gopinath, G.R.; Wu, G.R.; Matthews, L.; et al. Reactome: A Knowledgebase of Biological Pathways. Nucleic Acids Res. 2005, 33, 428–432. [Google Scholar] [CrossRef]



| Group, Year | Goal | Tear Sampling Method | Sample Preparation | MS Technology | Protein Identification Criteria | NIPs in HSs |
|---|---|---|---|---|---|---|
| de Souza et al., Genome Biol., 2006 [22] | Characterization of the protein content of the human tear fluid from a HSs | Microcapillary method | With pre-fractionation of proteins with one-dimensional SDS-PAGE (13 fractions) or without (in-solution digestion of the whole samples) | Hybrid linear ion trap–Fourier-transform (LTQ-FT) and linear ion trap-Orbitrap (LTQ-Orbitrap) | Two peptides with Mascot scores of >35, Two peptides with Mascot scores of >27 (p ≤ 0.01), or one peptide with an Mascot score of >54 (p ≤ 0.0001), when MS3 was performed | 491 |
| Zhou et al., J. Proteomics, 2012 [18] | Analysis of the human tear proteome from HSs | Schirmer strips | Offline SCX fractionation of peptides (6 fractions) | TripleTOF 5600 system | FDR <1% for peptides | 1543 |
| Aass et al., Anal. Biochem., 2015 [19] | Optimizing extraction method from Schirmer strips to study the tear proteome | Schirmer strips | Offline SCX fractionation of peptides (16 fractions) | LTQ-Orbitrap XL hybrid | Peptide and protein with FDRs of <1% (high) and 5% (relaxed) | 1526 |
| Kandhavelu et al., J. Proteomics, 2016 [20] | Comparison of tear proteins in control and fungal keratitis patients | Capillary method | N-linked glycoprotein enrichment or one-dimensional SDS-PAGE pre-fractionation of proteins (26 fractions) | LTQ-Orbitrap Velos Pro | One peptide with an FDR of <5% | 1873 |
| Dor et al., Exp. Eye Res., 2019 [21] | Characterization of healthy human tear protein composition | Schirmer strips | Off-gel electrophoresis of peptides (12 fractions) | LTQ-Orbitrap Velos Pro TripleTOF 5600+ in SWATH-MS mode | Two peptides with FDRs of <1% | 1351 |
| Nättinen et al., Trans. Vis. Sci. Tech., 2020 [23] | Investigation of protein profile differences between capillary and Schirmer strip tear fluid samples | Schirmer strips | No pre-fractionation (in-solution digestion of whole samples) | TripleTOF 5600 + in SWATH-MS mode | FDR <1% | 908 |
| Capillary method | 404 | |||||
| Hua et al., BMC Ophthalmol., 2020 [24] | Quantification of potential candidate biomarkers for HSV-1 epithelial keratitis | Microcapillary method | No pre-fractionation (in-solution digestion of whole samples) | LTQ-Orbitrap XL | FDR <1% | 949 |
| Ponzini et al., Int. J. Mol. Sci., 2021 [25] | Demonstration of feasibility of single-tear quantitative proteomics | Capillary method | No pre-fractionation (in-solution digestion of whole samples) | Orbitrap fusion | One peptide with an FDR of <1.0% | 932 |
| Zysset-Burri et al., Inv. Ophthalmol. Vis. Sci., 2021 [26] | Exploring the interplay between the ocular surface microbiome and the tear proteome | Schirmer strips | With pre-fractionation of (5 factions) proteins with one-dimensional SDS-PAGE | QExactive HF | FDR <1% | 2172 |
| Accession Number | Protein Name | W | B | R |
|---|---|---|---|---|
| Mean ± SD | ||||
| (1) Common in all batches | ||||
| P12273 | Prolactin-induced protein [1] | 142.3 ± 14 | 108 ± 15.4 | 121.7 ± 2.5 |
| P98160 | Heparan sulfate proteoglycan 2 [1,29] | 113.3 ± 5.5 | 56.3 ± 4.9 | 123.7 ± 1.5 |
| P61626 | Lysozyme C * [6,29,44] | 111.3 ± 6.7 | 104 ± 4.6 | 108.7 ± 5.5 |
| P01876 | Ig alpha-1 chain C region [6,29] | 107 ± 2.6 | 61.7 ± 3.2 | 74 ± 4.4 |
