LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction and Preparation of Samples
2.3. Measurement of TPC, TFC, and TCT
2.4. Measurement of Antioxidant Activities
2.5. LC-ESI-QTOF-MS/MS Characterization and Semi-Quantification of Phenolic Metabolites
2.6. Statistical Analysis
3. Results and Discussion
3.1. Measurement of Total Polyphenols (TPC, TFC, and TCT)
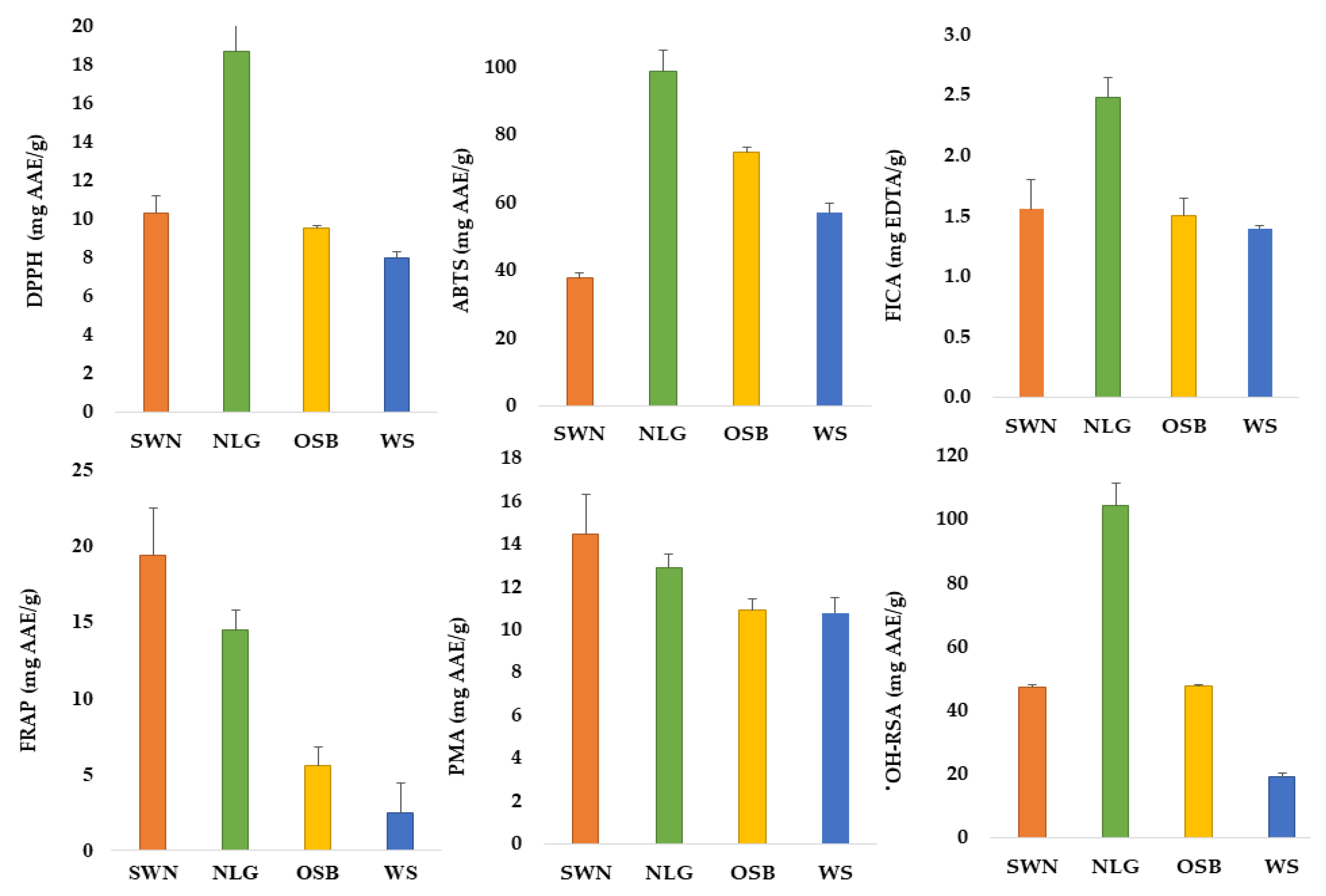
3.2. Antioxidant Potential of Australian Native Plants
3.3. Pearson Correlation and Biplot Analysi of Phenolic Contents and Antioxidant Activities
3.4. LC-MS/MS Identification of Bioactive Phenolic Metabolites from Australian Native Plants
3.4.1. Phenolic Acids
Benzoic Acids and Their Derivatives
Hydroxycinnamic Acids and Derivatives
3.4.2. Flavonoids
Flavanols
Flavones and Flavanones
Flavonols, Chalcones, and Dihydrochalcones
3.4.3. Isoflavonoids
3.4.4. Lignans and Stilbenes
3.4.5. Other Polyphenols
3.5. Distribution of Phenolic Metabolites in Australian Native Plants
3.6. Heatmap Hierarchical Clustering of Quantified Phenolic Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vélez, P.R.C.; Ali, A.; Fournier-Level, A.; Dunshea, F.; Jusuf, P.R. P06-04 Antioxidant activity and embryotoxicity of Citrus australasica, Tasmannia lanceolata and Diploglottis australis extracts in zebrafish. Toxicol. Lett. 2022, 368, S114. [Google Scholar] [CrossRef]
- Kiloni, S.M.; Akhtar, A.; Cáceres-Vélez, P.R.; Dunshea, F.; Jusuf, P. P06-05 Zebrafish embryo acute toxicity and antioxidant characterization of native Australian plants: Towards safe and effective glaucoma treatments. Toxicol. Lett. 2022, 368, S115. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Sze, D.M.Y. Antioxidant and cytoprotective activities of native Australian fruit polyphenols. Food Res. Int. 2011, 44, 2034–2040. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Haridas, V.; Arntzen, C.J.; Gutterman, J.U. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappaB by inhibiting both its nuclear localization and ability to bind DNA. Proc. Natl. Acad. Sci. USA 2001, 98, 11557–11562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottrell, J.J.; Le, H.H.; Artaiz, O.; Iqbal, Y.; Suleria, H.A.; Ali, A.; Celi, P.; Dunshea, F.R. Recent advances in the use of phytochemicals to manage gastrointestinal oxidative stress in poultry and pigs. Anim. Prod. Sci. 2022, 62, 1140–1146. [Google Scholar] [CrossRef]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A Natural Feed Additive for Poultry Health and Production-A Review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 111951, in press. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of Mango, Apple and Banana Fruit Peels as Prebiotics and Functional Ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. In vitro bioaccessibility of phenolic compounds and alpha-glucosidase inhibition activity in yoghurts enriched with mango peel powder. Food Biosci. 2022, 50, 102011. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; BK, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and Characterization of Phenolic Compounds from Australian Grown Bananas and Their Antioxidant Capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Ee, K.Y.; Agboola, S.; Rehman, A.; Zhao, J. Characterisation of phenolic components present in raw and roasted wattle (Acacia victoriae Bentham) seeds. Food Chem. 2011, 129, 816–821. [Google Scholar] [CrossRef]
