Metabolomic Profiles Associated with Obesity and Periodontitis during Pregnancy: Cross-Sectional Study with Proton Nuclear Magnetic Resonance (1H-NMR)-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval Statement
2.2. Sampling Method
2.3. Co-Variables
2.4. Collection and Storage of Saliva and Plasma Samples
2.5. Sample Preparation
2.6. 1H-NMR Spectroscopy Analysis
2.7. Statistical Analysis and Bioinformatics
3. Results
3.1. Characteristics of the Study Population
3.2. Metabolomic Profiling of Plasma and Saliva
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Barrington, G.; Bettiol, S.; Barnett, T.; Crocombe, L. Is Overweight/Obesity a Risk Factor for Periodontitis in Young Adults and Adolescents?: A Systematic Review. Obes. Rev. 2018, 19, 852–883. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.G.; Pola, N.M.; Casarin, M.; Muniz, F.W.M.G. Association between Clinical Measures of Gingival Inflammation and Obesity in Adults: Systematic Review and Meta-Analyses. Clin. Oral Investig. 2021, 25, 4281–4298. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontology 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- Fuhler, G.M. The Immune System and Microbiome in Pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Foratori-Junior, G.A.; Pereira, P.R.; Gasparoto, I.A.; de Carvalho Sales-Peres, S.H.; Storniolo de Souza, J.M.; Khan, S. Is Overweight Associated with Periodontitis in Pregnant Women? Systematic Review and Meta-Analysis. Jpn. Dent. Sci. Rev. 2022, 58, 41–51. [Google Scholar] [CrossRef]
- Barnes, V.M.; Ciancio, S.G.; Shibly, O.; Xu, T.; Devizio, W.; Trivedi, H.M.; Guo, L.; Jönsson, T.J. Metabolomics Reveals Elevated Macromolecular Degradation in Periodontal Disease. J. Dent. Res. 2011, 90, 1293–1297. [Google Scholar] [CrossRef]
- Aimetti, M.; Cacciatore, S.; Graziano, A.; Tenori, L. Metabonomic Analysis of Saliva Reveals Generalized Chronic Periodontitis Signature. Metabolomics 2012, 8, 465–474. [Google Scholar] [CrossRef]
