A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System
Abstract
:1. Introduction
2. Flavonoids: Classification, Metabolism, Absorption and Bioavailability
3. Canonical Mechanism of Flavonoids Regulating IBD
3.1. Antioxidant Property
3.2. Preservation of Epithelial Barrier Functon
3.3. Immunomodulatory Property in the Gut
3.4. Shaping Microbiota Composition and Function
4. Enteroendocrine System
4.1. Enteroendocrine Cells: Subtypes and Functions
4.2. Changes of Enteroendocrine System in IBD
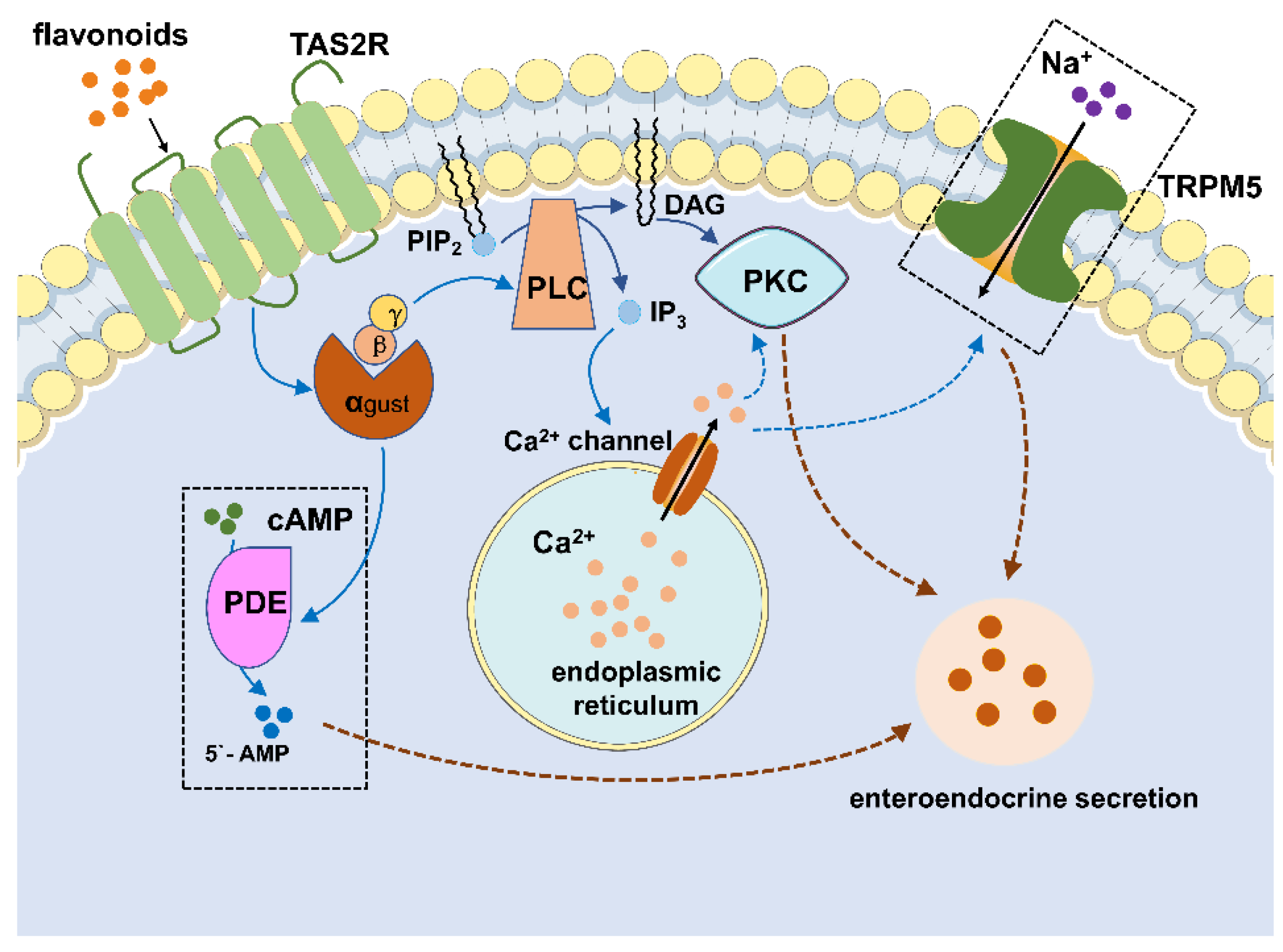
4.3. Flavonoids Regulate the Enteroendocrine System
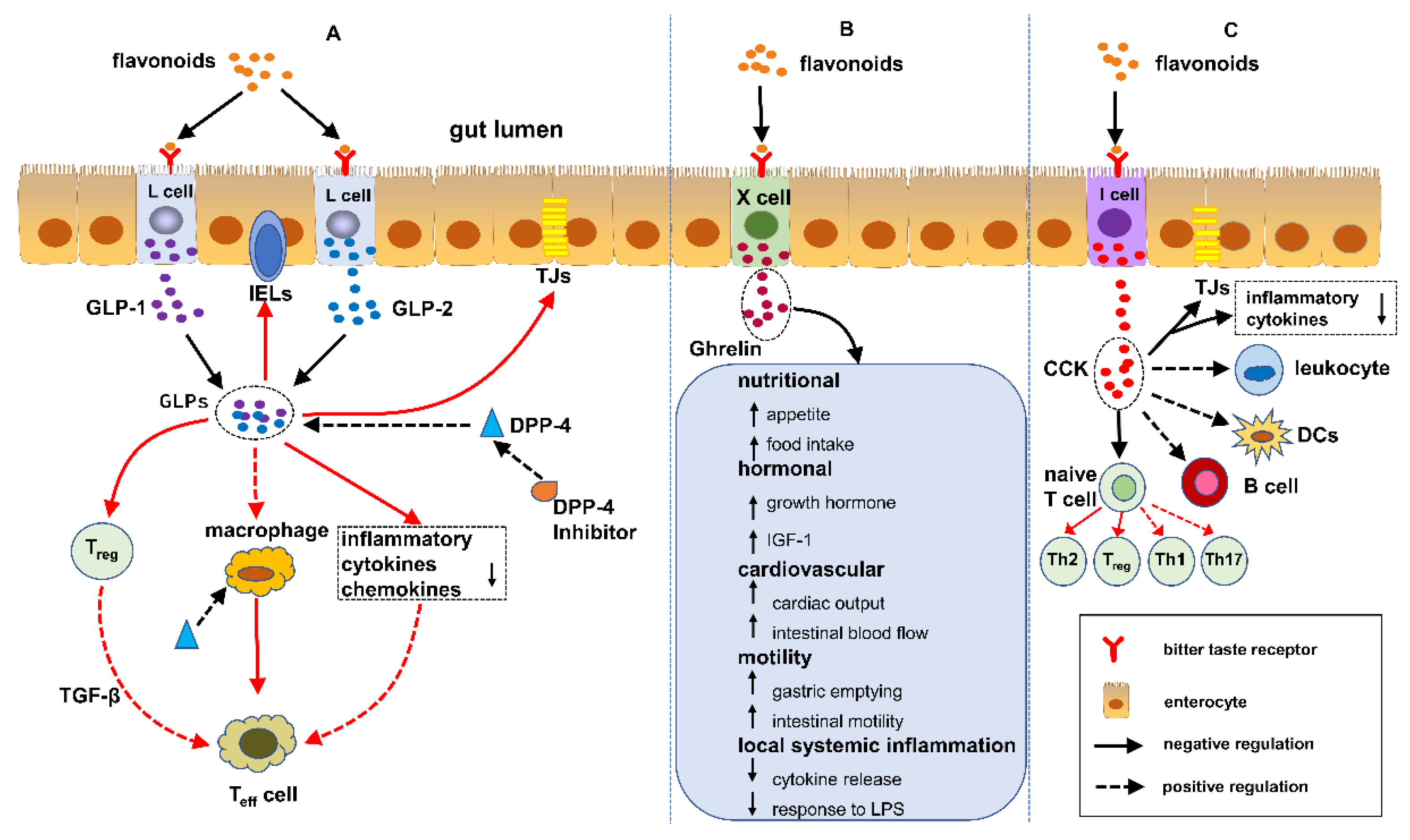
5. Flavonoids Regulate IBD Mediated by EECs
5.1. GLPs/DPP-4 Inhibitors and IBD
5.1.1. GLP-1
5.1.2. GLP-2
5.1.3. DPP-4 Inhibitors
5.2. Ghrelin and IBD
5.3. CCK and IBD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Pinent, M.; Blay, M.; Serrano, J.; Ardevol, A. Effects of flavanols on the enteroendocrine system: Repercussions on food intake. Crit. Rev. Food Sci. Nutr. 2017, 57, 326–334. [Google Scholar] [CrossRef]
- Hunt, J.E.; Holst, J.J.; Jeppesen, P.B.; Kissow, H. GLP-1 and Intestinal Diseases. Biomedicines 2021, 9, 383. [Google Scholar] [CrossRef]
- Villumsen, M.; Schelde, A.B.; Jimenez-Solem, E.; Jess, T.; Allin, K.H. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine 2021, 37, 100979. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Lima, J.L.; Fernandes, E. Proinflammatory Pathways: The Modulation by Flavonoids. Med. Res. Rev. 2015, 35, 877–936. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Ribeiro, D.; Proenca, C.; Rocha, S.; Lima, J.; Carvalho, F.; Fernandes, E.; Freitas, M. Immunomodulatory Effects of Flavonoids in the Prophylaxis and Treatment of Inflammatory Bowel Diseases: A Comprehensive Review. Curr. Med. Chem. 2018, 25, 3374–3412. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-infla.ammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Galsanov Sh, B.; Tourova, A.D.; Klimenko, E.D. Effect of quercitrin on structural changes in the large and small intestines in experimental enterocolitis. Biull. Exp. Biol. Med. 1976, 81, 623–625. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Veljaca, M.; Lesch, C.A.; Pllana, R.; Sanchez, B.; Chan, K.; Guglietta, A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1995, 272, 417–422. [Google Scholar] [PubMed]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Miller, M.J.; Sandoval, M. Nitric Oxide. III. A molecular prelude to intestinal inflammation. Am. J. Physiol. 1999, 276, G795–G799. [Google Scholar] [CrossRef] [PubMed]
- Sichel, G.; Corsaro, C.; Scalia, M.; Di Bilio, A.J.; Bonomo, R.P. In vitro scavenger activity of some flavonoids and melanins against O2-(.). Free Radic. Biol. Med. 1991, 11, 1–8. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodriguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Galvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Mankertz, J.; Schulzke, J.D. Altered permeability in inflammatory bowel disease: Pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Vivinus-Nebot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef]