- Hannachi, H.; Elfalleh, W.; Ennajeh, I.; Laajel, M.; Khouja, M.-L.; Ferchichi, A.; Nasri, N. Chemicals profiling and antioxidants activities of Acacia seeds. J. Med. Plants Res. 2011, 5, 6869–6875. [Google Scholar] [CrossRef]
- Sommano, S.; Caffin, N.; Kerven, G. Screening for antioxidant activity, phenolic content, and flavonoids from Australian native food plants. Int. J. Food Prop. 2013, 16, 1394–1406. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.M.A.N.; Nadeem, M.; Safdar, M.N.; Jabbar, S.; Mahmood, S.; Murtaza, M.A.; Ameer, K.; Ibrahim, S.A. Antioxidant Activity and Phenolic Content of Sonication- and Maceration-Assisted Ethanol and Acetone Extracts of Cymbopogon citratus Leaves. Separations 2022, 9, 244. [Google Scholar] [CrossRef]
- Godwin, A.; Daniel, G.A.; Shadrack, D.; Elom, S.A.; Afua, N.; Ab, K.; Godsway, B.; Joseph, K.G.; Sackitey, N.O.; Isaak, K.B. Determination of elemental, phenolic, antioxidant and flavonoid properties of Lemon grass (Cymbopogon citratus Stapf). Int. Food Res. J. 2014, 21, 1971–1979. [Google Scholar]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhandl, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Ali, A.; Fournier-Level, A.; Dunshea, F.R.; Jusuf, P.R. Phytochemical and Safety Evaluations of Finger Lime, Mountain Pepper, and Tamarind in Zebrafish Embryos. Antioxidants 2022, 11, 1280. [Google Scholar] [CrossRef]
- Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Gülçın, İ.; Oktay, M.; Kıreçcı, E.; Küfrevıoǧlu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Pyracantha coccinea M. Roem. and their antioxidant capacity. Cell. Mol. Biol. 2021, 67, 201–211. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Yang, W.-J.; Li, D.-P.; Li, J.-K.; Li, M.-H.; Chen, Y.-L.; Zhang, P.-Z. Synergistic antioxidant activities of eight traditional Chinese herb pairs. Biol. Pharm. Bull. 2009, 32, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Lin, C.-C.; Lin, W.-C.; Yang, C.-H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011, 75, 1977–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Yang, C.-H.; Wu, P.-S.; Kwan, C.-C.; Chen, Y.-S. Antimicrobial, anti-tyrosinase and antioxidant activities of aqueous aromatic extracts from forty-eight selected herbs. J. Med. Plants Res. 2011, 5, 6203–6209. [Google Scholar]
- Garg, D.; Muley, A.; Khare, N.; Marar, T. Comparative analysis of phytochemical profile and antioxidant activity of some Indian culinary herbs. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 845–854. [Google Scholar]
- Chen, I.-C.; Chang, H.-C.; Yang, H.-W.; Chen, G.-L. Evaluation of total antioxidant activity of several popular vegetables and Chinese herbs: A fast approach with ABTS/H2O2/HRP system in microplates. J. Food Drug. Anal. 2004, 12, 12. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goleniowski, M.; Bonfill, M.; Cusido, R.; Palazón, J. Phenolic acids. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1951–1973. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Passo Tsamo, C.V.; Herent, M.-F.; Tomekpe, K.; Happi Emaga, T.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C.M. Effect of boiling on phenolic profiles determined using HPLC/ESI-LTQ-Orbitrap-MS, physico-chemical parameters of six plantain banana cultivars (Musa sp). J. Food Compos. Anal. 2015, 44, 158–169. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Hou, K.; Wang, Z. Application of Nanotechnology to Enhance Adsorption and Bioavailability of Procyanidins: A Review. Food Rev. Int. 2021, 1–15. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-Based Screening and Identification of Flavonoids and Derived Metabolites in Human Urine after Oral Administration of Exocarpium Citri Grandis Extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.M.; Tian, Y.X.; Liu, Y.; Chen, C.H.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Comparison of flavonoids and isoflavonoids as antioxidants. J. Agric. Food Chem. 2009, 57, 3780–3785. [Google Scholar] [CrossRef]
