The Beneficial Effect of a Healthy Dietary Pattern on Androgen Deprivation Therapy-Related Metabolic Abnormalities in Patients with Prostate Cancer: A Meta-Analysis Based on Randomized Controlled Trials and Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Assessment of the Risk of Biases
2.5. Statistical Analysis
3. Results
3.1. Literature Data
3.2. Study Characteristics
3.3. Risk Assessment of Bias in Included Studies
3.4. Effect of Healthy DP on Glucose Metabolism
3.5. Effect of Healthy DP on Lipid Metabolism
3.5.1. BMI, BFM, and BLM
3.5.2. Plasma Lipid Parameters
3.6. Effect of Healthy DP on BP
3.7. Effect of Healthy DP on Fatigue
3.8. Effect of Healthy DP on PSA
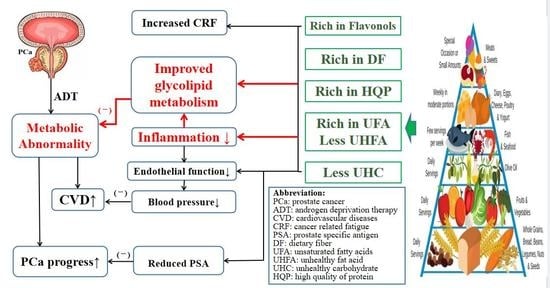
4. Discussion
4.1. Effect of Healthy DP on Glycolipid Metabolism
4.1.1. Glucose Metabolism
4.1.2. Lipid Metabolism
4.2. Effect of Healthy DP on Blood Pressure
4.3. Effect of Healthy DP on Cancer Related Fatigue
4.4. Effect of Healthy DP on PSA
5. Conclusions
6. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 6, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.M.; Kuo, Y.F.; Shahinian, V.B. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol. Oncol. 2011, 29, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, V.; Lorenzo, G.D.; Tartarone, A.; Aieta, M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: An unresolved controversy. Crit. Rev. Oncol. Hematol. 2013, 86, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cai Jianliang, D.; Gang, S.H. Review on metabolism and nutrition after androgen deprivation therapy in prostate patients. Electron. J. Metab. Nutr. Cancer 2020, 2, 236–239. [Google Scholar]
- Yeung, S.S.Y.; Kwan, M.; Woo, J. Healthy Diet for Healthy Aging. Nutrients 2021, 12, 4310. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 2, 496. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 2, 348. [Google Scholar] [CrossRef]
- Millen, B.E.; Abrams, S.; Adams-Campbell, L.; Anderson, C.A.; Brenna, J.T.; Campbell, W.W.; Clinton, S.; Hu, F.; Nelson, M.; Neuhouser, M.L.; et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv. Nutr. 2016, 7, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Nobes, J.P.; Langley, S.; Klopper, T.; Russell-Jones, D.; Laing, R.W. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012, 109, 1495–1502. [Google Scholar] [CrossRef]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.; Saxton, J.M.; Rosario, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 66, e51–e52. [Google Scholar] [CrossRef]
- Focht, B.C.; Lucas, A.R.; Grainger, E.; Simpson, C.; Fairman, C.M.; Thomas-Ahner, J.M.; Buell, J.; Monk, J.P.; Mortazavi, A.; Clinton, S.K. Effects of a Group-Mediated Exercise and Dietary Intervention in the Treatment of Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: Results From the IDEA-P Trial. Ann. Behav. Med. 2018, 52, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Jenkins, D.G.; Wright, O.R.L. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial. Clin. Nutr. 2021, 40, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Howard, L.; Allen, J.; Smith, J.; Stout, J.; Aronson, W.; Inman, B.A.; Armstrong, A.J.; George, D.; Westman, E.; et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: Carbohydrate and prostate study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019, 22, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.E.; Tew, G.A.; Fairhurst, C.; Bourke, L.; Saxton, J.M.; Winter, E.M.; Rosario, D.J. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br. J. Cancer 2016, 114, 401–408. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, Immunity, and Hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- O’Neill, R.F.; Haseen, F.; Murray, L.J.; O’Sullivan, J.M.; Cantwell, M.M. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J. Cancer Surviv. 2015, 9, 431–440. [Google Scholar] [CrossRef]
- Chaplow, Z.; Focht, B.; Lucas, A.; Grainger, E.; Simpson, C.; Fairman, C. Effects of a Lifestyle Intervention on Change in Body Composition in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy. JCSM Clin. Rep. 2020, 5, 52–60. [Google Scholar] [CrossRef]