- Barnes, V.M.; Kennedy, A.D.; Panagakos, F.; Devizio, W.; Trivedi, H.M.; Jönsson, T.; Guo, L.; Cervi, S.; Scannapieco, F.A. Global Metabolomic Analysis of Human Saliva and Plasma from Healthy and Diabetic Subjects, with and without Periodontal Disease. PLoS ONE 2014, 9, e105181. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Bamba, T.; Fukusaki, E.; Amano, A. Prediction of Periodontal Inflammation via Metabolic Profiling of Saliva. J. Dent. Res. 2016, 95, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Rzeznik, M.; Triba, M.N.; Levy, P.; Jungo, S.; Botosoa, E.; Duchemann, B.; le Moyec, L.; Bernaudin, J.-F.; Savarin, P.; Guez, D. Identification of a Discriminative Metabolomic Fingerprint of Potential Clinical Relevance in Saliva of Patients with Periodontitis Using 1H Nuclear Magnetic Resonance (NMR) Spectroscopy. PLoS ONE 2017, 12, e0182767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.P.; Saxena, M.; Saimbi, C.S.; Arif, J.M.; Roy, R. Metabolic Profiling by 1H NMR Spectroscopy of Saliva Shows Clear Distinction between Control and Diseased Case of Periodontitis. Metabolomics 2017, 13, 137. [Google Scholar] [CrossRef]
- García-Villaescusa, A.; Morales-Tatay, J.M.; Monleón-Salvadó, D.; González-Darder, J.M.; Bellot-Arcis, C.; Montiel-Company, J.M.; Almerich-Silla, J.M. Using NMR in Saliva to Identify Possible Biomarkers of Glioblastoma and Chronic Periodontitis. PLoS ONE 2018, 13, e0188710. [Google Scholar] [CrossRef]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Tenori, L.; Cacciatore, S.; Aimetti, M. Analysis of Salivary Phenotypes of Generalized Aggressive and Chronic Periodontitis through Nuclear Magnetic Resonance-Based Metabolomics. J. Periodontol. 2018, 89, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Gawron, K.; Wojtowicz, W.; Łazarz-Bartyzel, K.; Łamasz, A.; Qasem, B.; Mydel, P.; Chomyszyn-Gajewska, M.; Potempa, J.; Mlynarz, P. Metabolomic Status of The Oral Cavity in Chronic Periodontitis. In Vivo 2019, 33, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Romano, F.; Meoni, G.; Manavella, V.; Baima, G.; Mariani, G.M.; Cacciatore, S.; Tenori, L.; Aimetti, M. Effect of Non-Surgical Periodontal Therapy on Salivary Metabolic Fingerprint of Generalized Chronic Periodontitis Using Nuclear Magnetic Resonance Spectroscopy. Arch. Oral Biol. 2019, 97, 208–214. [Google Scholar] [CrossRef]