- Sajid, M.; Stone, S.R.; Kaur, P. Recent Advances in Heterologous Synthesis Paving Way for Future Green-Modular Bioindustries: A Review With Special Reference to Isoflavonoids. Front. Bioeng. Biotechnol. 2021, 9, 673270. [Google Scholar] [CrossRef]
- Liu, R.; Ye, M.; Guo, H.; Bi, K.; Guo, D.A. Liquid chromatography/electrospray ionization mass spectrometry for the characterization of twenty-three flavonoids in the extract of Dalbergia odorifera. Rapid Commun. Mass Spectrom. RCM 2005, 19, 1557–1565. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Moraes, M.N.; Rostagno, M.A.; Meireles, M.A.A. Fast analysis of phenolic terpenes by high-performance liquid chromatography using a fused-core column. Anal. Methods 2014, 6, 7457–7468. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, M.; Sun, M.; Xue, X.; Wang, T.; Cao, W.; Sun, L. Rapid determination of major compounds in the ethanol extract of geopropolis from Malaysian stingless bees, Heterotrigona itama, by UHPLC-Q-TOF/MS and NMR. Molecules 2017, 22, 1935. [Google Scholar] [CrossRef] [PubMed]



| Variables | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) |
|---|---|---|---|
| Sandalwood nuts | 8.54 ± 0.33 b | 2.81 ± 0.21 b | 1.12 ± 0.06 cd |
| Lemongrass | 15.09 ± 0.88 a | 3.07 ± 0.08 a | 1.36 ± 0.08 c |
| Old man saltbush | 6.39 ± 0.25 c | 2.32 ± 0.12 b | 1.99 ± 0.02 b |
| Wattle seeds | 4.17 ± 0.33 d | 0.67 ± 0.05 c | 2.88 ± 0.10 a |
| Variables | TPC | TFC | TCT | DPPH | ABTS | FRAP | RPA | PMA | FICA |
|---|---|---|---|---|---|---|---|---|---|
| TFC | 0.80 | ||||||||
| TCT | −0.72 | −0.96 | |||||||
| DPPH | 0.98 | 0.68 | −0.57 | ||||||
| ABTS | 0.64 | 0.31 | −0.06 | 0.76 | |||||
| FRAP | 0.65 | 0.81 | −0.94 | 0.49 | −0.15 | ||||
| RPA | 0.99 | 0.78 | −0.74 | 0.96 | 0.54 | 0.71 | |||
| PMA | 0.55 | 0.70 | −0.88 | 0.38 | −0.29 | 0.99 | 0.63 | ||
| FICA | 0.97 | 0.64 | −0.52 | 1.00 | 0.78 | 0.45 | 0.94 | 0.34 | |
| •OH-RSA | 0.98 | 0.80 | −0.67 | 0.98 | 0.75 | 0.54 | 0.94 | 0.41 | 0.97 |
| No. | Proposed Compounds | Molecular Formula | RT (min) | Mode of Ionization | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS | Samples |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| Hydroxybenzoic acid | |||||||||
| 1 | * Protocatechuic acid | C7H6O4 | 6.821 | [M−H]− | 153.0193 | 153.0186 | −4.6 | 109 | LG, SWN |
| 2 | * Gallic acid | C7H6O5 | 6.913 | [M−H]− | 169.0142 | 169.0134 | 4.6 | 125 | WS, SWN |
| 3 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 10.169 | [M−H]− | 315.0721 | 315.0735 | 4.6 | 153, 109 | LG |
| 4 | * p-Hydroxybenzoic acid | C7H6O3 | 16.180 | [M−H]− | 137.0244 | 137.0240 | −2.9 | 93 | LG, SWN |
| 5 | * Vanillic acid | C8H8O4 | 17.114 | [M−H]− | 167.0350 | 167.0345 | −3.0 | 152, 123, 108 | LG, SWN, WS |
| 6 | * Ellagic acid | C14H6O8 | 25.216 | ** [M−H]− | 300.9990 | 300.9988 | −0.7 | 284, 257 | SWN, OSB |
| 7 | Punicalin | C34H22O22 | 48.689 | [M+H]+ | 783.0676 | 783.0646 | −3.8 | 765, 737, 675, 617, 169 | OSB |
| Hydroxycinnamic acids | |||||||||
| 8 | Verbascoside A | C31H40O16 | 3.866 | [M−H]− | 667.2243 | 667.2268 | 3.7 | 283, 94 | SWN |
