Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications
Abstract
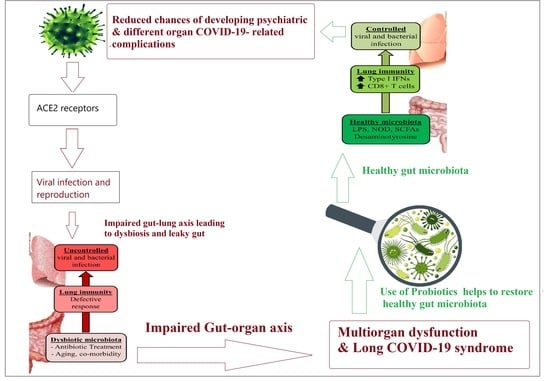
1. Introduction
2. Oral Health as Contributor to Long COVID-19
3. Role of Gut Microbiota–Organ Axis in Long-COVID-19 Multi-Organ Dysfunction
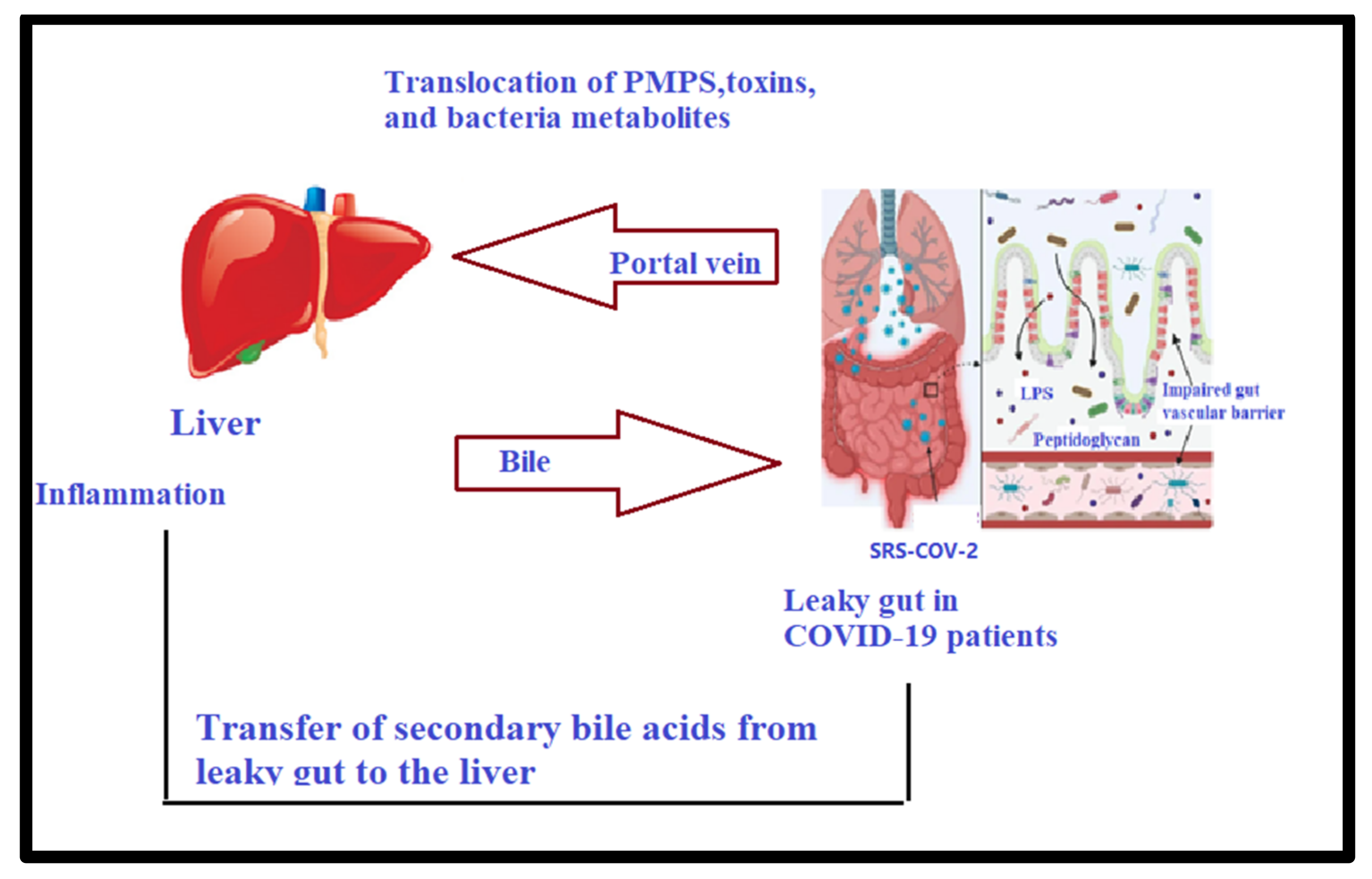
3.1. Gut–Liver Axis and Long COVID-19
3.2. Gut–Heart Axis in Long COVID-19
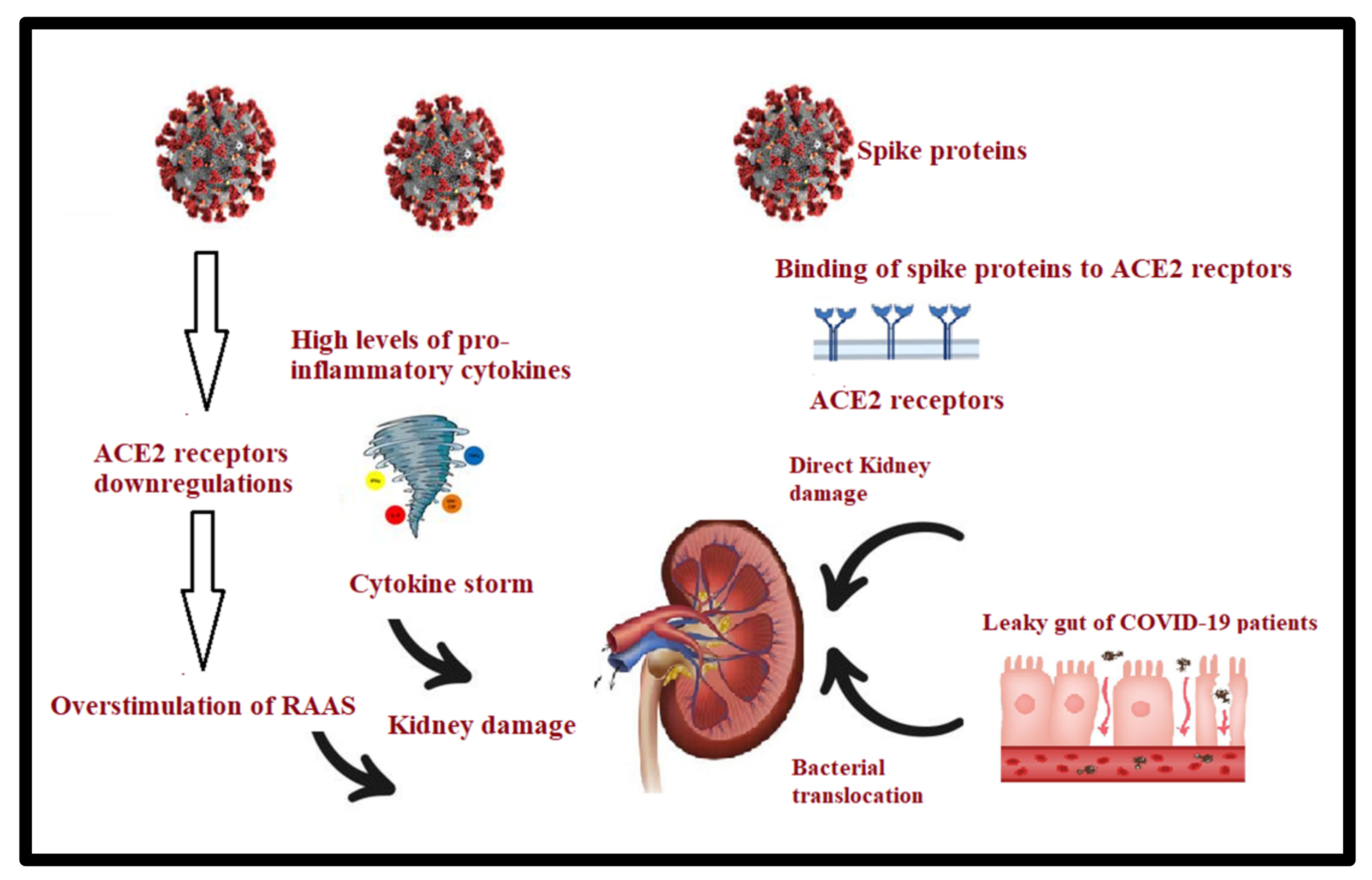
3.3. Gut–Kidney Axis in Long COVID-19
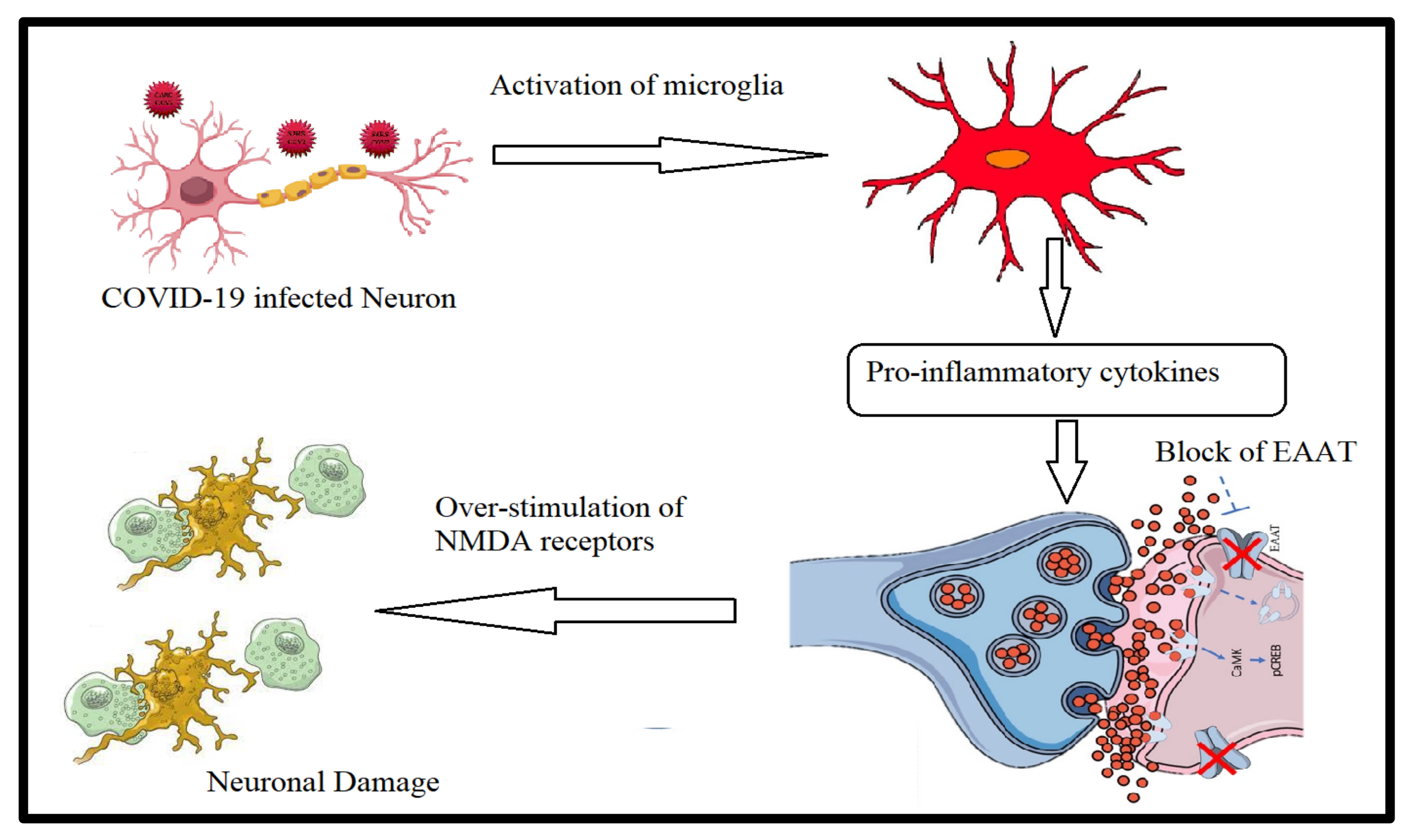
3.4. Gut–Brain Axis in Long COVID-19
Neuropsychiatric Complications and Gut–Brain Axis
3.5. Gut–Spleen Axis in Long COVID-19
4. Immune Perspective on Long COVID-19 and Proposed Mechanisms
| Description | Terms | References |
|---|---|---|
| Symptoms lasting 4 weeks post-acute infection | Long COVID | [104] |
| Symptoms 3 months post-acute infections | Post COVID | [130] |
| Long-term COVID-19 is said to be cyclical, progressive, and multiphasic | Long COVID | [131] |
| Multi-organ indications that continue for months after acute COVID-19 | Long-hauler COVID-19 | [132,133] |
| Chronic COVID syndrome | Long-COVID | |
| Symptoms lasting more than 100 days | Long-haul COVID | [134] |
| Long-tail COVID | ||
| Symptoms lasting more than 2 months | Long COVID | [135,136] |
| Symptoms to last more than 4 weeks | Late sequelae of SARS-CoV-2 infection | [137,138] |
| Long-haulers | ||
| Long-COVID | ||
| Symptoms persist more than 4 weeks after acute infection diagnosis | Post-acute COVID-19 syndrome | [130] |
| Symptoms continue for 5–12 weeks | Acute post-COVID symptoms | [139] |
| Symptoms continue for 12–24 weeks | Long post-COVID symptoms | |
| Symptoms continue for >24 weeks | Persistent post-COVID symptoms | |
| Symptoms continue for 1–3 months | Post-acute COVID-19 | [106,140] |
| On-going symptomatic COVID-19 | ||
| Symptoms continue more than 3 months | Chronic COVID-19 | |
| Long COVID | ||
| Post-COVID-19 syndrome |
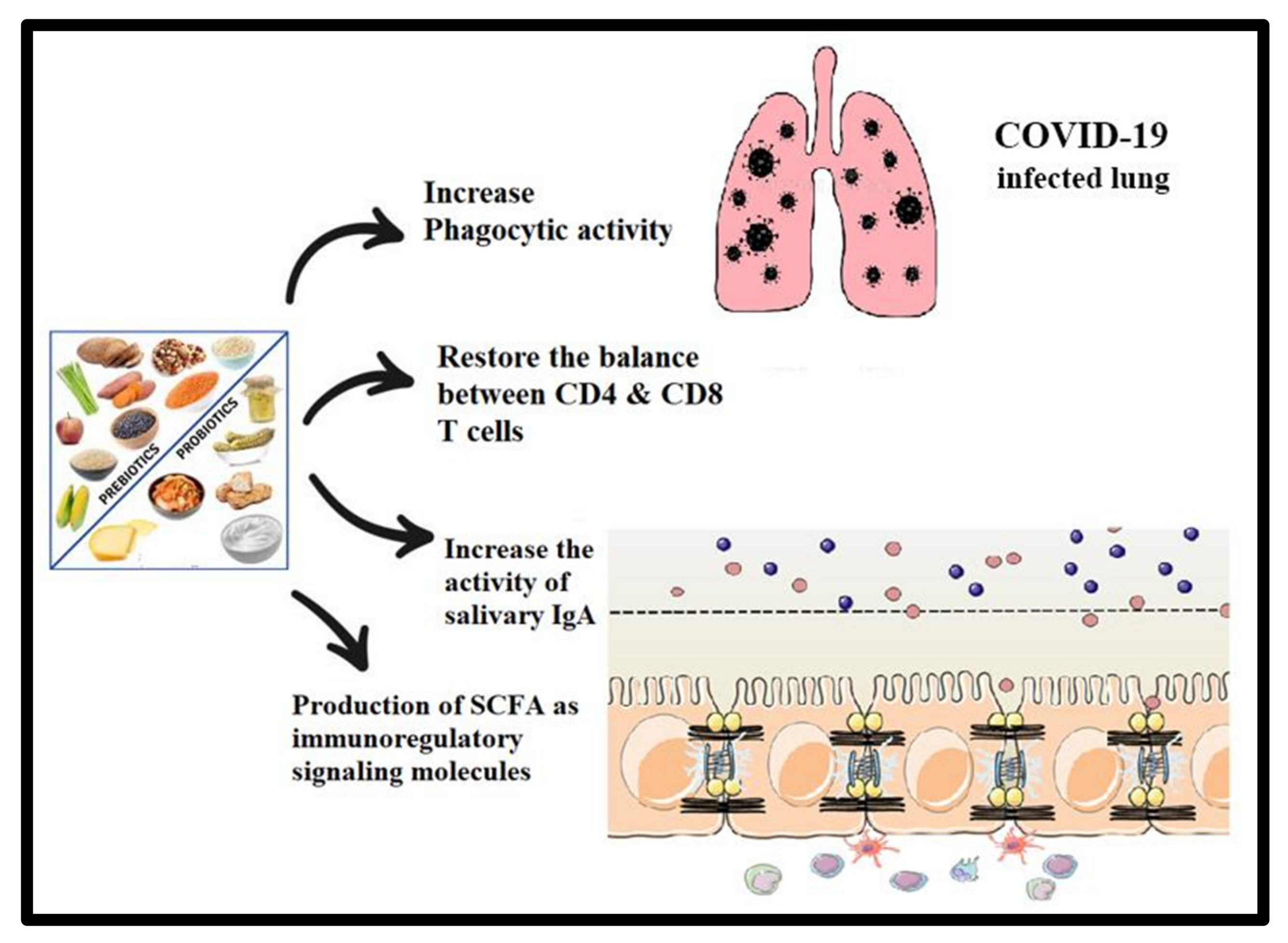
5. Potential Preventive and/or Therapeutic Effects of Prebiotic- and Probiotic-Related Strategies in COVID-19
6. Conclusions
7. Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after Acute COVID-19. JAMA 2020, 324, 603–6052020. [Google Scholar] [PubMed]
- Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the inflammatory response to severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, S.; Chen, Y.; Lu, H.; Shi, D.; Guo, J.; Wu, W.R.; Yang, Y.; Li, Y.; Xu, K.J.; et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut 2022, 71, 222. [Google Scholar] [CrossRef]
- Ng, S.C.; Tilg, H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 2020, 69, 973. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.Y.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.L.; Chan, P.K.S.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the human gut microbiota for immunomodulatory organisms. Cell 2017, 168, 928–943. [Google Scholar] [CrossRef]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shutting of PPAR-γ and ReIA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamel, A.H.M.; Basuoni, A.; Salem, Z.A.; AbuBakr, N. The impact of oral health status on COVID-19 severity, recovery period and C-reactive protein values. Br. Dent. J. 2021. [Google Scholar] [CrossRef]