- Azuma, T.; Shigeshiro, M.; Kodama, M.; Tanabe, S.; Suzuki, T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2013, 143, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J. Agric. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Bruckner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lugering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohn’s Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascaraque, C.; Aranda, C.; Ocon, B.; Monte, M.J.; Suarez, M.D.; Zarzuelo, A.; Marin, J.J.; Martinez-Augustin, O.; de Medina, F.S. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef]
- Yamaji, O.; Nagaishi, T.; Totsuka, T.; Onizawa, M.; Suzuki, M.; Tsuge, N.; Hasegawa, A.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; et al. The development of colitogenic CD4(+) T cells is regulated by IL-7 in collaboration with NK cell function in a murine model of colitis. J. Immunol. 2012, 188, 2524–2536. [Google Scholar] [CrossRef] [Green Version]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Kikut, J.; Konecka, N.; Zietek, M.; Kulpa, D.; Szczuko, M. Diet supporting therapy for inflammatory bowel diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef]
- Hong, Z.; Piao, M. Effect of Quercetin Monoglycosides on Oxidative Stress and Gut Microbiota Diversity in Mice with Dextran Sodium Sulphate-Induced Colitis. Biomed. Res. Int. 2018, 2018, 8343052. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. The new biology of gastrointestinal hormones. Physiol. Rev. 1998, 78, 1087–1108. [Google Scholar] [CrossRef] [Green Version]
- Solcia, E.; Capella, C.; Buffa, R.; Usellini, L.; Fiocca, R.; Frigerio, B.; Tenti, P.; Sessa, F. The diffuse endocrine-paracrine system of the gut in health and disease: Ultrastructural features. Scand. J. Gastroenterol. Suppl. 1981, 70, 25–36. [Google Scholar]
- Fothergill, L.J.; Furness, J.B. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: The need for a new classification scheme. Histochem. Cell Biol. 2018, 150, 693–702. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.M.; Richards, P.; Cairns, L.S.; Rogers, G.J.; Bannon, C.A.; Parker, H.E.; Morley, T.C.; Yeo, G.S.; Reimann, F.; Gribble, F.M. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Eissa, N.; Hussein, H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.E. Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem. Pharmacol. 2018, 152, 315–326. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Koshimizu, H.; Cawley, N.X.; Kim, T.; Yergey, A.L.; Loh, Y.P. Serpinin: A novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol. Endocrinol. 2011, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.P.; Cheng, Y.; Mahata, S.K.; Corti, A.; Tota, B. Chromogranin A and derived peptides in health and disease. J. Mol. Neurosci. 2012, 48, 347–356. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef]
- Moran, G.W.; Pennock, J.; McLaughlin, J.T. Enteroendocrine cells in terminal ileal Crohn’s disease. J. Crohn’s Colitis 2012, 6, 871–880. [Google Scholar] [CrossRef] [Green Version]