- Citterio, F.; Romano, F.; Meoni, G.; Iaderosa, G.; Grossi, S.; Sobrero, A.; Dego, F.; Corana, M.; Berta, G.N.; Tenori, L.; et al. Changes in the Salivary Metabolic Profile of Generalized Periodontitis Patients after Non-Surgical Periodontal Therapy: A Metabolomic Analysis Using Nuclear Magnetic Resonance Spectroscopy. J. Clin. Med. 2020, 9, 3977. [Google Scholar] [CrossRef]
- Goodson, J.M. Disease Reciprocity between Gingivitis and Obesity. J. Periodontol. 2020, 91, S26–S34. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Song, Y.; Lee, H.A.; Kim, S.; Chung, J. Metabolic Phenotyping of Saliva to Identify Possible Biomarkers of Periodontitis Using Proton Nuclear Magnetic Resonance. J. Clin. Periodontol. 2021, 48, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Na, H.S.; Kim, S.; Kim, S.; Yu, Y.; Kim, S.Y.; Kim, H.-J.; Lee, J.Y.; Lee, J.-H.; Chung, J. Molecular Subgroup of Periodontitis Revealed by Integrated Analysis of the Microbiome and Metabolome in a Cross-Sectional Observational Study. J. Oral Microbiol. 2021, 13, 1902707. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, A.; Kuboniwa, M.; Katakami, N.; Furuno, M.; Nishizawa, H.; Omori, K.; Taya, N.; Ishikawa, A.; Mayumi, S.; Tanaka Isomura, E.; et al. Saliva and Plasma Reflect Metabolism Altered by Diabetes and Periodontitis. Front. Mol. Biosci. 2021, 8, 742002. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, T.; Liang, Z.; Zhao, L.; Xiong, X.; Xie, K.; Yu, W.; Zeng, X.; Gao, J.; Zhou, Y.; et al. Untargeted and Targeted Gingival Metabolome in Rodents Reveal Metabolic Links between High-fat Diet-induced Obesity and Periodontitis. J. Clin. Periodontol. 2021, 48, 1137–1148. [Google Scholar] [CrossRef]
- Pereira, K.K.Y.; Jara, C.M.; Antunes, G.L.; Gomes, M.S.; Rösing, C.K.; Cavagni, J.; Haas, A.N. Effects of Periodontitis and Periodontal Treatment on Systemic Inflammatory Markers and Metabolic Profile in Obese and Non-obese Rats. J. Periodontol. 