- Franziska, B. Good oral health may prevent severe COVID-19 progression. Ger. Soc. Dent. Oral Med. GSDOM 2020, 22, 6527. [Google Scholar]
- Buunk-Werkhoven, Y.A.B.; Reyerse, E. What is the impact of oral (Public) health promotion, and of interventions for oral (self) care awareness raising and behavior change? J. Dent. Oral Disord. Ther. 2020, 8, 1–4. [Google Scholar] [CrossRef]
- France, K.; Glick, M. Long COVID and oral health care considerations. J. Am. Dent. Assoc. 2022, 153, 167–174. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Polizzi, E.; Tetè, G.; De Lorenzo, R.; Magnaghi, C.; Rovere Querini, P.; Ciceri, F. Frequent and persistent salivary gland ectasia and oral disease after COVID-19. J. Dent. Res. 2021, 100, 464–471. [Google Scholar] [CrossRef]
- Neto, C.L.D.M.M.; Bannwart, L.C.; de Melo Moreno, A.L.; Goiato, M.C. SARS-CoV-2 and Dentistry-Review. Eur. J. Dent. 2020, 14, S130–S139. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.M. Oral manifestations of COVID-19: Brief review. Dent. Med. Probl. 2021, 58, 123–126. [Google Scholar] [CrossRef]
- Zangrillo, A.; Beretta, L.; Scandroglio, A.M.; Monti, G.; Fominskiy, E.; Colombo, S.; Morselli, F.; Belletti, A.; Silvani, P.; Crivellari, M.; et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit. Care Resusc. 2020, 22, 200–211. [Google Scholar]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huan, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Deiana, G.; de Riu, G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope 2020, 130, 1787. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef]
- Pisanic, N.; Randad, P.R.; Kruczynski, K.; Manabe, Y.C.; Thomas, D.L.; Pekosz, A.; Klein, S.L.; Betenbaugh, M.J.; Clarke, W.A.; Laeyendecker, O.; et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J. Clin. Microbiol. 2020, 59, e02204-20. [Google Scholar] [CrossRef]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zheng, K.I.; Wang, X.B.; Yan, H.D.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Ma, H.L.; Chen, Y.P.; George, J.; et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J. Hepatol. 2020, 73, 719–721. [Google Scholar] [CrossRef]
- Assante, G.; Williams, R.; Youngson, N.A. Is the increased risk for MAFLD patients to develop severe COVID-19 linked to perturbation of the gut-liver axis? J. Hepatol. 2021, 74, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Mu, M.I.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Roberts, L.M.; Buford, T.W. Lipopolysaccharide binding protein is associated with CVD risk in older adults. Aging Clin. Exp. Res. 2021, 33, 1651–1658. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Cai, X.P.; Deng, J.W.; Zheng, L.; Zhu, H.H.; Yang, B.; Zheng, M.; Chen, Z. An overview of COVID-19. J. Zhejiang Univ. Sci. B 2020, 21, 343–360. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzim, E. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Sarfraz, A.; Barrios, A.; Garimella, R.; Dominari, A.; Kc, M.; Pandav, K.; Pantoja, J.C.; Retnakumar, V.; Cherrez-Ojeda, I. Cardio-Pulmonary sequelae in recovered COVID-19 patients: Considerations for primary care. J. Prim. Care Community Health 2021, 12, 21501327211023726. [Google Scholar] [CrossRef]