- El-Salhy, M.; Danielsson, A.; Stenling, R.; Grimelius, L. Colonic endocrine cells in inflammatory bowel disease. J. Intern. Med. 1997, 242, 413–419. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G. Changes in enteroendocrine and immune cells following colitis induction by TNBS in rats. Mol. Med. Rep. 2016, 14, 4967–4974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H. Abnormalities in endocrine and immune cells are correlated in dextransulfatesodiuminduced colitis in rats. Mol. Med. Rep. 2017, 15, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strid, H.; Simren, M.; Lasson, A.; Isaksson, S.; Stridsberg, M.; Ohman, L. Fecal chromogranins and secretogranins are increased in patients with ulcerative colitis but are not associated with disease activity. J. Crohn’s Colitis 2013, 7, e615–e622. [Google Scholar] [CrossRef]
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol. Life Sci. 2005, 62, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Bendet, N.; Scapa, E.; Cohen, O.; Bloch, O.; Aharoni, D.; Ramot, Y.; Weiss, M.; Halevi, A.; Rapoport, M.J. Enhanced glucose-dependent glucagon-like peptide-1 and insulin secretion in Crohn patients with terminal ileum disease is unrelated to disease activity or ileal resection. Scand. J. Gastroenterol. 2004, 39, 650–656. [Google Scholar] [CrossRef]
- Binimelis, J.; Webb, S.M.; Mones, J.; Serrano, J.; Casamitjana, R.; Elena, M.; Peinado, M.A.; Vilardell, F.; De Leiva, A. Circulating immunoreactive somatostatin in gastrointestinal diseases. Decrease after vagotomy and enhancement in active ulcerative colitis, irritable bowel syndrome, and duodenal ulcer. Scand. J. Gastroenterol. 1987, 22, 931–937. [Google Scholar] [CrossRef]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef]
- Keller, J.; Beglinger, C.; Holst, J.J.; Andresen, V.; Layer, P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G861–G868. [Google Scholar] [CrossRef] [Green Version]
- Koch, T.R.; Roddy, D.R.; Go, V.L. Abnormalities of fasting serum concentrations of peptide YY in the idiopathic inflammatory bowel diseases. Am. J. Gastroenterol. 1987, 82, 321–326. [Google Scholar] [PubMed]
- Moran, G.W.; Leslie, F.C.; McLaughlin, J.T. Crohn’s disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin. Nutr. 2013, 32, 404–411. [Google Scholar] [CrossRef]
- Nishi, Y.; Isomoto, H.; Ueno, H.; Ohnita, K.; Wen, C.Y.; Takeshima, F.; Mishima, R.; Nakazato, M.; Kohno, S. Plasma leptin and ghrelin concentrations in patients with Crohn’s disease. World J. Gastroenterol. 2005, 11, 7314–7317. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food in.ntake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmanov, A.A.; Beauchamp, G.K. Taste receptor genes. Annu. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef] [Green Version]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.; Zuker, C.S. A novel family of mammalian taste receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Casanova-Marti, A.; Depoortere, I.; Blay, M.T.; Terra, X.; Pinent, M.; Ardevol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef]