2022, 93, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos Fusco, N.; Foratori-Junior, G.A.; Missio, A.L.T.; Jesuino, B.G.; de Carvalho Sales-Peres, S.H. Systemic and Oral Conditions of Pregnant Women with Excessive Weight Assisted in a Private Health System. Int. Dent. J. 2019, 69, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Caracho, R.A.; Foratori-Junior, G.A.; dos Santos Fusco, N.; Jesuino, B.G.; Missio, A.L.T.; de Carvalho Sales-Peres, S.H. Systemic Conditions and Oral Health-Related Quality of Life of Pregnant Women of Normal Weight and Who Are Overweight. Int. Dent. J. 2020, 70, 287–295. [Google Scholar] [CrossRef]
- Foratori-Junior, G.A.; da Silva, B.M.; da Silva Pinto, A.C.; Honório, H.M.; Groppo, F.C.; de Carvalho Sales-Peres, S.H. Systemic and Periodontal Conditions of Overweight/Obese Patients during Pregnancy and after Delivery: A Prospective Cohort. Clin. Oral Investig. 2020, 24, 157–165. [Google Scholar] [CrossRef]
- Foratori-Junior, G.A.; Jesuino, B.G.; Caracho, R.A.; Orenha, E.S.; Groppo, F.C.; SALES-PERES; SHDC. Association between Excessive Maternal Weight, Periodontitis during the Third Trimester of Pregnancy, and Infants’ Health at Birth. J. Appl. Oral Sci. 2020, 28, e20190351. [Google Scholar] [CrossRef]
- Jesuino, B.G.; Foratori-Junior, G.A.; Missio, A.L.T.; Mascoli, L.S.; de Carvalho Sales-Peres, S.H. Periodontal Status of Women with Excessive Gestational Weight Gain and the Association with Their Newborns’ Health. Int. Dent. J. 2020, 70, 396–404. [Google Scholar] [CrossRef]
- Foratori-Junior, G.A.; Missio, A.L.T.; Orenha, E.S.; de Carvalho Sales-Peres, S.H. Systemic Condition, Periodontal Status, and Quality of Life in Obese Women During Pregnancy and After Delivery. Int. Dent. J. 2021, 71, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Foratori-Junior, G.A.; Mosquim, V.; Buzalaf, M.A.R.; de Carvalho Sales-Peres, S.H. Salivary Cytokines Levels, Maternal Periodontitis and Infants’ Weight at Birth: A Cohort Study in Pregnant Women with Obesity. Placenta 2021, 115, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainamo, J.; Bay, I. Problems and Proposals for Recording Gingivitis and Plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-Induced Gingivitis: Case Definition and Diagnostic Considerations. J. Clin. Periodontol. 2018, 45, S44–S67. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.; Parkes, H.G.; Carpenter, G.H.; So, P.