- Stefanini, A.M.; Fidelis, T.O.; Penna, G.M.; Pessanha, G.R.G.; Marques, R.A.G.; Oliveira, D.C.D. Tomographic identification and evaluation of pulmonary involvement due to SARS-CoV-2 infection using artificial intelligence and image segmentation technique. In Bioengineering and Biomedical Signal and Image Processing; Rojas, I., Castillo-Secilla, D., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 405–416. [Google Scholar]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus. Cell 2020, 38, 1190–1191. [Google Scholar] [CrossRef]
- Hoel, H.; Heggelund, L.; Reikvam, D.H.; Stiksrud, B.; Ueland, T.; Michelsen, A.E.; Otterdal, K.; Muller, K.E.; Lind, A.; Muller, F.; et al. Elevated markers of gut leakage and inflammasome activation in COVID-19 patients with cardiac involvement. J. Intern. Med. 2021, 289, 523–531. [Google Scholar] [CrossRef]
- Mielcarek, C.; Romond, P.C.; Romond, M.B.; Bezirtzoglou, E. Modulation of bacterial translocation in mice mediated through lactose and human milk oligosaccharides. Anaerobe 2011, 17, 361–366. [Google Scholar] [CrossRef]
- Gebbers, J.-O.; Laissue, J.-A. Bacterial Translocation in the Normal Human Appendix Parallels the Development of the Local Immune System. Ann. N. Y. Acad. Sci. 2004, 1029, 337–343. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Ren. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and outcomes of individuals with pre-existing kidney disease and covid-19 admitted to intensive care units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203.e1. [Google Scholar] [CrossRef]
- Silva, M.A.; da Silva, A.R.P.A.; do Amaral, M.A.; Fragas, M.G.; Câmara, N.O.S. Metabolic Alterations in SARS-CoV-2 Infection and Its Implication in Kidney Dysfunction. Front. Physiol. 2021, 12, 624698. [Google Scholar] [CrossRef]
- Robinson, F.A.; Mihealsick, R.P.; Wagener, B.M.; Hanna, P.; Poston, M.D.; Efimov, I.R.; Shivkumar, K.; Hoover, D.B. Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection. Am. J. Physiology. Heart Circ. Physiol. 2020, 319, H1059–H1068. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Renal complications in COVID-19: A systematic review and meta-analysis. Ann. Med. 2020, 52, 345–353. [Google Scholar] [CrossRef]
- Voorend, C.G.N.; Van Oevelen, M.; Nieberg, M.; Meuleman, Y.; Franssen, C.F.M.; Joosten, H.; Berkhout-Byrne, N.C.; Abrahams, A.C.; Mooijaart, S.P.; Bos, W.J.W.; et al. Impact of the COVID-19 pandemic on symptoms of anxiety and depression and health-related quality of life in older patients with chronic kidney disease. BMC Geriatr. 2021, 21, 650. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J.; Lu, J.; Xu, X.; Long, W.; Yan, G.; Tang, M.; Zou, L.; Xu, Z.; Zhuo, P.; et al. Investigation of COVID-19-related symptoms based on factor analysis. Ann Palliat Med. 2020, 9, 1851–1858. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohanam, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. New Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; MacKay, C.R.; Marinõ, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef]
- Fukuuchi, F.; Hida, M.; Aiba, Y.; Koga, Y.; Endoh, M.; Kurokawa, K.; Sakai, H. Intestinal bacteria-derived putrefactants in chronic renal failure. Clin. Exp. Nephrol. 2002, 6, 99–104. [Google Scholar] [CrossRef]