- Di Pizio, A.; Niv, M.Y. Promiscuity and selectivity of bitter molecules and their receptors. Bioorg. Med. Chem. 2015, 23, 4082–4091. [Google Scholar] [CrossRef]
- Englander, E.W.; Gomez, G.A.; Greeley, G.H., Jr. Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech. Ageing Dev. 2004, 125, 871–875. [Google Scholar] [CrossRef]
- Wang, Q.; Liszt, K.I.; Deloose, E.; Canovai, E.; Thijs, T.; Farre, R.; Ceulemans, L.J.; Lannoo, M.; Tack, J.; Depoortere, I. Obesity alters adrenergic and chemosensory signaling pathways that regulate ghrelin secretion in the human gut. FASEB J. 2019, 33, 4907–4920. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Margalef, M.; Blay, M.; Arola-Arnal, A.; Muguerza, B.; Ardevol, A.; Pinent, M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014, 5, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Na, X.; Zhang, Y.; Li, L.; Zhao, X.; Cui, H. Isoflavone reduces body weight by decreasing food intake in ovariectomized rats. Ann. Nutr. Metab. 2009, 54, 163–170. [Google Scholar] [CrossRef]
- Matvienko, O.A.; Alekel, D.L.; Genschel, U.; Ritland, L.; Van Loan, M.D.; Koehler, K.J. Appetitive hormones, but not isoflavone tablets, influence overall and central adiposity in healthy postmenopausal women. Menopause 2010, 17, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Weickert, M.O.; Reimann, M.; Otto, B.; Hall, W.L.; Vafeiadou, K.; Hallund, J.; Ferrari, M.; Talbot, D.; Branca, F.; Bugel, S.; et al. Soy isoflavones increase preprandial peptide YY (PYY), but have no effect on ghrelin and body weight in healthy postmenopausal women. J. Negat. Results Biomed. 2006, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Shukor, N.; Ravallec, R.; Van Camp, J.; Raes, K.; Smagghe, G. Flavonoids stimulate cholecystokinin peptide secretion from the enteroendocrine STC-1 cells. Fitoterapia 2016, 113, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Grau-Bove, C.; Gonzalez-Quilen, C.; Terra, X.; Blay, M.T.; Beltran-Debon, R.; Jorba-Martin, R.; Espina, B.; Pinent, M.; Ardevol, A. Effects of Flavanols on Enteroendocrine Secretion. Biomolecules 2020, 10, 844. [Google Scholar] [CrossRef]
- Duan, L.; Rao, X.; Braunstein, Z.; Toomey, A.C.; Zhong, J. Role of Incretin Axis in Inflammatory Bowel Disease. Front. Immunol. 2017, 8, 1734. [Google Scholar] [CrossRef] [Green Version]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.; et al. GLP-1R Agonists Modulate Enteric Immune Responses Through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Sokilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunner’s Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef] [Green Version]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine 2017, 13, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Lourie, J. A Novel Use of Liraglutide: Induction of Partial Remission in Ulcerative Colitis and Ankylosing Spondylitis. Clin. Med. Rev. Case Rep. 2019, 6, 6–8. [Google Scholar] [CrossRef]