| P02768 | Serum albumin * [6,45] | 101.7 ± 2.1 | 204.3 ± 8.1 | 67 ± 4 |
| P02788 | Lactotransferrin * [29,44] | 100.7 ± 5.6 | 130.3 ± 7.1 | 101.7 ± 3.5 |
| P01024 | Complement C3 [22,44] | 72.7 ± 3.2 | 69.7 ± 1.5 | 62.7 ± 2.1 |
| Q9UGM3 | Salivary agglutinin [1,44] | 50.7 ± 4 | 48 ± 2 | 54.3 ± 0.6 |
| P31025 | Lipocalin-1 * [6,29,44] | 45.3 ± 2.1 | 50 ± 3.6 | 36.7 ± 2.3 |
| P00352 | Retinal dehydrogenase 1 [23,44] | 35.3 ± 2.1 | 46 ± 4 | 24.7 ± 2.1 |
| O75556 | Mammaglobin-B [1,44] | 35.3 ± 0.6 | 18 ± 1 | 6.3 ± 1.2 |
| P06733 | Alpha-enolase [1,44] | 31.3 ± 1.2 | 52 ± 4.6 | 27.3 ± 0.6 |
| P02787 | Serotransferrin [46] | 24.7 ± 3.1 | 59 ± 4 | 26.7 ± 1.5 |
| P07900 | Heat shock protein HSP 90-alpha [23,47] | 22.3 ± 1.5 | 8 ± 2.6 | 24.3 ± 1.5 |
| P04083 | Annexin A1 [23,44] | 22.3 ± 0.6 | 24.7 ± 0.6 | 18 ± 1 |
| P06702 | Protein S100-A9 [1] | 19 ± 1 | 28.7 ± 2.1 | 22 ± 1 |
| P07858 | Cathepsin B [1] | 18.3 ± 1.5 | 19.7 ± 1.5 | 22 ± 1.7 |
| P47895 | Aldehyde dehydrogenase family 1 member A3 [23,48] | 18 ± 2.6 | 23.7 ± 1.5 | 6.3 ± 2.1 |
| P00738 | Haptoglobin [1] | 18 ± 2 | 19 ± 1.7 | 14 ± 0 |
| P30740 | Serpin B1 [22,23] | 15.7 ± 2.1 | 24.3 ± 1.2 | 8.7 ± 3.2 |
| P05109 | Protein S100-A8 [1] | 15.7 ± 0.6 | 22.3 ± 3.2 | 22 ± 2.6 |
| P25311 | Zinc-alpha-2-glycoprotein [1] | 12.7 ± 1.5 | 14.3 ± 1.5 | 16.3 ± 1.5 |
| P07355 | Annexin A2 [23,44] | 12.7 ± 1.2 | 36.7 ± 3.5 | 25 ± 1 |
| P18510 | Interleukin 1 receptor antagonist protein [49] | 9.7 ± 1.5 | 12.3 ± 1.5 | 7 ± 1 |
| Q8WUM4 | Programmed cell death 6-interacting protein [50,51] | 9 ± 0 | 2.7 ± 0.6 | 5 ± 1.7 |
| P23528 | Cofilin-1 [1] | 8.7 ± 3.8 | 15.3 ± 2.1 | 8.7 ± 2.1 |
| P07476 | Involucrin [52] | 6 ± 1 | 3 ± 1.7 | 20.3 ± 2.3 |
| P01023 | Alpha-2-macroglobulin [22,52] | 5.3 ± 1.5 | 15.7 ± 2.3 | 7.7 ± 1.2 |
| Q6UXB2 | C-X-C motif chemokine ligand 17 [53] | 1 ± 1 | 0.7 ± 1.2 | 1 ± 1 |
| (2) Common to two batches | ||||
| P98088 | Mucin-5AC [44,54,55] | 10.3 ± 0.6 | 54.3 ± 3.8 | 0 |
| Q14116 | Interleukin 18 [56] | 0.7 ± 0.6 | 2 ± 1 | 0 |
| P02778 | C-X-C motif chemokine ligand 10 [57] | 2 ± 0 | 0 | 0.3 ± 0.6 |
| (3) Unique to one batch | ||||
| Q9UHD0 | Interleukin 19 [58] | 0.7 ± 1.2 | 0 | 0 |
| P14780 | Matrix metallopeptidase 9 * [49,59,60] | 0 | 0.3 ± 0.6 | 0 |
| P29508 | Serpin B3 [61] | 0 | 0 | 23.7 ± 2.5 |
| P48594 | Serpin B4 [61] | 0 | 0 | 12 ± 1 |
| Q9UHA7 | Interleukin 36 alpha [62] | 0 | 0 | 0.3 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkurt Arslan, M.; Kolman, I.; Pionneau, C.; Chardonnet, S.; Magny, R.; Baudouin, C.; Brignole-Baudouin, F.; Kessal, K. Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations. Metabolites 2022, 12, 2. https://doi.org/10.3390/metabo12010002
Akkurt Arslan M, Kolman I, Pionneau C, Chardonnet S, Magny R, Baudouin C, Brignole-Baudouin F, Kessal K. Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations. Metabolites. 2022; 12(1):2. https://doi.org/10.3390/metabo12010002
Chicago/Turabian StyleAkkurt Arslan, Murat, Ioannis Kolman, Cédric Pionneau, Solenne Chardonnet, Romain Magny, Christophe Baudouin, Françoise Brignole-Baudouin, and Karima Kessal. 2022. "Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations" Metabolites 12, no. 1: 2. https://doi.org/10.3390/metabo12010002
APA StyleAkkurt Arslan, M., Kolman, I., Pionneau, C., Chardonnet, S., Magny, R., Baudouin, C., Brignole-Baudouin, F., & Kessal, K. (2022). Proteomic Analysis of Tears and Conjunctival Cells Collected with Schirmer Strips Using timsTOF Pro: Preanalytical Considerations. Metabolites, 12(1), 2. https://doi.org/10.3390/metabo12010002