- Nalewajska, M.; Przybyciński, J.; Marchelek-Myśliwiec, M.; Dziedziejko, V.; Ciechanowski, K. Gut Microbiota In Chronic Kidney Disease. Postępy Mikrobiol. Adv. Microbiol. 2019, 58, 237–245. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: A viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Dogan, L.; Sarikaya, Z.T.; Kara, S.; Akinci, C.; Kaya, D.; Kaya, Y.; Yildirim, D.; Tuzuner, F.; Yildirim, M.S.; et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology 2020, 297, E232–E235. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Le Guennec, L.; Devianne, J.; Jalin, L.; Cao, A.; Galanaud, D.; Navarro, V.; Boutolleau, D.; Rohaut, B.; Weiss, N.; Demeret, S. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia 2020, 61, e90–e94. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Kwan, P.; Zhou, D.; Del Felice, A.; Duncan, J.S.; Sander, J.W. Acute and late neurological complications of COVID19: The quest for evidence. Brain 2020, 143, e99. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hanif, M.; Ali, M.J.; Haider, M.A.; Kherani, D.; Memon, G.M.; Karim, A.H.; Sattar, A. Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front. Neurol. 2020, 11, 518. [Google Scholar] [CrossRef]
- Rudroff, T.; Kamholz, J.; Fietsam, A.C.; Deters, J.R.; Bryant, A.D. Post-covid-19 fatigue: Potential contributing factors. Brain Sci. 2020, 10, 1012. [Google Scholar] [CrossRef]
- Egbert, A.R.; Cankurtaran, S.; Karpiak, S. Brain abnormalities in COVID-19 acute/subacute phase: A rapid systematic review. Brain Behav. Immun. 2020, 89, 543–554. [Google Scholar] [CrossRef]
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Kim, J.E.; Heo, J.H.; Kim, H.O.; Song, S.H.; Park, S.S.; Park, T.H.; Ahn, J.Y.; Kim, M.K.; Choi, J.P. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017, 13, 227–233. [Google Scholar] [CrossRef]
- Savarin, C.; Bergmann, C.C. Viral-induced suppression of self-reactive T cells: Lessons from neurotropic coronavirus-induced demyelination. J. Neuroimmunol. 2017, 308, 12–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Skinner, D.D.; Lane, T.E. Innate immune responses and viral-induced neurologic disease. J. Clin. Med. 2019, 8, 3. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Cunming, L.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Ye, M.; Ren, Y.; Lv, T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020, 88, 945–946. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome associated with SARS-CoV-2. New Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Pincherle, A.; Jöhr, J.; Pancini, L.; Leocani, L.; Dalla Vecchia, L.; Ryvlin, P.; Schiff, N.D.; Diserens, K. Intensive Care admission and early Neuro-Rehabilitation. Lessons for COVID-19? Front. Neurol. 2020, 11, 880. [Google Scholar] [CrossRef]
- Yin, C.H.; Wang, C.; Tang, Z.; Wen, Y.; Zhang, S.W.; Wang, B.E. Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004, 16, 646–650. [Google Scholar]
- Klein, R.S.; Garber, C.; Howard, N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017, 18, 132–141. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 2019, 12, 14. [Google Scholar] [CrossRef]