-W. Developing and Standardizing a Protocol for Quantitative Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy of Saliva. J. Proteome Res. 2018, 17, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.; Parkes, H.G.; So, P.-W.; Carpenter, G.H. Determining Bacterial and Host Contributions to the Human Salivary Metabolome. J. Oral Microbiol. 2019, 11, 1617014. [Google Scholar] [CrossRef] [Green Version]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Piotto, M.; Saudek, V.; Sklenář, V. Gradient-Tailored Excitation for Single-Quantum NMR Spectroscopy of Aqueous Solutions. J. Biomol. NMR 1992, 2, 661–665. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Clendinen, C.S.; Lee-McMullen, B.; Williams, C.M.; Stupp, G.S.; Vandenborne, K.; Hahn, D.A.; Walter, G.A.; Edison, A.S. 13C NMR Metabolomics: Applications at Natural Abundance. Anal. Chem. 2014, 86, 9242–9250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Wishart, D.S. MSEA: A Web-Based Tool to Identify Biologically Meaningful Patterns in Quantitative Metabolomic Data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [Green Version]
- Bhatta, A.; Yao, L.; Xu, Z.; Toque, H.A.; Chen, J.; Atawia, R.T.; Fouda, A.Y.; Bagi, Z.; Lucas, R.; Caldwell, R.B.; et al. Obesity-Induced Vascular Dysfunction and Arterial Stiffening Requires Endothelial Cell Arginase 1. Cardiovasc. Res. 2017, 113, 1664–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Liebsch, C.; Pitchika, V.; Pink, C.; Samietz, S.; Kastenmüller, G.; Artati, A.; Suhre, K.; Adamski, J.; Nauck, M.; Völzke, H.; et al. The Saliva Metabolome in Association to Oral Health Status. J. Dent. Res. 2019, 98, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.M.; Lima, P.L.; Alves, M.R.A.; Soares, R.D.L.; Kanufre, V.D.C.; Rodrigues, V.D.M.; Starling, A.L.P.; Norton, R.D.C.; Aguiar, M.J.B.D. Overweight/Obesity in Adolescents with Phenylketonuria: Protective and Predisposing Factors. J. Pediatr. 2022, 98, 104–110. [Google Scholar] [CrossRef]
- Baima, G.; Iaderosa, G.; Citterio, F.; Grossi, S.; Romano, F.; Berta, G.N.; Buduneli, N.; Aimetti, M. Salivary Metabolomics for the Diagnosis of Periodontal Diseases: A Systematic Review with Methodological Quality Assessment. Metabolomics 2021, 17, 1. [Google Scholar] [CrossRef]
