Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
2.3. Study Procedures
2.4. Sample Collection and Analysis
2.5. Data Realignment and Imputation
2.6. Data Analysis
3. Results
3.1. Realignment and Imputation Results and Study Participants
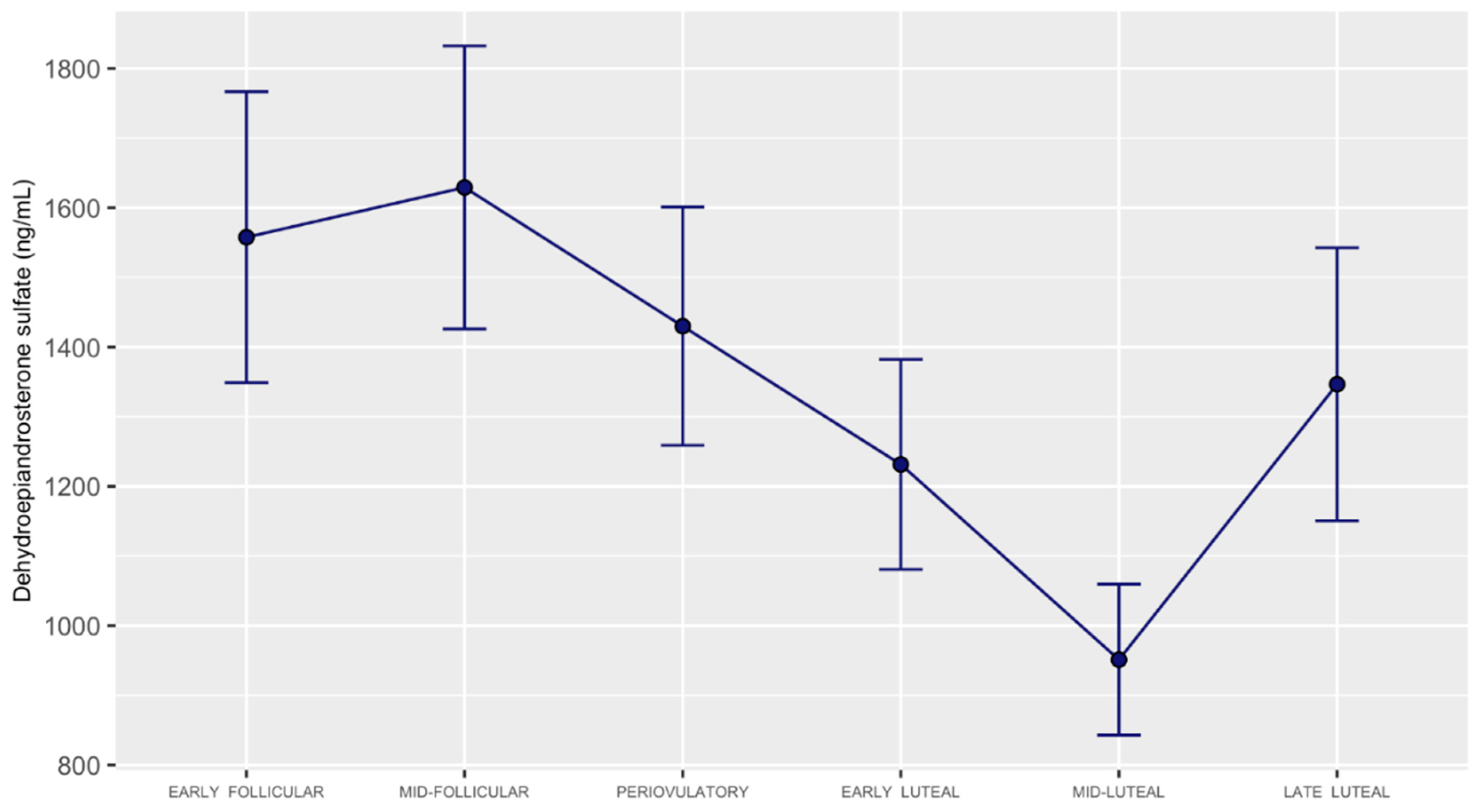
3.2. DHEA, DHEA-Sulfate, and DHEA-Sulfate: DHEA Ratio Concentrations across the Menstrual Cycle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baulieu, E.-E.; Robel, P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. USA 1998, 95, 4089–4091. [Google Scholar] [CrossRef] [Green Version]
- Berr, C.; Lafont, S.; Debuire, B.; Dartigues, J.-F.; Baulieu, E.-E. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: A French community-based study. Proc. Natl. Acad. Sci. USA 1996, 93, 13410–13415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naelitz, B.D.; Sharifi, N. Through the Looking-Glass: Reevaluating DHEA Metabolism Through HSD3B1 Genetics. Trends Endocrinol. Metab. 2020, 31, 680–690. [Google Scholar] [CrossRef]
- Kroboth, P.D.; Salek, F.S.; Pittenger, A.L.; Fabian, T.J.; Frye, R. DHEA and DHEA-S: A Review. J. Clin. Pharmacol. 1999, 39, 327–348. [Google Scholar] [CrossRef]
- Turcu, A.F.; Rege, J.; Auchus, R.J.; Rainey, W.E. 11-Oxygenated androgens in health and disease. Nat. Rev. Endocrinol. 2020, 16, 284–296. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocriol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Chatterton, R.T. Functions of dehydroepiandrosterone in relation to breast cancer. Steroids 2022, 179, 108970. [Google Scholar] [CrossRef]
- Mumford, S.L.; Schisterman, E.F.; Gaskins, A.J.; Pollack, A.Z.; Perkins, N.J.; Whitcomb, B.W.; Ye, A.; Wactawski-Wende, J. Realignment and multiple imputation of longitudinal data: An application to menstrual cycle data. Paediatr. Perinat. Epidemiol. 2011, 25, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, C.F.; Duisters, K.; Weger, B.D.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef] [Green Version]
- van Zuiden, M.; Haverkort, S.Q.; Tan, Z.; Daams, J.; Lok, A.; Olff, M. DHEA and DHEA-S levels in posttraumatic stress disorder: A meta-analytic review. Psychoneuroendocrinology 2017, 84, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Genud, R.; Merenlender Wagner, A.; Gispan, I.; Maayan, R.; Weizman, A.; Yadid, G. DHEA Lessens Depressive-Like Behavior via GABA-ergic Modulation of the Mesolimbic System. Neuropsychopharmacology 2008, 34, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, Y.; Hiraki, K. Prefrontal cortex and executive function in young children: A review of NIRS studies. Front. Hum. Neurosci. 