| 9 | 1-Feruloyl-5-caffeoylquinic acid | C26H26O12 | 3.943 | [M−H]− | 529.1351 | 529.1343 | −1.5 | 193, 191, 179, 135 | OSB, LG |
| 10 | 1,2,2′-Triferuloylgentiobiose | C42H46O20 | 3.951 | [M−H]− | 869.2509 | 869.2495 | −1.6 | 693, 517 | WS |
| 11 | 1-Sinapoyl-2-feruloylgentiobiose | C33H40O18 | 3.951 | [M−H]− | 723.2142 | 723.2149 | 1.0 | 529, 499 | WS, OSB |
| 12 | 3-p-Coumaroylquinic acid | C16H18O8 | 4.271 | [M−H]− | 337.0929 | 337.0927 | −0.6 | 191, 119 | WS, LG, OSB, SWN |
| 13 | 3-Sinapoylquinic acid | C18H22O10 | 4.366 | [M−H]− | 397.1140 | 397.1116 | −6.0 | 223, 191 | SWN, WS |
| 14 | Ferulic acid 4-O-glucuronide | C16H18O10 | 6.634 | [M−H]− | 369.0827 | 369.0847 | 5.4 | 193 | WS |
| 15 | * Cinnamic acid | C9H8O2 | 7.678 | [M−H]− | 147.0451 | 147.0446 | −2.2 | 103 | LG |
| 16 | * 3-Caffeoylquinic acid | C16H18O9 | 13.294 | ** [M−H]− | 353.0878 | 353.0874 | −1.1 | 191, 179, 161 | SWN, WS, LG, OSB |
| 17 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 14.524 | ** [M−H]− | 325.0929 | 325.0920 | −2.8 | 163 | LG, OSB |
| 18 | * Ferulic acid | C10H10O4 | 15.335 | [M−H]− | 193.0506 | 193.0499 | −3.6 | 178, 149, 134 | LG |
| 19 | 3-Feruloylquinic acid | C17H20O9 | 15.335 | [M−H]− | 367.1034 | 367.1038 | 1.1 | 193, 191, 134 | LG, SWN, WS |
| 20 | 1-O-Sinapoyl-ꞵ-D-glucose | C17H22O10 | 16.295 | [M−H]− | 385.1140 | 385.1145 | 1.3 | 223, 193 | LG |
| 21 | * Caffeic acid | C9H8O4 | 18.553 | [M−H]− | 179.0350 | 179.0358 | 2.9 | 161, 135 | LG |
| 22 | * Sinapic acid | C11H12O5 | 22.223 | [M−H]− | 223.0612 | 223.0606 | −2.7 | 193, 179, 149, 134 | SWN |
| 23 | * p-Coumaric acid | C9H8O3 | 24.583 | [M−H]− | 163.0400 | 163.0395 | −3.1 | 119 | SWN, LG |
| 24 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 24.731 | ** [M−H]− | 515.1195 | 515.1204 | 2.6 | 191, 179, 135 | LG, OSB |
| 25 | Verbascoside | C29H36O15 | 26.408 | ** [M−H]− | 623.1981 | 623.1985 | 0.6 | 462, 461, 161 | OSB, LG |
| 26 | Hydroxycaffeic acid | C9H8O5 | 26.690 | [M−H]− | 195.0299 | 195.0299 | 0.0 | 177, 151 | SWN |
| 27 | * Syringic Acid | C9H10O5 | 28.680 | [M−H]− | 197.0455 | 197.0455 | 0.0 | 182, 153, 138, 121 | SWN |
| 28 | * Rosmarinic acid | C18H16O8 | 29.245 | [M−H]− | 359.0772 | 359.0781 | 2.5 | 197, 179, 161, 135 | OSB, SWN, LG |
| 29 | 1,2-Diferuloylgentiobiose | C32H38O17 | 29.283 | ** [M−H]− | 693.2036 | 693.2055 | 2.7 | 193, 134 | LG, OSB |
| 30 | Chicoric acid | C22H18O12 | 37.085 | ** [M+H]+ | 475.0871 | 475.0873 | 0.4 | 293, 311 | LG, WS |
| 31 | Caffeic acid 4-O-glucoside | C15H18O9 | 45.661 | [M+H]+ | 343.1024 | 343.1016 | −2.3 | 179 | WS, LG |
| Hydroxyphenylacetic acids | |||||||||
| 32 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 14.301 | [M−H]− | 151.0400 | 151.0400 | 0.0 | 136, 92 | LG, SWN |
| 33 | Homovanillic acid | C9H10O4 | 32.463 | [M−H]− | 181.0506 | 181.0497 | −5.0 | 163 | SWN |
| Flavonoids | |||||||||
| Flavanols | |||||||||
| 34 | Procyanidin trimer C1 | C45H38O18 | 3.871 | [M−H]− | 865.1985 | 865.2049 | 7.4 | 739, 713, 695, 577, 451 | WS |
| 35 | (+)-Catechin 3-O-gallate | C22H18O10 | 3.940 | ** [M−H]− | 441.0827 | 441.0818 | −2.0 | 289 | SWN, WS, LG, OSB |
| 36 | Catechin 3′-glucoside | C21H24O11 | 3.977 | [M−H]− | 451.1246 | 451.1236 | −2.2 | 289 | WS, SWN, OSB, LG |
| 37 | 4′-O-Methylepigallocatechin | C16H16O7 | 4.527 | [M−H]− | 319.0823 | 319.0804 | −6.0 | 289, 245 | WS |
| 38 | 4″-O-Methylepigallocatechin 3-O-gallate | C23H20O11 | 9.014 | ** [M−H]− | 471.0933 | 471.0927 | −1.3 | 305, 183, 139 | LG, OSB, WS |