- Coolen, T.; Lolli, V.; Sadeghi, N.; Rovai, A.; Trotta, N.; Taccone, F.S.; Creteur, J.; Henrard, S.; Goffard, J.C.; Dewitte, O.; et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 2020, 95, e2016–e2027. [Google Scholar] [CrossRef]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.C.; Griffith, B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Cureus 2020, 296, E119–E120. [Google Scholar]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.; et al. Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Bottemanne, H.; Delaigue, F.; Lemogne, C. SARS-CoV-2 Psychiatric Sequelae: An Urgent Need of Prevention. Front. Psychiatry 2021, 2, 1479. [Google Scholar] [CrossRef]
- Grover, S.; Kumar, V.; Chakrabarti, S.; Hollikatti, P.; Singh, P.; Tyagi, S.; Kulhara, P.; Avasthi, A. Prevalence and Type of Functional Somatic Complaints in Patients with First-episode Depression. East Asian Arch Psychiatry. East Asian Arch. Psychiatry 2012, 22, 146–153. [Google Scholar]
- Perna, G.; Caldirola, D. COVID-19 and panic disorder: Clinical considerations for the most physical of mental disorders. Braz. J. Psychiatry 2021, 43, 110–111. [Google Scholar] [CrossRef]
- Ezzat, M.M.; Elsherif, A.A. Prevalence of Fatigue in Patients Post COVID-19. Eur. J. Mol. Clin. Med. 2021, 08, 1330–1340. [Google Scholar]
- Katafuchi, T.; Kondo, T.; Take, S.; Yoshimura, M. Brain Cytokines and the 5-HT System during Poly I:C-Induced Fatigue. Ann. N. Y. Acad. Sci. 2006, 1088, 230–237. [Google Scholar] [CrossRef]
- Costa, L.H.A.; Santos, B.M.; Branco, L.G.S. Can selective serotonin reuptake inhibitors have a neuroprotective effect during COVID-19? Eur. J. Pharmacol. 2020, 889, 173629. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Al-Sarraj, S.; Troakes, C.; Hanley, B.; Osborn, M.; Richardson, M.P.; Hotopf, M.; Bullmore, E.; Everall, I. Invited Review: The spectrum of neuropathology in COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 3–16. [Google Scholar] [CrossRef]
- Donegani, M.I.; Miceli, A.; Pardini, M.; Bauckneht, M.; Chiola, S.; Pennone, M.; Marini, C.; Massa, F.; Raffa, S.; Ferrarazzo, G.; et al. Brain metabolic correlates of persistent olfactory dysfunction after SARS-CoV-2 infection. Biomedicines 2021, 9, 287. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef]
- Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease: FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 486–510. [Google Scholar] [CrossRef]
- Zoghi, A.; Ramezani, M.; Roozbeh, M.; Darazam, I.A.; Sahraian, M.A. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult. Scler. Relat. Disord. 2020, 44, 102324. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Hepburn, M.; Mullaguri, N.; George, P.; Hantus, S.; Punia, V.; Bhimraj, A.; Newey, C.R. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit. Care 2021, 34, 139–143. [Google Scholar] [CrossRef]
- Afshar, H.; Yassin, Z.; Kalantari, S.; Aloosh, O.; Lotfi, T.; Moghaddasi, M.; Sadeghipour, A.; Emamikhah, M. Evolution and resolution of brain involvement associated with SARS-CoV-2 infection: A close Clinical—Paraclinical follow up study of a case. Mult. Scler. Relat. Disord. 2020, 43, 102216. [Google Scholar] [CrossRef]