2013, 7, 867. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.D.; Duman, R.S. Peripheral BDNF Produces Antidepressant-Like Effects in Cellular and Behavioral Models. Neuropsychopharmacology 2010, 35, 2378–2391. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Reus, V.I.; Keebler, A.; Nelson, N.; Friedland, M.; Brizendine, L.; Roberts, E. Double-Blind Treatment of Major Depression with Dehydroepiandrosterone. Am. J. Psychiatry 1999, 156, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Strous, R.D.; Stryjer, R.; Maayan, R.; Gal, G.; Viglin, D.; Katz, E.; Eisner, D.; Weizman, A. Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Psychoneuroendocrinology 2007, 32, 96–105. [Google Scholar] [CrossRef]
- Heuser, I.; Deuschle, M.; Luppa, P.; Schweiger, U.; Standhardt, H.; Weber, B. Increased Diurnal Plasma Concentrations of Dehydroepiandrosterone in Depressed Patients. J. Clin. Endocrinol. Metab. 1998, 83, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, I.M.; Herbert, J.; Altham, P.M.E. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol. Med. 1998, 28, 265–273. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Von Mühlen, D.; Laughlin, G.A.; Kripke, A. Endogenous Levels of Dehydroepiandrosterone Sulfate, but Not Other Sex Hormones, Are Associated with Depressed Mood in Older Women: The Rancho Bernardo Study. J. Am. Geriatr. Soc. 1999, 47, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, S.-Y.; Liu, F.; Zhang, Y.-L.; Zhu, D.-M.; Zang, Y.-Y. Clinical significance of decreased protein expression of dehydroepiandrosterone sulfate in the development of depression: A meta-analysis. J. Affect. Disord. 2015, 174, 416–423. [Google Scholar] [CrossRef]
- Mocking, R.; Pellikaan, C.; Lok, A.; Assies, J.; Ruhé, H.; Koeter, M.; Visser, I.; Bockting, C.; Olff, M.; Schene, A. DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Young, A.H.; Gallagher, P.; Porter, R.J. Elevation of the Cortisol-Dehydroepiandrosterone Ratio in Drug-Free Depressed Patients. Am. J. Psychiatry 2002, 159, 1237–1239. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Reuter, J.M.; Gaikwad, N.W.; Rotroff, D.; Kucera, H.R.; Motsinger-Reif, A.; Smith, C.P.; Nieman, L.K.; Rubinow, D.R.; Kaddurah-Daouk, R.; et al. The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: Effects of estradiol and progesterone addback. Transl. Psychiatry 2017, 7, e1193. [Google Scholar] [CrossRef] [Green Version]