| 39 | * Procyanidin B2 | C30H26O12 | 18.412 | ** [M−H]− | 577.1351 | 577.1366 | 2.6 | 451, 425, 407, 289 | WS, LG |
| 40 | Epigallocatechin 3-O-gallate-7-O-glucoside-4″-O-glucuronide | C34H36O22 | 18.553 | ** [M−H]− | 795.1625 | 795.1625 | 0.0 | 305, 289, 245 | LG, WS, OSB |
| 41 | 4′,4″-Dimethylepigallocatechin gallate | C24H22O11 | 19.568 | [M−H]− | 485.1089 | 485.1106 | 3.5 | 305, 289 | LG, WS |
| 42 | * (+)-Catechin | C15H14O6 | 41.850 | ** [M−H]− | 289.0717 | 289.0710 | −2.4 | 245, 205, 179 | SWN, WS |
| 43 | 4′-O-Methyl-(-)-epicatechin 3′-O-glucuronide | C22H24O12 | 45.631 | [M−H]− | 479.1195 | 479.1208 | 2.7 | 461, 435, 303 | LG, SWN |
| 44 | (-)-Epigallocatechin 3′-O-glucuronide | C21H22O13 | 57.250 | ** [M+H]+ | 483.1133 | 483.1142 | 1.9 | 307 | OSB, WS, LG |
| 45 | (+)-Gallocatechin | C15H14O7 | 68.325 | [M+H]+ | 307.0813 | 307.0803 | −3.3 | 291 | OSB |
| Flavanones | |||||||||
| 46 | Hesperidin | C28H34O15 | 4.210 | [M−H]− | 609.1825 | 609.1821 | −0.7 | 301 | LG |
| 47 | Hesperetin 3′-O-glucuronide | C22H22O12 | 4.828 | [M−H]− | 477.1038 | 477.1030 | −1.7 | 301 | SWN, WS |
| 48 | Narirutin 4′-O-glucoside | C33H42O19 | 4.924 | [M−H]− | 741.2247 | 741.2230 | −2.3 | 579 | LG, OSB, WS |
| 49 | Neoeriocitrin | C27H32O15 | 15.335 | [M−H]− | 595.1668 | 595.1655 | −2.2 | 459, 287, 151 | LG, SWN, WS |
| 50 | Didymin | C28H34O14 | 25.479 | [M−H]− | 593.1876 | 593.1880 | 0.7 | 447, 285, 151 | SWN, WS, LG, OSB |
| 51 | Naringin | C27H32O14 | 28.576 | [M−H]− | 579.1719 | 579.1699 | −3.5 | 459, 313, 271 | LG, OSB, SWN |
| 52 | Naringin 6′-malonate | C30H34O17 | 38.465 | [M−H]− | 665.1723 | 665.1711 | −1.8 | 579 | OSB |
| 53 | Naringenin | C15H12O5 | 44.409 | [M−H]− | 271.0612 | 271.0623 | 4.1 | 151, 119 | SWN |
| 54 | Naringenin 7-O-glucoside | C21H22O10 | 49.237 | [M−H]− | 433.1140 | 433.1140 | 0.0 | 271 | LG, OSB, WS |
| 55 | Hesperetin 5,7-O-diglucuronide | C28H30O18 | 60.331 | ** [M+H]+ | 655.1505 | 655.1518 | 2.0 | 303 | WS, OSB |
| Flavones | |||||||||
| 56 | Tetramethylscutellarein | C19H18O6 | 4.050 | ** [M−H]− | 341.1030 | 341.1030 | 0.0 | 341 | LG, OSB, WS |
| 57 | Syringetin-3-O-glucoside | C23H24O13 | 7.166 | [M−H]− | 507.1144 | 507.1165 | 4.1 | 345 | LG, OSB, SWN |
| 58 | * Swertisin | C22H22O10 | 10.402 | [M−H]− | 445.1140 | 445.1171 | 7.0 | 325, 297, 282 | WS |
| 59 | Apigenin 6,8-C-arabinoside-C-glucoside | C26H28O14 | 18.932 | [M−H]− | 563.1406 | 563.1402 | −0.7 | 269 | LG, OSB, WS |
| 60 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 18.932 | ** [M−H]− | 447.0933 | 447.0931 | −0.4 | 429, 301, 163 | LG, WS |
| 61 | Chrysoeriol 7-O-glucoside | C22H22O11 | 21.244 | [M−H]− | 461.1089 | 461.1101 | 2.6 | 289 | WS |
| 62 | Rhoifolin | C27H30O14 | 22.656 | [M−H]− | 577.1563 | 577.1615 | 9.0 | 431, 269 | LG |
| 63 | Tricin 7-neohesperidoside | C29H34O16 | 22.886 | [M−H]− | 637.1774 | 637.1785 | 1.7 | 329 | OSB, WS |
| 64 | 8-Methoxyluteolin | C16H12O7 | 25.360 | [M−H]− | 315.0510 | 315.0513 | 1.0 | 300 | SWN, WS |
| 65 | 3,4′,7-Tetrahydroxyflavone | C15H10O6 | 26.486 | [M−H]− | 285.0404 | 285.0404 | 0.0 | SWN, LG | |
| 66 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 27.921 | ** [M−H]− | 593.1512 | 593.1517 | 0.8 | 269 | LG, OSB |
| 67 | 6-Hydroxyluteolin | C15H10O7 | 28.680 | ** [M−H]− | 301.0353 | 301.0348 | −1.7 | 285 | SWN, LG |
| 68 | 6-Hydroxyflavone | C15H10O3 | 36.774 | [M−H]− | 237.0557 | 237.0568 | 4.6 | 237 | LG |
| 69 | Lonicerjaponin B | C34H44O17 | 41.301 | [M−H]− | 723.2505 | 723.2499 | −0.8 | 723 | LG |