- Drucker, D.J.; Yusta, B.; Boushey, R.P.; DeForest, L.; Brubaker, P.L. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G79–G91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivory, C.P.; Wallace, L.E.; McCafferty, D.M.; Sigalet, D.L. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G1202–G1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.Y.; Zou, H.; Lee, C.; Muppidi, A.; Chao, E.; Fu, Q.; Luo, X.; Wang, D.; Schultz, P.G.; Shen, W. Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models. J. Med. Chem. 2018, 61, 3218–3223. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.K.; Lv, J.J.; Wu, J.; Xu, Z.W. Therapeutic effects of different doses of polyethylene glycosylated porcine glucagon-like peptide-2 on ulcerative colitis in male rats. BMC Gastroenterol. 2017, 17, 34. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.L.; Katz, S.; Fang, J.C.; Bernstein, C.N.; Abou-Assi, S.G.; Teduglutide Study, G. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 962–973. [Google Scholar] [CrossRef]
- Detel, D.; Buljevic, S.; Pucar, L.B.; Kucic, N.; Pugel, E.P.; Varljen, J. Influence of CD26/dipeptidyl peptidase IV deficiency on immunophenotypic changes during colitis development and resolution. J. Physiol. Biochem. 2016, 72, 405–419. [Google Scholar] [CrossRef]
- Salaga, M.; Mokrowiecka, A.; Zielinska, M.; Malecka-Panas, E.; Kordek, R.; Kamysz, E.; Fichna, J. New Peptide Inhibitor of Dipeptidyl Peptidase IV, EMDB-1 Extends the Half-Life of GLP-2 and Attenuates Colitis in Mice after Topical Administration. J. Pharmacol. Exp. Ther. 2017, 363, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Abrahami, D.; Douros, A.; Yin, H.; Yu, O.H.Y.; Renoux, C.; Bitton, A.; Azoulay, L. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: Population based cohort study. BMJ 2018, 360, k872. [Google Scholar] [CrossRef] [Green Version]
- Radel, J.A.; Pender, D.N.; Shah, S.A. Dipeptidyl Peptidase-4 Inhibitors and Inflammatory Bowel Disease Risk: A Meta-analysis. Ann. Pharmacother. 2019, 53, 697–704. [Google Scholar] [CrossRef]
- Ban, H.; Bamba, S.; Imaeda, H.; Inatomi, O.; Kobori, A.; Sasaki, M.; Tsujikawa, T.; Andoh, A.; Fujiyama, Y. The DPP-IV inhibitor ER-319711 has a proliferative effect on the colonic epithelium and a minimal effect in the amelioration of colitis. Oncol. Rep. 2011, 25, 1699–1703. [Google Scholar] [CrossRef] [Green Version]
- Yazbeck, R.; Abbott, C.A.; Howarth, G.S. The use of GLP-2 and related growth factors in intestinal diseases. Curr. Opin. Investig. Drugs 2010, 11, 440–446. [Google Scholar] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar] [PubMed]
- De Smet, B.; Thijs, T.; Moechars, D.; Colsoul, B.; Polders, L.; Ver Donck, L.; Coulie, B.; Peeters, T.L.; Depoortere, I. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol. Motil. 2009, 21, 59–70. [Google Scholar] [CrossRef]
- Deboer, M.D. Use of ghrelin as a treatment for inflammatory bowel disease: Mechanistic considerations. Int. J. Pept. 2011, 2011, 189242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufresne, M.; Seva, C.; Fourmy, D. Cholecystokinin and gastrin receptors. Physiol. Rev. 2006, 86, 805–847. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, A.; Cakir, B.; Ercan, F.; Yegen, B.C. Anti-inflammatory effects of leptin and cholecystokinin on acetic acid-induced colitis in rats: Role of capsaicin-sensitive vagal afferent fibers. Regul. Pept. 2003, 116, 109–118. [Google Scholar] [CrossRef]