- Baguley, B.J.; Adlard, K.; Jenkins, D.; Wright, O.R.L.; Skinner, T.L. Mediterranean Style Dietary Pattern with High Intensity Interval Training in Men with Prostate Cancer Treated with Androgen Deprivation Therapy: A Pilot Randomised Control Trial. Int. J. Environ. Res. Public Health 2022, 19, 5709. [Google Scholar] [CrossRef]
- Muthusamy, T.; Murugesan, P.; Balasubramanian, K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective akt phosphorylation in target tissues of adult male rat. Metabolism 2009, 58, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V. Testosterone a key factor in gender related metabolic syndrome. Obes. Rev. 2018, 19, 557. [Google Scholar] [CrossRef] [PubMed]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.M.; Mourtzakis, M. Glucose impairments and insulin resistance in prostate cancer: The role of obesity, nutrition and exercise. Obes. Rev. 2018, 19, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Chuy, K.L.; Ji, C.Y.; Bates, M.; Steingart, R.M. Cardiovascular and Metabolic Effects of Androgen-Deprivation Therapy for Prostate Cancer. J. Oncol. Pract. 2018, 14, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, J.; Wang, Z.; Li, Y.; Li, M. Effects of a normal caloric high-protein diet on metabolic parameters and gastrointestinal hormones in obese and type 2 diabetic patients. Chin. J. Clin. Nutr. 2013, 3, 172–177. [Google Scholar]
- Wang, L.L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.Y.; Huang, Y.H.; Wang, X.H. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Doughty, K.N.; Del Pilar, N.X.; Audette, A.; Katz, D.L. Lifestyle Medicine and the Management of Cardiovascular Disease. Curr. Cardiol. Rep. 2017, 19, 116. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Weight Loss Associated With Consumption of Apples: A Review. J. Am. Coll. Nutr. 2018, 37, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.; Basu, A.; Kremers, W.K.; et al. Randomized Controlled Trial of a MUFA or Fiber-Rich Diet on Hepatic Fat in Prediabetes. J. Clin. Endocr. Metab. 2017, 102, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.; Bhat, R. Quercetin: A Bioactive Compound Imparting Cardiovascular and Neuroprotective Benefits: Scope for Exploring Fresh Produce, Their Wastes, and By-Products. Biology 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Ciumrnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, T.C.; Rchian, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Carr, R.D.; Larsen, M.O.; Jelic, K.; Lindgren, O.; Vikman, J.; Holst, J.J.; Deacon, C.F.; Ahrén, B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J. Clin. Endocr. Metab. 2010, 95, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Knop, F.K.; Aaboe, K.; Vilsbøll, T.; Vølund, A.; Holst, J.J.; Krarup, T.; Madsbad, S. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes. Metab. 2012, 14, 500–510. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, G.; Sun, B.; Zhao, G.; Liu, D.; Sun, J.; Liu, C.; Guo, H. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol. Lett. 2015, 9, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.H.; Wu, S.; Zhang, M. Metabolic complications of androgen deprivation therapy and its intervention management. Natl. J. Androl. 2018, 3, 277–281. [Google Scholar]
- Neefjes, E.C.W.; van den Hurk, R.M.; Blauwhoff-Buskermolen, S.; van der Vorst, M.J.D.L.; Becker-Commissaris, A.; de van der Schueren, M.A.E.; Buffart, L.M.; Verheul, H.M. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 623–629. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory Biomarkers and Fatigue during Radiation Therapy for Breast and Prostate Cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef] [Green Version]
- He, H.B.; Zhao, Z.G.; Pu, Y.F.; Chen, J.; Ni, Y.X.; Zhong, J.; Liu, H.Y.; Li, Y.S.; Yan, Z.C.; Liu, D.Y.; et al. Relationship of different types of abdominal obesity to risk of metabolic syndrom. Natl. Med. J. 2008, 18, 1251–1254. [Google Scholar]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Chinese Group of Clinical Guidelines for Prevention and Treatment of Type Diabetes Mellitus in the Elderly GEAM. Clinical Guidelines for Prevention and Treatment of Type 2 Diabetes Mellitus in the Elderly in China (2022 Edition). Chin. J. Intern. Med. 2022, 61, 12–50. [Google Scholar]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: Two large prospective studies in US men and women. Eur. J. Epidemiol. 2018, 33, 1113–1123. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2010, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 6, 965–983. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yan, G.S. Effects of Dietary Fiber on Intestinal Microecology Related to Obesity. Food Nutr. China 2020, 9, 12–16. [Google Scholar]