| 70 | Cirsilineol | C18H16O7 | 65.460 | [M+H]+ | 345.0969 | 345.0956 | −3.8 | 303, 312, 297, 284 | LG, OSB, WS |
| 71 | * Diosmin | C28H32O15 | 69.786 | ** [M+H]+ | 609.1814 | 609.1783 | −5.1 | 301 | WS, LG |
| Flavonols | |||||||||
| 72 | Myricetin 3-O-arabinoside | C20H18O12 | 4.950 | ** [M−H]− | 449.0725 | 449.0736 | 2.4 | 317 | LG, OSB |
| 73 | Quercetin 3-O-arabinoside | C20H18O11 | 5.145 | ** [M−H]− | 433.0776 | 433.0792 | 3.7 | 301 | SWN, OSB, WS |
| 74 | 3-Methoxysinensetin | C21H22O8 | 12.608 | [M−H]− | 401.1242 | 401.1241 | −0.2 | 327, 209 | OSB, SWN, LG |
| 75 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 14.751 | [M−H]− | 609.1461 | 609.1464 | 0.5 | 447, 285 | LG, SWN |
| 76 | Kaempferol 3-O-xylosyl-glucoside | C26H28O15 | 16.783 | [M−H]− | 579.1355 | 579.1353 | −0.3 | 285 | LG, OSB |
| 77 | 3-Methoxynobiletin | C22H24O9 | 17.998 | [M−H]− | 431.1347 | 431.1357 | 2.3 | 401, 387 | OSB |
| 78 | Kaempferide | C16H11O6 | 18.000 | [M−H]− | 298.0483 | 298.0498 | 5.0 | 283, 151 | LG |
| 79 | Myricetin 3-O-rhamnoside | C21H20O12 | 24.447 | [M−H]− | 463.0882 | 463.0896 | 3.0 | 317 | SWN |
| 80 | Isorhamnetin 3-O-rutinoside | C28H32O16 | 24.961 | ** [M−H]− | 623.1617 | 623.1667 | 8.0 | 315 | OSB |
| 81 | Spinacetin 3-O-glucosyl-(1->6)-glucoside | C29H34O18 | 28.842 | [M−H]− | 669.1672 | 669.1671 | −0.1 | 669 | OSB, SWN, LG |
| 82 | 6,8-Dihydroxykaempferol | C15H10O8 | 33.805 | [M−H]− | 317.0303 | 317.0314 | 3.5 | 285 | SWN |
| 83 | Taxifolin 4′,7-diglucoside | C27H32O17 | 35.838 | ** [M−H]− | 627.1567 | 627.1542 | −4.0 | 303 | OSB |
| 84 | Kaempferol 3,7,4′-O-triglucoside | C33H40O21 | 35.986 | ** [M−H]− | 771.1989 | 771.1989 | 0.0 | 285 | LG, OSB |
| 85 | Myricetin 3-O-glucoside | C21H20O13 | 50.735 | ** [M+H]+ | 481.0977 | 481.0980 | 0.6 | 319 | OSB, LG |
| 86 | Kaempferol 3-O-rhamnoside | C21H19O10 | 52.448 | ** [M+H]+ | 432.1051 | 432.1037 | −3.2 | 287 | OSB, LG |
| 87 | Quercetin 4′-O-glucuronide | C21H18O13 | 54.978 | [M+H]+ | 479.0820 | 479.0814 | −1.3 | 303 | OSB |
| 87 | 3,7-Dimethylquercetin | C17H14O7 | 44.677 | ** [M+H]+ | 331.0813 | 331.0804 | −2.7 | 316, 301 | LG, OSB, SWN |
| 88 | * Tricin | C17H14O7 | 44.677 | ** [M−H]− | 331.0813 | 331.0804 | −2.7 | 316, 301 | LG |
| 89 | Quercetin 3-O-(6″-malonyl)-glucoside | C24H22O15 | 64.747 | ** [M−H]− | 549.0886 | 549.0900 | 2.5 | 301 | WS |
| 90 | Quercetin 3-O-xylosyl-glucuronide | C26H26O17 | 64.800 | [M+H]+ | 611.1243 | 611.1224 | −3.1 | 303 | LG |
| 91 | (-)-Epicatechin-epicatechin-galactoside | C36H34O15 | 66.810 | ** [M+H]+ | 707.1971 | 707.2000 | 4.1 | 291 | WS, LG |
| 92 | Quercetin 3-O-glucosyl-xyloside | C26H28O16 | 66.838 | [M+H]+ | 597.1450 | 597.1469 | 3.2 | 303 | LG |
| 93 | Kaempferol 3-O-glucoside | C21H20O11 | 23.730 | [M−H]− | 447.093261 | 447.0947 | 3.2 | 285 | LG |
| 94 | Isorhamnetin 3-O-glucuronide | C22H20O13 | 68.303 | [M+H]+ | 493.0977 | 493.0994 | 3.4 | 317 | LG |
| 95 | Quercetin 3-O-xylosyl-rutinoside | C32H38O20 | 68.764 | ** [M+H]+ | 743.2029 | 743.2019 | −1.3 | 303 | WS, SWN, OSB |
| 96 | Kaempferol 3-O-glucuronide | C21H18O12 | 69.376 | [M+H]+ | 463.0871 | 463.0880 | 1.9 | 287 | WS, OSB |
| Isoflavonoids | |||||||||
| 97 | Daidzin 4′-O-glucuronide | C27H28O15 | 3.075 | ** [M+H]+ | 593.1501 | 593.1491 | −1.7 | 431 | OSB, LG |
| 98 | 6″-O-Malonyldaidzin | C24H22O12 | 6.634 | ** [M−H]− | 501.1038 | 501.1015 | −4.6 | 253 | WS, LG |
| 99 | 3′-O-Methylviolanone | C18H18O6 | 11.024 | [M−H]− | 329.1030 | 329.1025 | −1.5 | 285, 163 | OSB |