- Howards, P.P.; Schisterman, E.F.; Wactawski-Wende, J.; Reschke, J.E.; Frazer, A.A.; Hovey, K.M. Timing Clinic Visits to Phases of the Menstrual Cycle by Using a Fertility Monitor: The BioCycle Study. Am. J. Epidemiology 2008, 169, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidovic, A.D.N.; Khalil, D.; Sun, J. Association between Neuroticism and Premenstrual Affective/Psychological Symptomatology. Psychiatry Int. 2022, 3, 52–64. [Google Scholar] [CrossRef]
- Endicott, J.; Nee, J.; Harrison, W. Daily Record of Severity of Problems (DRSP): Reliability and validity. Arch. Women’s Ment. Health 2005, 9, 41–49. [Google Scholar] [CrossRef]
- Leiva, R.A.; Bouchard, T.P.; Abdullah, S.H.; Ecochard, R. Urinary Luteinizing Hormone Tests: Which Concentration Threshold Best Predicts Ovulation? Front. Public Health 2017, 5, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
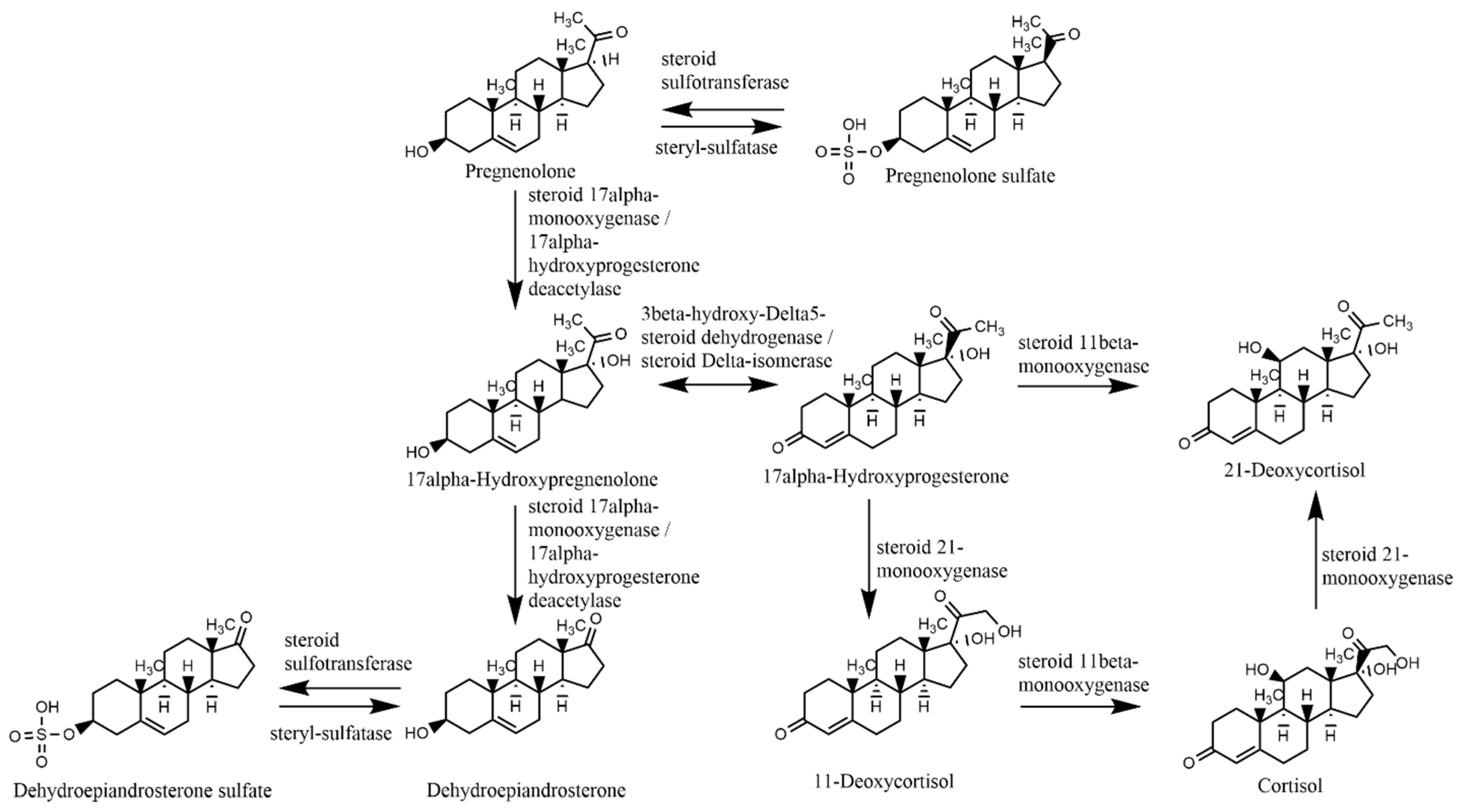
- Rižner, T.L. The Important Roles of Steroid Sulfatase and Sulfotransferases in Gynecological Diseases. Front. Pharmacol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Colette, S.; Defrère, S.; Van Kerk, O.; Van Langendonckt, A.; Dolmans, M.-M.; Donnez, J. Differential expression of steroidogenic enzymes according to endometriosis type. Fertil. Steril. 2013, 100, 1642–1649. [Google Scholar] [CrossRef]
- Dassen, H.; Punyadeera, C.; Kamps, R.; Delvoux, B.; Van Langendonckt, A.; Donnez, J.; Husen, B.; Thole, H.; Dunselman, G.; Groothuis, P. Estrogen metabolizing enzymes in endometrium and endometriosis. Hum. Reprod. 2007, 22, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, M.; Stribinskis, V.; Klinge, C.M.; Ramos, K.S.; Colburn, N.H.; Li, Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008, 27, 4373–4379. [Google Scholar] [CrossRef] [Green Version]
- Geese, W.J.; Raftogianis, R.B. Biochemical Characterization and Tissue Distribution of Human SULT2B1. Biochem. Biophys. Res. Commun. 2001, 288, 280–289. [Google Scholar] [CrossRef]
- Koizumi, M.; Momoeda, M.; Hiroi, H.; Hosokawa, Y.; Tsutsumi, R.; Osuga, Y.; Yano, T.; Taketani, Y. Expression and regulation of cholesterol sulfotransferase (SULT2B1b) in human endometrium. Fertil. Steril. 2010, 93, 1538–1544. [Google Scholar] [CrossRef]