- Adam, C.L.; Thomson, L.M.; Williams, P.A.; Ross, A.W. Soluble Fermentable Dietary Fibre (Pectin) Decreases Caloric Intake, Adiposity and Lipidaemia in High-Fat Diet-Induced Obese Rats. PLoS ONE 2015, 10, e140392. [Google Scholar] [CrossRef] [Green Version]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, H.; Nishizawa, M.; Nakagawa, A.; Nakano, S.; Kigoshi, T.; Uchida, K. Testosterone inhibits tumor necrosis factor-α-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002, 530, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Turner, L.; Poole, K.; Faithfull, S.; Griffin, B.A. Current and future strategies for the nutritional management of cardiometabolic complications of androgen deprivation therapy for prostate cancer. Nutr Res. Rev. 2017, 30, 220–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, D.W.; Davis, C.K.; Irving, B.A.; Rodriguez, J.; Barrett, E.J.; Weltman, A.; Taylor, A.G.; Gaesser, G.A. A High-Carbohydrate, High-Fiber Meal Improves Endothelial Function in Adults With the Metabolic Syndrome. Diabetes Care 2006, 29, 2313–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qinhui, Y.; Wei, Z.; Xiaolei, Z.; Susu, D.; Fan, Y.; Xiaonan, L. Omega 3-long chain polyunsaturated fatty acids ameliorate metabolic abnormalities in adulthood induced by early overnutrition in rats. Chin. J. Endocrinol. Metab. 2020, 1, 63–71. [Google Scholar]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef] [Green Version]
- Paschoin, D.; Hassan, B.J.; Riechelmann, R.; Giglio, A.D. Cancer-related fatigue: A review. Rev. Assoc. Méd. Bras. 2011, 57, 206–214. [Google Scholar]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef] [Green Version]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2013, 1, 51–59. [Google Scholar] [CrossRef]
- Huang, J. Guidelines for the Diagnosis and Treatment of Urological and Andrological Diseases in China (2019); Science Press: Beijing, China, 2020. [Google Scholar]









| Author Year Country | Patients (n) | Age (y) (M ± SD)/ Median (IQR) | Duration ADT (Months) (M ± SD)/ Median (IQR) | Measures of Intervention | DOI (weeks) | Outcomes | |
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Bourke et al. [11] (2014, UK) | 50/50 | D: 71 ± 6 C: 71 ± 8 | D: 33 ± 33 C: 30 ± 30 | Diet: reduction in fat <25% of total energy, ≥5 portions/d fruit and vegetables, increased fiber consumption, decreased refined carbohydrates and limiting alcohol intake to 1–2 units/d; Others: nutrition advice pack provided to participants; small-group healthy-eating seminars lasting 20 min/2 weeks; Exercise: supervised and self-directed 3 aerobic and resistance exercise sessions/week. | Usual care | 12 | BMI, BFM, BLM, BP, Fatigue, PSA |
| Chaplow et al. [20] (2020, USA) | 14/16 | D: 67.9 ± 7.9 C: 64.3 ± 6.1 | D: 26.0 ± 25.5 C: 20.4 ± 21.0 | Diet: rich in whole grains, vegetables, and fruits; limited processed high-fat, low-nutrient dense foods; reduced red and processed meats; and overall caloric intake levels. Others: eight group-based nutritional counselling sessions with a registered dietitian and two individualized phone sessions. Exercise: resistance and aerobic exercise. | Usual care | 12 | BFM, BLM |
| Focht et al. [12] (2018, USA) | 16/16 | D: 69.4 ± 9.0 C: 64.5 ± 8.6 | D: 32.2 ± 27.3 C: 15.3 ± 19.4 | Diet: rich in whole grains, vegetables, and fruits; limited processed high-fat, low-nutrient dense foods; reduced intake of red and processed meats; overall caloric intake levels. Others: ten (30 min) nutritional counseling sessions with a registered dietitian. Setting a group including four to eight patients. Exercise: resistance and aerobic exercise. | Usual care | 12 | BMI |