| 100 | Violanone | C17H16O6 | 11.105 | * [M−H]− | 315.0874 | 315.0867 | −2.2 | 300, 285, 135 | OSB, LG |
| 101 | Equol 7-O-glucuronide | C21H22O9 | 12.021 | [M−H]− | 417.1191 | 417.1198 | 1.7 | 241 | OSB, SWN |
| 102 | Dihydrobiochanin A | C16H14O5 | 12.216 | [M−H]− | 285.0768 | 285.0767 | −0.4 | 203, 175 | OSB, WS |
| 103 | Sativanone | C17H16O5 | 12.341 | ** [M−H]− | 299.0925 | 299.0933 | 2.7 | 284, 269, 225 | OSB |
| 104 | Glycitein 4′-O-glucuronide | C22H20O11 | 15.551 | ** [M−H]− | 459.0933 | 459.0918 | −3.3 | 441, 283, 267 | WS, OSB, LG |
| 105 | 6″-O-Acetyldaidzin | C23H22O10 | 16.735 | ** [M−H]− | 457.1140 | 457.1155 | 3.3 | 439, 253 | LG, SWN, WS |
| 106 | Genistein 4′,7-O-diglucuronide | C27H26O17 | 18.356 | ** [M−H]− | 621.1097 | 621.1073 | −3.9 | 445, 427, 269 | LG, OSB |
| 107 | 3′-Hydroxymelanettin | C16H12O6 | 18.519 | [M−H]− | 299.0561 | 299.0556 | −1.7 | 284 | LG |
| 108 | 6″-O-Acetylgenistin | C23H22O11 | 18.553 | ** [M−H]− | 473.1089 | 473.1120 | 6.6 | 269, 59 | LG |
| 109 | Daidzein 7-O-glucuronide | C21H18O10 | 19.221 | [M−H]− | 429.0827 | 429.0855 | 6.5 | 253 | LG, WS |
| 110 | Formononetin 7-O-glucuronide | C22H20O10 | 30.443 | [M−H]− | 443.0983 | 443.0994 | 2.5 | 269 | OSB, LG |
| 111 | 3′,4′,5,7-Tetrahydroxyisoflavanone | C15H12O6 | 33.384 | [M−H]− | 287.0561 | 287.0547 | −4.9 | 269, 179 | SWN |
| 112 | 3′-Hydroxy-O-desmethylangolensin | C15H14O5 | 43.831 | [M−H]− | 273.0768 | 273.0769 | 0.4 | 273 | SWN |
| 113 | 6″-O-Malonylglycitin | C25H24O13 | 44.248 | [M−H]− | 531.1144 | 531.1160 | 3.0 | 283, 267 | LG |
| 114 | 6″-O-Malonylgenistin | C24H22O13 | 44.567 | ** [M+H]+ | 519.1133 | 519.1126 | −1.3 | 271 | WS, OSB, WS |
| Chalcones and Dihydrochalcones | |||||||||
| 115 | Phloridzin | C21H24O10 | 4.309 | [M−H]− | 435.1297 | 435.1305 | 1.8 | WS, SWN | |
| 116 | Phloretin 2′-O-xylosyl-glucoside | C26H32O14 | 17.703 | ** [M−H]− | 567.1719 | 567.1726 | 1.2 | 273 | LG, SWN |
| 117 | Phloretin 2′-O-glucuronide | C21H22O11 | 23.564 | [M−H]− | 449.1089 | 449.1073 | −3.6 | 273, 149 | LG, OSB, SWN |
| 118 | Xanthohumol | C21H22O5 | 26.055 | [M−H]− | 353.1394 | 353.1392 | −0.6 | 233, 119 | LG |
| Stilbenes | |||||||||
| 119 | Dihydroresveratrol | C14H14O3 | 4.160 | [M−H]− | 229.0870 | 229.0880 | 4.4 | 229 | WS |
| 120 | Piceatannol 3-O-glucoside | C20H22O9 | 4.428 | [M−H]− | 405.1191 | 405.1161 | −7.4 | 243 | WS |
| 121 | Piceatannol | C14H12O4 | 4.674 | [M−H]− | 243.0663 | 243.0679 | 6.6 | 225, 201 | WS |
| 122 | trans-Resveratrol 3-O-glucuronide | C20H20O9 | 10.023 | [M−H]− | 403.1034 | 403.1041 | 1.7 | 227 | OSB, WS |
| 123 | Resveratrol 3-O-glucoside | C20H22O8 | 23.802 | [M−H]− | 389.1242 | 389.1248 | 1.5 | 227 | LG, SWN |
| Lignans | |||||||||
| 124 | Todolactol A | C20H24O7 | 5.816 | [M−H]− | 375.1449 | 375.1443 | −1.6 | 357, 329 | WS |
| 125 | Sesamin | C20H18O6 | 13.905 | [M−H]− | 353.1030 | 353.1015 | −4.2 | 338, 163 | OSB, SWN, LG |
| 126 | 7-Oxomatairesinol | C20H20O7 | 15.308 | [M−H]− | 371.1136 | 371.1127 | −2.4 | 358, 343, 328 | OSB |
| 127 | Conidendrin | C20H20O6 | 16.050 | [M−H]− | 355.1187 | 355.1175 | −3.4 | 337, 311, 309, 295 | OSB |
| 128 | Sesaminol 2-O-triglucoside | C36H46O22 | 17.812 | [M−H]− | 829.2408 | 829.2448 | 4.8 | 369 | LG |
| 129 | Trachelogenin | C21H24O7 | 21.905 | [M−H]− | 387.1449 | 387.1464 | 3.9 | 343, 329, 137 | OSB, LG |
| 130 | 1-Acetoxypinoresinol | C22H24O8 | 21.957 | [M−H]− | 415.1398 | 415.1394 | −1.0 | 357 | LG |