- Lubbers, T.; De Haan, J.J.; Hadfoune, M.; Zhang, Y.; Luyer, M.D.; Grundy, D.; Buurman, W.A.; Greve, J.W. Lipid-enriched enteral nutrition controls the inflammatory response in murine Gram-negative sepsis. Crit. Care Med. 2010, 38, 1996–2002. [Google Scholar] [CrossRef]
- Lubbers, T.; Kox, M.; de Haan, J.J.; Greve, J.W.; Pompe, J.C.; Ramakers, B.P.; Pickkers, P.; Buurman, W.A. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit. Care Med. 2013, 41, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Saia, R.S.; Ribeiro, A.B.; Giusti, H. Cholecystokinin Modulates the Mucosal Inflammatory Response and Prevents the Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction. Shock 2020, 53, 242–251. [Google Scholar] [CrossRef]
- Jia, X.; Cong, B.; Zhang, J.; Li, H.; Liu, W.; Chang, H.; Dong, M.; Ma, C. CCK8 negatively regulates the TLR9-induced activation of human peripheral blood pDCs by targeting TRAF6 signaling. Eur. J. Immunol. 2014, 44, 489–499. [Google Scholar] [CrossRef]
- Zhang, J.G.; Cong, B.; Li, Q.X.; Chen, H.Y.; Qin, J.; Fu, L.H. Cholecystokinin octapeptide regulates lipopolysaccharide-activated B cells co-stimulatory molecule expression and cytokines production in vitro. Immunopharmacol. Immunotoxicol. 2011, 33, 157–163. [Google Scholar] [CrossRef]
- Zhang, J.G.; Liu, J.X.; Jia, X.X.; Geng, J.; Yu, F.; Cong, B. Cholecystokinin octapeptide regulates the differentiation and effector cytokine production of CD4(+) T cells in vitro. Int. Immunopharmacol. 2014, 20, 307–315. [Google Scholar] [CrossRef]

| Class | Forms Existed | Dietary Sources |
|---|---|---|
| Flavones | Apigenin, Chrysin, Luteolin, Baicalin | Buckwheat, Fruit peel, Tomato skin |
| Isoflavones | Daidzein, Genistein | Chinese herb, Soybeans |
| Flavanols | Epicatechin, Catechin | Chocolate, Tea, Fruits |
| Flavanonols | Taxifolin, Astilbin | Onion |
| Flavanones | Naringin, Hesperidin, Naringenin | Grape, Citrus fruits |
| Flavonols | Kaempferol, Fisetin, Quercetin, Myricetin | Red wine, Olive oil, Grapes |
| Anthocyanidins | Delphinidin, Malvidin, Cyanide, Pelargonidin | Berries, Vegetables, Red wine |
| Hormone | Stimulating Factor | Model | Effects | Reference |
|---|---|---|---|---|
| GLP-1 | DSS | GLP-1R knockout colitis mice | Increased weight loss, disease activity and intestinal epithelial damage. | [91] |
| GLP-1 | DSS | Colitis mice | No decrease in the level of intestinal inflammation. | [92] |
| GLP-1 | CD4+CD25− T cells | GLP-1R knockout colitis mice | Decreased the inflammatory score in histopathology and the level of proinflammatory cytokines. | [93] |
| GLP-1 | DSS | Colitis mice | Decreased weight loss, histological destruction, improved stool consistency, reduced IL-1β and increased the expression of the intestinal chloride transporter. | [94] |