| Gilbert et al. [15] (2016, UK) | 25/25 | D: 70.1 ± 5.3 C: 70.4 ± 9.2 | D: 19 (12, 36) C: 18 (9, 25) | Diet: reduction in fat intake <25% of total energy intake, ≥5 portions/d fruit and vegetables, increased fiber consumption, decreased refined carbohydrates and limiting alcohol intake to 1–2 units/d; Others: nutrition-advice pack provided to participants; small-group healthy-eating seminars lasting 20 min/2 weeks; exercise: supervised and self-directed three aerobic and resistance exercise sessions/week. | Usual care | 12 | BMI, BFM, Lipids, BP |
| Nobes et al. [10] (2011, UK) | 20/20 | D: 71 (58, 80)C: 70 (56–84) | NA | Dietary advice was given in concordance with the low glycemic index diet; Others: a comprehensive guidebook; Exercise: aerobic exercise. | Usual care | 24 | HbA1c, HOMA-IR, BMI, BFM, Lipids, BP |
| Baguley et al. [13] (2020, Australia) | 12/11 | D: 66.6 ± 7.6 C: 65.1 ± 7.9 | D: 36.4 ± 38.3 C: 31.0 ± 32.2 | MED: total energy composition of 45–65% carbohydrate, 20–35% fat, saturated fat <10%, and 15–25% protein sources. A dietary energy reduction if BMI ≥25 kg/m2; Others: face-to-face, 30–45 min nutrition consultations with an accredited practicing dietitian every 2 weeks for 12 weeks to promote dietary behavior change. | Usual care | 12 | BMI, BFM, BLM, Fatigue |
| Baguley et al. [21] (2022, Australia) | 12/11 | D: 66.6 ± 7.6 C: 65.1 ± 7.9 | D: 36.4 ± 38.3 C: 31.0 ± 32.2 | 0–20 w: MED: the content of diet intervention was the same as MED of Baguley et al. (2020). Exercise: high intensity interval training three times/week for 12–20 weeks: | Usual care | 20 | BMI, BFM, BLM, Fatigue |
| Freedland et al. [14] (2019, USA) | 11/18 | D: 66 (61, 76) C: 66 (56, 70) | NA | LCD: limiting carbohydrate ≤20 g/d, providing a list of LCD foods and a list of moderate/high carbohydrate foods to limit. Sample menus and recipes were also provided. Others: coached by the dietitian in person or by phone weekly for months 0–3 and biweekly for months 4–6; Exercise: walking ≥30 min/d for ≥5 d/week. | Usual care | 24 | HOMA-IR, BMI, BFM, Lipids, PSA |
| O’Neill et al. [19] (2015, UK) | 47/47 | D: 69.7 ± 6.8 C: 69.9 ± 7.0 | D: 26.4 ± 32.4 C: 19.3 ± 18.6 | Diet: ≥5 servings/d vegetables and fruits, fat 30–35% and saturated fat <10%, polyunsaturated fat 10%, limited processed meats, 25–35 g of fiber/d, ≤28 units/week of alcohol, limited foods high in salt and/or sugar. Others: individually tailored dietary guidebook, phone contact every 2 weeks for months 0–3 and every 3 weeks thereafter; Exercise: walking at a brisk pace ≥30 min/d and ≥5 d/week in line with UK physical activity guidelines. | Usual care | 24 | BMI, BFM, Fatigue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wu, L.; Qian, C.; Ju, Y.; Liu, T.; Chen, Y.; Wang, X. The Beneficial Effect of a Healthy Dietary Pattern on Androgen Deprivation Therapy-Related Metabolic Abnormalities in Patients with Prostate Cancer: A Meta-Analysis Based on Randomized Controlled Trials and Systematic Review. Metabolites 2022, 12, 969. https://doi.org/10.3390/metabo12100969
Wang L, Wu L, Qian C, Ju Y, Liu T, Chen Y, Wang X. The Beneficial Effect of a Healthy Dietary Pattern on Androgen Deprivation Therapy-Related Metabolic Abnormalities in Patients with Prostate Cancer: A Meta-Analysis Based on Randomized Controlled Trials and Systematic Review. Metabolites. 2022; 12(10):969. https://doi.org/10.3390/metabo12100969
Chicago/Turabian StyleWang, Lili, Lifen Wu, Chunya Qian, Yang Ju, Ting Liu, Yushan Chen, and Xiaohua Wang. 2022. "The Beneficial Effect of a Healthy Dietary Pattern on Androgen Deprivation Therapy-Related Metabolic Abnormalities in Patients with Prostate Cancer: A Meta-Analysis Based on Randomized Controlled Trials and Systematic Review" Metabolites 12, no. 10: 969. https://doi.org/10.3390/metabo12100969
APA StyleWang, L., Wu, L., Qian, C., Ju, Y., Liu, T., Chen, Y., & Wang, X. (2022). The Beneficial Effect of a Healthy Dietary Pattern on Androgen Deprivation Therapy-Related Metabolic Abnormalities in Patients with Prostate Cancer: A Meta-Analysis Based on Randomized Controlled Trials and Systematic Review. Metabolites, 12(10), 969. https://doi.org/10.3390/metabo12100969