- Falany, J.L.; Falany, C.N. Regulation of estrogen sulfotransferase in human endometrial adenocarcinoma cells by progesterone. Endocrinology 1996, 137, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.; Mahfoudi, A.; Mularoni, A.; Nicollier, M.; Adessi, G.L. Progesterone stimulates sulfate uptake in subcultured endometrial epithelial cells. Mol. Cell Endocrinol. 1992, 90, 95–102. [Google Scholar] [CrossRef]
- Mahfoudi, A.; Beck, L.; Nicollier, M.; Coosemans, V.; Adessi, G. Progesterone effect on intracellular inorganic sulphate in uterine epithelial cells. Mol. Cell Endocrinol. 1991, 79, R15–R20. [Google Scholar] [CrossRef]
- Beck, L.; Mularoni, A.; Cardis, P.; Adessi, G.L.; Nicollier, M. Adenosine 3’,5’-monophosphate mediates progesterone effect on sulfate uptake in endometrial epithelial cells. Endocrinology 1995, 136, 1737–1743. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.; Idkowiak, J.; Arlt, W.; Foster, P.A. The Regulation of Steroid Action by Sulfation and Desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef]
- Chen, B.; Wen, Y.; Zhang, Z.; Wang, H.; Warrington, J.A.; Polan, M.L. Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. Am. J. Obstet. Gynecol. 2003, 189, 89–97. [Google Scholar] [CrossRef]
- Tise, C.G.; Anforth, L.E.; Zhou, A.E.; Perry, J.A.; McArdle, P.F.; Streeten, E.A.; Shuldiner, A.R.; Yerges-Armstrong, L.M. Sex-specific effects of serum sulfate level and SLC13A1 nonsense variants on DHEA homeostasis. Mol. Genet. Metab. Rep. 2017, 10, 84–91. [Google Scholar] [CrossRef]
- Chapman, A.B.; Zamudio, S.; Woodmansee, W.; Merouani, A.; Osorio, F.; Johnson, A.; Moore, L.G.; Dahms, T.; Coffin, C.; Abraham, W.T.; et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am. J. Physiol. Physiol. 1997, 273, F777–F782. [Google Scholar] [CrossRef]
- Sung, E.; Han, A.; Hinrichs, T.; Vorgerd, M.; Manchado, C.; Platen, P. Effects of follicular versus luteal phase-based strength training in young women. SpringerPlus 2014, 3, 668. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, I.; Luisi, S.; Quirici, B.; Monteleone, P.; Bernardi, F.; Liut, M.; Casarosa, E.; Palumbo, M.; Petraglia, F.; Genazzani, A.R. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol. Endocrinol. 2004, 18, 79–87. [Google Scholar] [CrossRef]
- Duskova, M.; Simunkova, K.; Hill, M.; Velíková, M.; Kubátová, J.; Kancheva, L.; Kazihnitková, H.; Hruškovičová, H.; Pospíšilová, H.; Rácz, B.; et al. Chronic Cigarette Smoking Alters Circulating Sex Hormones and Neuroactive Steroids in Premenopausal Women. Physiol. Res. 2012, 61, 97–111. [Google Scholar] [CrossRef]