- Benedetti, F.; Palladini, M.; Paolini, M.; Melloni, E.; Vai, B.; De Lorenzo, R.; Furlan, R.; Rovere-Querini, P.; Falini, A.; Mazza, M.G. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav. Immun. Health 2021, 18, 100387. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef]
- Anzalone, N.; Castellano, A.; Scotti, R.; Scandroglio, A.M.; Filippi, M.; Ciceri, F.; Tresoldi, M.; Falini, A. Multifocal laminar cortical brain lesions: A consistent MRI finding in neuro-COVID-19 patients. J. Neurol. 2020, 267, 2806–2809. [Google Scholar] [CrossRef]
- Naeije, R.; Caravita, S. Phenotyping long COVID. Eur. Respir. J. 2021, 58, 2101763. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Pogue, A.; Hill, J.M. SARS-CoV-2 Infectivity and Neurological Targets in the Brain. Cell Mol. Neurobiol. 2022, 42, 42,217–224. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. The BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone system inhibitors in patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Kim, K.D.; Zhao, J.; Auh, S.; Yang, X.; Du, P.; Tang, H.; Fu, Y.X. Adaptive immune cells temper initial innate responses. Nat. Med. 2007, 13, 1248–1252. [Google Scholar] [CrossRef]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 8063, 5. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenov, N.; Howard Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Kathryn Choon Beng Tan, K.C.B.; et al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.; Cannavaro, D.; Dazzi, D.; Covelli, D.; Mantovani, G.; Muscatello, A.; Ferrante, E.; Orsi, E.; Resi, V.; Longari, V.; et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020, 8, 739–741. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Jacqueline A Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, 3876. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, k.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 1979, 370. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Zhang, H.; Cao, X.; Mao, X.; Lu, Z. Higher level of Neutrophil-to-Lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 2020, 148, e139. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Rezaei, N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020, 44, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Chen, F.; Ou, Z.; Fan, O.; Tan, X.; Wang, Y.; Pan, Y.; Ke, B.; Li, L.; Guan, Y.; et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol. Immunol. 2020, 17, 1119–1125. [Google Scholar] [CrossRef]
- Amato, M.K.; Hennessy, C.; Shah, K.; Mayer, J. Multisystem inflammatory syndrome in an adult. J. Emerg. Med. 2021, 61, e1–e3. [Google Scholar] [CrossRef]
- Love, C.; Tomas, M.B.; Bronco, G.G.; Palestro, C.J. FDG PET of infection and inflammation. Radiographics 2005, 25, 1357–1368. [Google Scholar] [CrossRef]
- Sollini, M.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Gelardi, F.; Chiti, A. Vasculitis changes in COVID-19 survivors with persistent symptoms: An [18 F] FDG-PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1460–1466. [Google Scholar] [CrossRef]
- Kucuk, A.; Cumhur, C.M.; Cure, E. Can COVID-19 cause myalgia with a completely different mechanism? A hypothesis. Clin. Rheumatol. 2020, 39, 2103–2104. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Congqing Wu, C.; Wei Lu, W.; Zhang, Y.; Guoying Zhang, G.; Shi, X.; Hisada, Y.; Grover, S.P.; Xinyi Zhang, X.; Li, L.; Xiang, B.; et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 2019, 50, 1401–1411.e4. [Google Scholar]