| 131 | Schisandrin | C24H32O7 | 28.132 | [M−H]− | 431.2075 | 431.2064 | −2.6 | 431 | OSB |
| 132 | Deoxyschisandrin | C24H32O6 | 51.605 | [M−H]− | 415.2126 | 415.2126 | 0.0 | 402, 347, 361, 301 | SWN, OSB |
| 133 | Schisandrin C | C22H24O6 | 61.129 | [M−H]− | 383.1500 | 383.1505 | 1.3 | 367, 339, 311 | WS, LG |
| 134 | 7-Hydroxysecoisolariciresinol | C22H30O5 | 66.881 | [M−H]− | 373.2020 | 373.2017 | −0.8 | 357, 355, 327, 221 | SWN |
| Other polyphenols | |||||||||
| Coumarins and derivatives | |||||||||
| 135 | Bergapten | C12H8O4 | 6.562 | ** [M−H]− | 215.0350 | 215.0353 | 1.4 | 171 | WS, OSB |
| 136 | Scopoletin | C10H8O4 | 14.304 | [M−H]− | 191.0350 | 191.0340 | −5.2 | 175, 147 | SWN, WS |
| 137 | Umbelliferone | C9H6O3 | 17.236 | [M−H]− | 161.0244 | 161.0246 | 1.2 | 133, 117 | LG |
| 138 | Esculetin | C9H6O4 | 18.068 | [M−H]− | 177.0193 | 177.0205 | 6.8 | 133, 105 | LG, SWN |
| 139 | Esculin | C15H16O9 | 19.490 | [M−H]− | 339.0721 | 339.0731 | 2.9 | 177 | LG |
| 140 | Isopimpinellin | C13H10O5 | 26.790 | [M−H]− | 245.0455 | 245.0456 | 0.4 | 215, 201 | SWN, OSB, WS |
| Cyclitol | |||||||||
| 141 | Quinic Acid | C7H12O6 | 15.086 | [M−H]− | 191.0561 | 191.0551 | −5.2 | 173, 127, 85 | LG, SWN |
| Hydroxybenzoketones | |||||||||
| 142 | Norathyriol | C13H8O6 | 3.909 | [M−H]− | 259.0248 | 259.0255 | 2.7 | 241, 231 | OSB, SWN, WS |
| Hydroxyphenylpropenes | |||||||||
| 143 | [6]-Gingerol | C17H32O4 | 65.884 | [M−H]− | 299.2228 | 299.2220 | −2.7 | 299 | WS, OSB, LG |
| Phenolic terpenes | |||||||||
| 144 | Carnosic acid | C20H28O4 | 59.906 | [M−H]− | 331.1915 | 331.1930 | 4.5 | 287 | OSB, LG |
| 145 | Carvacrol | C10H14O | 66.059 | [M−H]− | 149.0972 | 149.0966 | −4.0 | 131, 105 | WS, LG |
| 146 | Rosmanol | C20H26O5 | 66.950 | [M−H]− | 345.1707 | 345.1716 | 2.6 | 301 | LG, WS, OSB |
| Tyrosols | |||||||||
| 147 | Hydroxytyrosol | C8H10O3 | 17.703 | ** [M−H]− | 153.0557 | 153.0557 | 0.0 | 123, 109 | LG, WS |
| 148 | Oleoside dimethylester | C18H26O11 | 24.097 | [M−H]− | 417.1402 | 417.1407 | 1.2 | 255, 223 | LG |
| 149 | Oleoside 11-methylester | C17H24O11 | 25.331 | [M−H]− | 403.1246 | 403.1229 | −4.2 | 223, 165 | LG, OSB |
| 150 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 65.148 | [M+H]+ | 317.1231 | 317.1240 | 2.8 | 153, 123 | OSB, WS |
| Other polyphenols | |||||||||
| 151 | * Pyrogallol | C6H6O3 | 6.821 | [M−H]− | 125.0244 | 125.0242 | −1.6 | 107, 97, 79 | LG, WS, OSB |
| 152 | Catechol | C6H6O2 | 6.927 | [M−H]− | 109.0295 | 109.0295 | 0.0 | 65 | LG, SWN |
| 153 | Salvianolic acid B | C36H30O16 | 32.818 | [M−H]− | 717.1461 | 717.1497 | 5.0 | 520, 357, 179, 161 | SWN |
| 154 | Phlorin | C12H16O8 | 63.601 | [M+H]+ | 289.0918 | 289.0907 | −3.8 | 125 | LG |
| 155 | Salvianolic acid G | C20H18O10 | 69.870 | [M+H]+ | 419.0973 | 419.0993 | 4.8 | 399, 237, 219, 179 | LG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites 2022, 12, 1016. https://doi.org/10.3390/metabo12111016
Ali A, Cottrell JJ, Dunshea FR. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites. 2022; 12(11):1016. https://doi.org/10.3390/metabo12111016
Chicago/Turabian StyleAli, Akhtar, Jeremy J. Cottrell, and Frank R. Dunshea. 2022. "LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants" Metabolites 12, no. 11: 1016. https://doi.org/10.3390/metabo12111016
APA StyleAli, A., Cottrell, J. J., & Dunshea, F. R. (2022). LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites, 12(11), 1016. https://doi.org/10.3390/metabo12111016