- Hirko, K.A.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E.; Eliassen, A.H. Alcohol Consumption in Relation to Plasma Sex Hormones, Prolactin, and Sex Hormone–Binding Globulin in Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2943–2953. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, K.A.; Eliassen, A.H.; Hankinson, S.E.; Rosner, B.A.; Tamimi, R.M. Circulating Hormones and Mammographic Density in Premenopausal Women. Horm. Cancer 2018, 9, 117–127. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Potischman, N.; Malone, K.E.; Dorgan, J.F.; Daling, J.; Schairer, C.; Brinton, L.A. Serum levels of sex hormones and breast cancer risk in premenopausal women: A case–control study (USA). Cancer Causes Control. 2004, 15, 45–53. [Google Scholar] [CrossRef]
- Alberg, A.J.; Gordon, G.B.; Genkinger, J.M.; Hoffman, S.C.; Selvin, E.; Comstock, G.W.; Helzlsouer, K.J. Serum dehydroepiandrosterone and dehydroepiandrosterone sulfate and risk of melanoma or squamous cell carcinoma of the skin. Anticancer Res. 2002, 21, 4051–4054. [Google Scholar]
- Kellner, M.; Muhtz, C.; Weinås, Å.; Ćurić, S.; Yassouridis, A.; Wiedemann, K. Impact of physical or sexual childhood abuse on plasma DHEA, DHEA-S and cortisol in a low-dose dexamethasone suppression test and on cardiovascular risk parameters in adult patients with major depression or anxiety disorders. Psychiatry Res. 2018, 270, 744–748. [Google Scholar] [CrossRef]
- Porsová-Dutoit, I.; Sulcová, J.; Stárka, L. Do DHEA/DHEAS play a protective role in coronary heart disease? Physiol. Res. 2000, 49 (Suppl. 1), S43–S56. [Google Scholar]
- Bednarek-Tupikowska, G.; Tworowska-Bardzińska, U.; Tupikowski, K. Effects of estrogen and estrogen-progesteron on serum nitric oxide metabolite concentrations in post-menopausal women. J. Endocrinol. Investig. 2008, 31, 877–881. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef]

| Timepoint Comparison | Class | Difference | p Value | Adjusted p Value | Significance | |
|---|---|---|---|---|---|---|
| Early Follicular | Mid-Follicular | Realigned | −1.5863 | 0.1340 | 0.2010 | ns |
| Early Follicular | Periovulatory | Realigned | −1.0568 | 0.3090 | 0.3635 | ns |
| Early Follicular | Early Luteal | Realigned | 3.8236 | 0.0020 | 0.0050 | ** |
| Early Follicular | Mid-Luteal | Realigned | 5.6265 | 0.0001 | 0.0004 | *** |
| Early Follicular | Late Luteal | Realigned | 0.6995 | 0.5000 | 0.5357 | ns |