- Bochenek, M.L.; Schäfer, K. Role of endothelial cells in acute and chronic thrombosis. Hamostaseologie 2019, 39, 128–139. [Google Scholar] [CrossRef]
- Rauch, U.; Nemerson, Y. Tissue Factor, the Blood, and the Arterial Wall. Trends. Cardiovasc. Med. 2000, 10, 139–143. [Google Scholar] [CrossRef]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.; Righy, C.; Franco, S.; Souza, T.M.; Pedro Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Altmann, D.M.; Boyton, R.J. Decoding the unknowns in long covid. The BMJ 2021, 372, n132. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- Nath, A. Long-Haul COVID. Neurology 2020, 95, 559–560. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Davido, B.; Seang, S.; Tubiana, R.; de Truchis, P. Post–COVID-19 chronic symptoms: A postinfectious entity? Clin. Microbiol. Infect. 2020, 26, 1448–1449. [Google Scholar] [CrossRef]
- Datta, S.D.; Talwar, A.; Lee, J.T. A Proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 Infection: Illness beyond acute infection and public health implications. JAMA J. Am. Med. Assoc. 2020, 324, 2251–2252. [Google Scholar] [CrossRef]
- Sivan, M.; Taylor, S. NICE guideline on long COVID: Research must be done urgently to fill the many gaps in this new ‘living guideline’. BMJ 2020, 371, m4938. [Google Scholar] [CrossRef]
- Fernández-De-las-peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-covid symptoms (Post-acute covid, long covid, persistent post-covid): An integrative classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long-term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136, Erratum in BMJ 2022, 376, o126. [Google Scholar] [CrossRef] [PubMed]
- Corcionivoschi, N.; Drinceanu, D.; Stef, L.; Luca, I.; Julean, C. Probiotics-identification and ways of action. Innov. Rom. Food Biotechnol. 2010, 6, 1. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Agri, V.D.; Gibson, G.R.; Reid, G.; Giannoni, E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Batista, K.S.; de Albuquerque, J.G.; Vasconcelos, M.; Bezerra, M.; da Silva Barbalho, M.B.; Pinheiro, R.O.; Aquino, J.S. Probiotics and prebiotics: Potential prevention and therapeutic target for nutritional management of COVID-19? Nutr. Res. Rev. 2021, 1–18, Epub ahead of print.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.Y.; Zhang, Z.; Feng, Y. The Crosstalk between gut microbiota and lungs in common lung diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef]
- National Health Committee of the People’s Republic of China. National Administration of Traditional Chinese Medicine Diagnostic and Therapeutic Guidance for 2019 Novel Coronavirus Disease (Version 5). 2019. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf (accessed on 5 June 2022).