| GLP-1 | Null | UC patient | Symptomatic remission of UC. | [95] |
| GLP-1 | Null | IBD patients | Improved the process of IBD. | [13] |
| GLP-2 | DSS | Colitis mice | Reduced IL-1 and increased the colon length, crypt depth and both mucosal area and integrity in the colon. | [96] |
| GLP-2 | Null | IL-10-deficient colitis mouse | Decreased the inflammation score in histopathology and lowered the MPO, IL-1β, IFN-γ and TNF-α. | [97] |
| GLP-2 | DSS | Colitis mice | Decreased weight loss and increased colon length. | [98] |
| GLP-2 | DSS | Colitis rats | Decreased colonic damage score and expression of IL-1, IL-7 and TNF-α. | [99] |
| GLP-2 | Null | CD patients | Induced remission and mucosal healing in CD patients. | [100] |
| DPP-4 inhibitors | DSS | DPP-4-deficient colitis mice | Increased MPO and expression of the NF-κB p65 subunit. | [101] |
| DPP-4 inhibitors | DSS and TNBS | Colitis mice | Increased GLP-2 and decreased MPO, weight loss and histological destruction. | [102] |
| DPP-4 inhibitors | Null | IBD patients | Increased risk of IBD. | [103] |
| DPP-4 inhibitors | Null | IBD patients | Did not augment the risk of IBD. | [104] |
| Hormone | Stimulating Factor | Model | Effects | Reference |
|---|---|---|---|---|
| Ghrelin | TNBS | Colitis mice | Reduced weight loss, histological colitis score and MPO; increased IL-10 and decreased TNF-α, IL-1β and IL-6. | [107] |
| Ghrelin | TNBS | Colitis rats | Accelerated the healing of TNBS colitis and increased the expression of iNOS and COX-2. | [108] |
| Ghrelin | 3% DSS | Colitis mice | Increased the activity score of colitis, neutrophil infiltration, IL-1β and MPO. | [109] |
| Hormone | Stimulating Factor | Model | Effects | Reference |
|---|---|---|---|---|
| CCK | Acetic acid | Colitis rats | Decreased inflammation parameters (WWI, histological colitis score and MPO). | [112] |
| CCK | LPS | Sepsis mice | Relieved intestinal epithelium damage and prevented bacterial displacement. | [113] |
| CCK | LPS | Healthy men | Decreased TNF-α, IL-6, IL-1 and increased IL-10. | [114] |
| CCK | LPS | Sepsis rats | Decreased TNF-α, IL-1ß, prevented bacterial displacement and increased tight junction. | [115] |
| CCK | CpG ODN | Dendritic cells | Decreased IFN-α and inhibited TNF receptor-associated factor 6. | [116] |
| CCK | LPS | B cells | Inhibited CD86 and CD80. | [117] |
| CCK | Null | T cells | Inhibited Th1 and Th17 and boosted Th2 and Treg. | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Weigmann, B. A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites 2022, 12, 31. https://doi.org/10.3390/metabo12010031
Li M, Weigmann B. A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites. 2022; 12(1):31. https://doi.org/10.3390/metabo12010031
Chicago/Turabian StyleLi, Mingrui, and Benno Weigmann. 2022. "A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System" Metabolites 12, no. 1: 31. https://doi.org/10.3390/metabo12010031
APA StyleLi, M., & Weigmann, B. (2022). A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites, 12(1), 31. https://doi.org/10.3390/metabo12010031