| Mid-Follicular | Periovulatory | Realigned | 0.6328 | 0.5360 | 0.5360 | ns |
| Mid-Follicular | Early Luteal | Realigned | 5.0998 | 0.0002 | 0.0008 | ** |
| Mid-Follicular | Mid-Luteal | Realigned | 7.1353 | 0.0000 | 0.0001 | *** |
| Mid-Follicular | Late Luteal | Realigned | 1.7301 | 0.1090 | 0.1817 | ns |
| Periovulatory | Early Luteal | Realigned | 3.3780 | 0.0050 | 0.0107 | ** |
| Periovulatory | Mid-Luteal | Realigned | 5.7557 | 0.0001 | 0.0004 | *** |
| Periovulatory | Late Luteal | Realigned | 1.4247 | 0.1850 | 0.2523 | ns |
| Early Luteal | Mid-Luteal | Realigned | 2.1868 | 0.0510 | 0.0956 | ns |
| Early Luteal | Late Luteal | Realigned | −1.0646 | 0.3150 | 0.3635 | ns |
| Mid-Luteal | Late Luteal | Realigned | −4.9848 | 0.0006 | 0.0017 | ** |
| Early Follicular | Mid-Follicular | Imputed | −1.5191 | 0.1528 | 0.2495 | ns |
| Early Follicular | Periovulatory | Imputed | 0.0491 | 0.9619 | 0.9619 | ns |
| Early Follicular | Early Luteal | Imputed | 2.2421 | 0.0586 | 0.1256 | ns |
| Early Follicular | Mid-Luteal | Imputed | 4.5609 | 0.0004 | 0.0026 | ** |
| Early Follicular | Late Luteal | Imputed | 0.8492 | 0.4148 | 0.4786 | ns |
| Mid-Follicular | Periovulatory | Imputed | 1.0254 | 0.3411 | 0.4651 | ns |
| Mid-Follicular | Early Luteal | Imputed | 2.9968 | 0.0312 | 0.0937 | ns |
| Mid-Follicular | Mid-Luteal | Imputed | 5.4979 | 0.0000 | 0.0007 | ** |
| Mid-Follicular | Late Luteal | Imputed | 1.8137 | 0.0910 | 0.1706 | ns |
| Periovulatory | Early Luteal | Imputed | 2.3709 | 0.0496 | 0.1240 | ns |
| Periovulatory | Mid-Luteal | Imputed | 3.7107 | 0.0045 | 0.0168 | * |
| Periovulatory | Late Luteal | Imputed | 0.7108 | 0.5007 | 0.5365 | ns |
| Early Luteal | Mid-Luteal | Imputed | 1.5299 | 0.1664 | 0.2495 | ns |
| Early Luteal | Late Luteal | Imputed | −0.8781 | 0.4139 | 0.4786 | ns |
| Mid-Luteal | Late Luteal | Imputed | −4.7800 | 0.0014 | 0.0068 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J.; Sun, J.; Dang, N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites 2022, 12, 941. https://doi.org/10.3390/metabo12100941
Hamidovic A, Soumare F, Naveed A, Davis J, Sun J, Dang N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites. 2022; 12(10):941. https://doi.org/10.3390/metabo12100941
Chicago/Turabian StyleHamidovic, Ajna, Fatimata Soumare, Aamina Naveed, John Davis, Jiehuan Sun, and Nhan Dang. 2022. "Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research" Metabolites 12, no. 10: 941. https://doi.org/10.3390/metabo12100941