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral administration of compound probiotics ameliorates HFD-Induced gut microbe dysbiosis and chronic metabolic inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic fatty liver disease rats. Probiotics Antimicrob Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, P.; Meng, W.; Jin, W.; Li, X.; Li, X. Modulated gut microbiota for potential COVID-19 prevention and treatment. Front. Med. 2022, 9, 811176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2015, 5, 719–728. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, F.; Xu, Q.; Su, Y.; Cai, X.; Zeng, F.; Zhang, Y. The role of probiotics in coronavirus disease-19 infection in Wuhan: A retrospective study of 311 severe patients. Int. Immunopharmacol. 2021, 95, 107531. [Google Scholar] [CrossRef]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of COVID-19: The Zhejiang experience the: Zhejiang experience. J. Zhejiang University. Med. Sci. 2020, 49, 147–157. [Google Scholar]
- Dhar, D.; Mohanty, A. Gut microbiota and COVID-19- possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Gogineni, V.K.; Morrow, L.E.; Malesker, M.A. Probiotics: Mechanisms of action and clinical applications. J. Probiotics Health 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Amer, M.; Nadeem, M.; Nazir, S.; Fakhar, M.; Abid, F.; Ain, Q.U.; Asif, E. Probiotics and their use in inflammatory bowel disease. Altern. Ther. Health Med. 2018, 24, 16–23. [Google Scholar]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 92, 8. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef]
- Wiciński, M.; Gębalski, J.; Mazurek, E.; Podhorecka, M.; Śniegocki, M.; Szychta, P.; Sawicka, E.; Malinowski, B. The influence of polyphenol compounds on human gastrointestinal tract microbiota. Nutrients 2020, 350, 12. [Google Scholar] [CrossRef]
- Ranucci, G.; Buccigrossi, V.; Borgia, E.; Piacentini, D.; Visentin, F.; Cantarutti, L.; Baiardi, P.; Felisi, M.; Spagnuolo, M.I.; Zanconato, S.; et al. Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: A randomized clinical trial. Nutrients 2018, 10, 286. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J. Funct. Foods 2020, 66, 103838. [Google Scholar] [CrossRef]
- West, C.E.; Dzidic, M.; Prescott, S.L.; Jenmalm, M.C. Bugging allergy; role of pre-, pro- and synbiotics in allergy prevention. Allergol. Int. 2017, 66, 529–538. [Google Scholar] [CrossRef]
- Din, A.U.; Mazhar, M.; Waseem, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021, 133, 110947. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenazy, M.F.; Aljohar, H.I.; Alruwaili, A.R.; Daghestani, M.H.; Alonazi, M.A.; Labban, R.S.; El-Ansary, A.K.; Balto, H.A. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites 2022, 12, 912. https://doi.org/10.3390/metabo12100912
Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, El-Ansary AK, Balto HA. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites. 2022; 12(10):912. https://doi.org/10.3390/metabo12100912
Chicago/Turabian StyleAlenazy, Maha F., Haya I. Aljohar, Ashwag R. Alruwaili, Maha H. Daghestani, Mona A. Alonazi, Ranyah S. Labban, Afaf K. El-Ansary, and Hanan A. Balto. 2022. "Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications" Metabolites 12, no. 10: 912. https://doi.org/10.3390/metabo12100912
APA StyleAlenazy, M. F., Aljohar, H. I., Alruwaili, A. R., Daghestani, M. H., Alonazi, M. A., Labban, R. S., El-Ansary, A. K., & Balto, H. A. (2022). Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites, 12(10), 912. https://doi.org/10.3390/metabo12100912