- Oktay, S.; Bal, O.O.; Kuru, L.; Yarat, A.; Noyan, U. Is Sialic Acid a Promising Marker for Periodontal Diseases? Niger. J. Clin. Pract. 2020, 23, 603–609. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Cope, K.; Risby, T.; Diehl, A.M. Increased Gastrointestinal Ethanol Production in Obese Mice: Implications for Fatty Liver Disease Pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-H.H.; Astley, C.M.; Cole, J.B.; Vedantam, S.; Mercader, J.M.; Metspalu, A.; Fischer, K.; Fortney, K.; Morgen, E.K.; Gonzalez, C.; et al. Integrating Untargeted Metabolomics, Genetically Informed Causal Inference, and Pathway Enrichment to Define the Obesity Metabolome. Int. J. Obes. 2020, 44, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin Resistance and Glycine Metabolism in Humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.F.; Ennis, M.A.; Dyer, R.A.; Lim, K.; Elango, R. Glycine, a Dispensable Amino Acid, Is Conditionally Indispensable in Late Stages of Human Pregnancy. J. Nutr. 2021, 151, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Shi, J.; Zhao, Y.; Yan, F.; Lei, L.; Li, H. Porphyromonas Gingivalis Triggers Inflammatory Responses in Periodontal Ligament Cells by Succinate-Succinate Dehydrogenase–HIF–1α Axis. Biochem. Biophys. Res. Commun. 2020, 522, 184–190. [Google Scholar] [CrossRef] [PubMed]

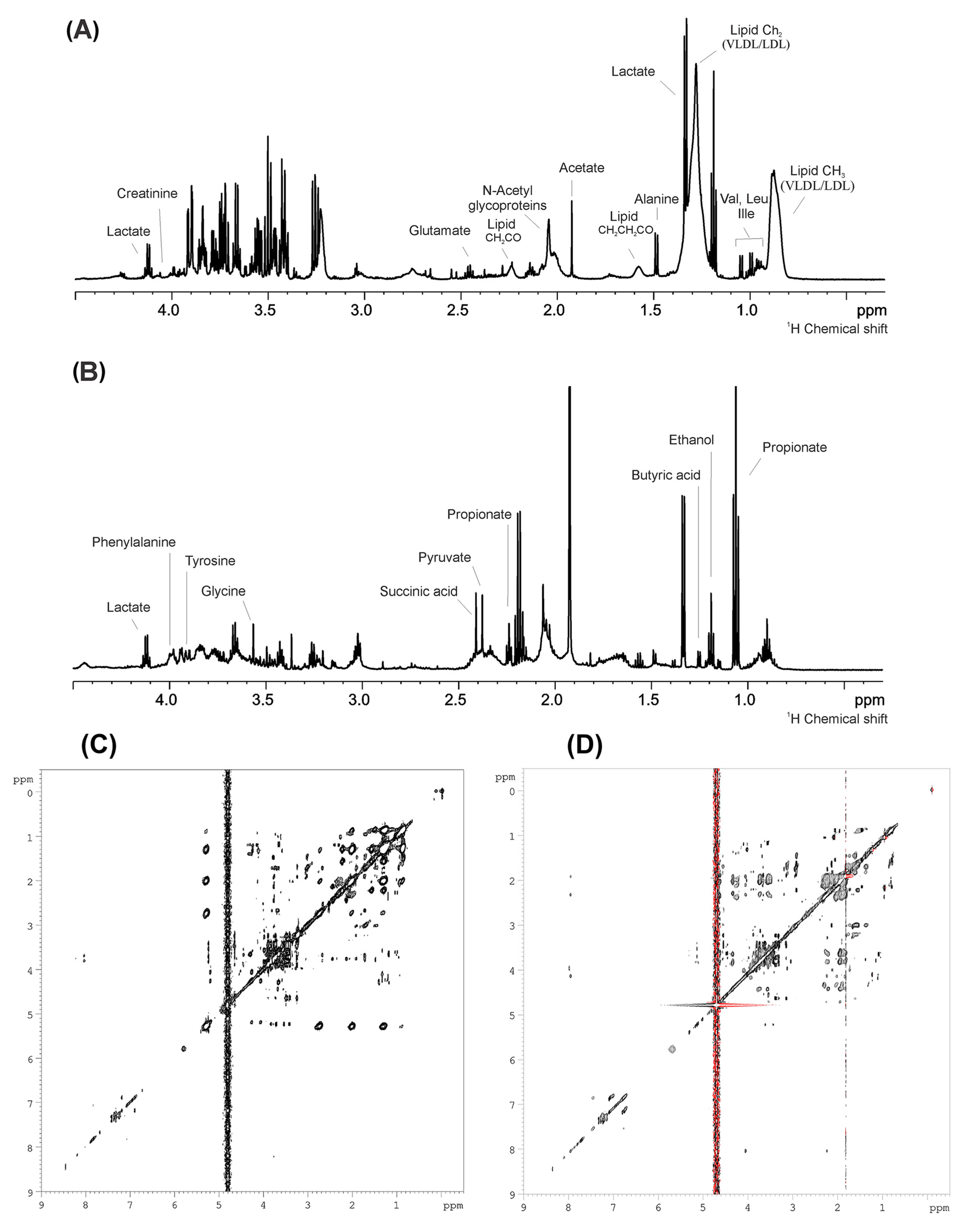

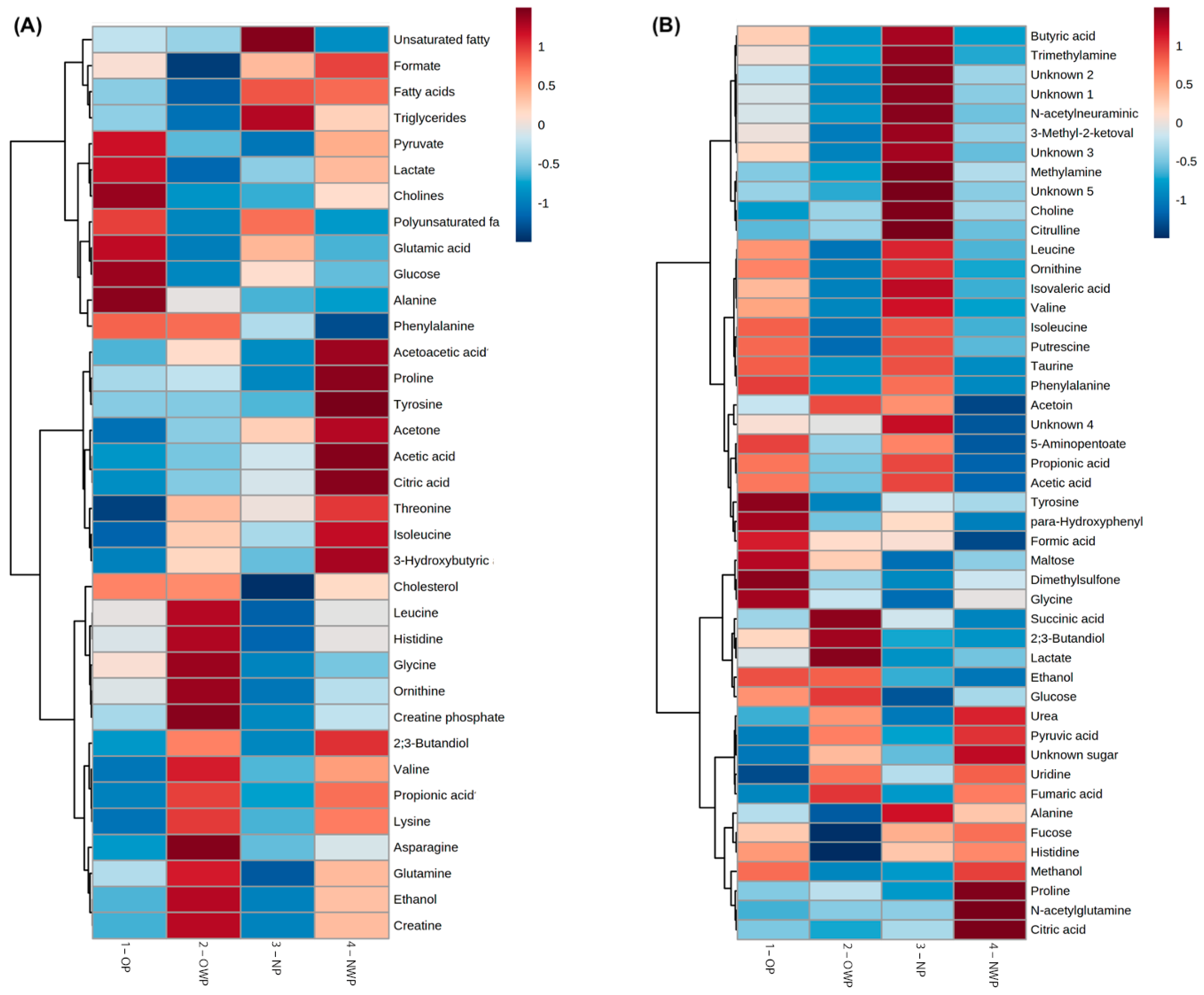
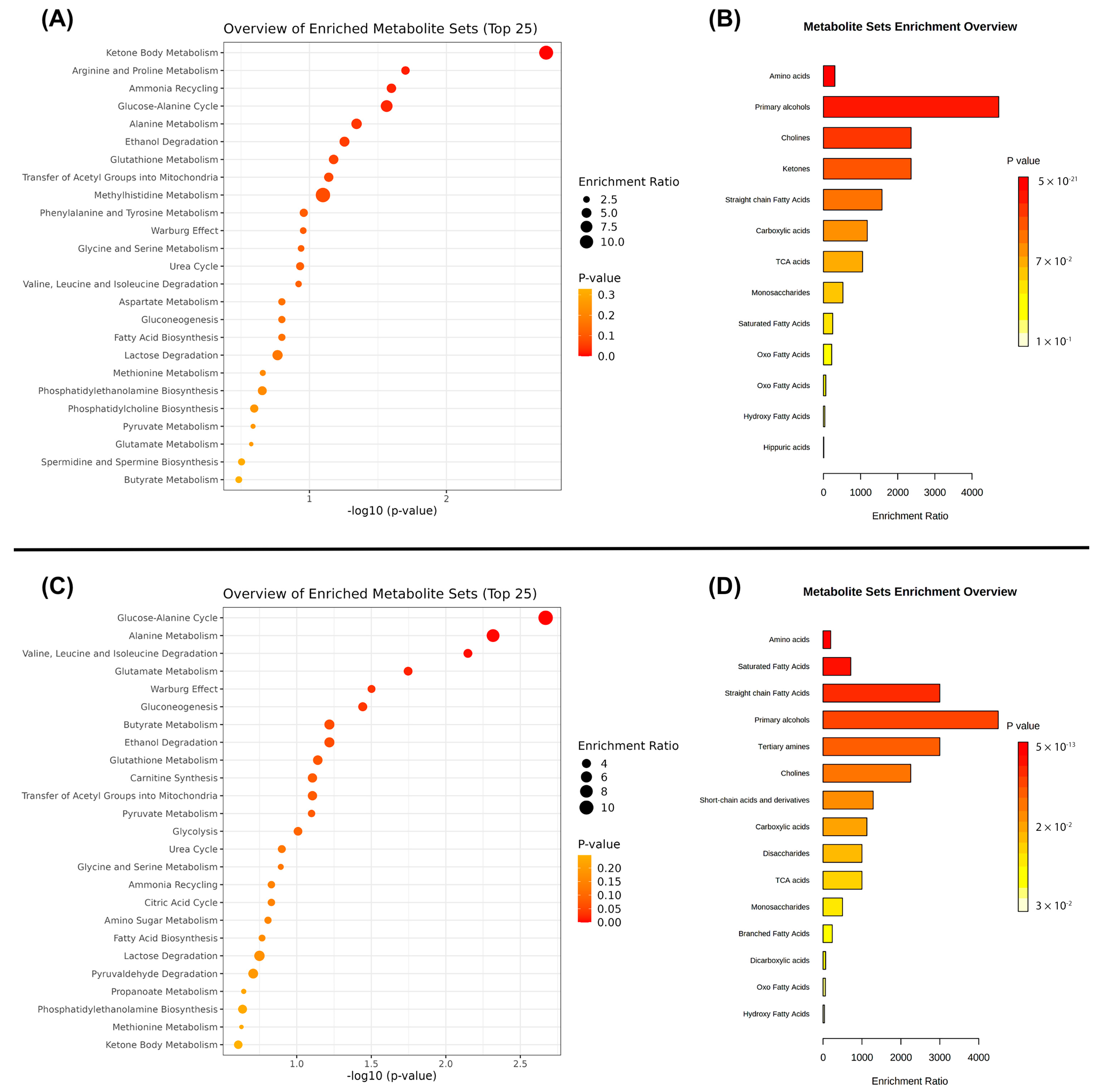
| Variables | OP (n = 20) | OWP (n = 27) | NP (n = 21) | NWP (n = 30) | p |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Median | Median | Median | Median | ||
| (1st–3rd Quartiles) | (1st–3rd Quartiles) | (1st–3rd Quartiles) | (1st–3rd Quartiles) | ||
| Age (years) | 29.85 ± 5.32 | 27.38 ± 6.05 | 25.33 ± 5.06 | 27.16 ± 5.80 | 0.082 * |
| Pre-pregnancy BMI (kg/m2) | 31.43 | 32.51 | 23.30 | 23.27 | <0.001 † |
| (30.09–35.04) | (30.30–36.51) | (20.43–24.80) | (22.26–24.65) | ||
| A | A | B | B | ||
| Pregnancy BMI (kg/m2) | 36.01 | 35.96 | 26.67 | 25.69 | <0.001 † |
| (31.50–39.36) | (32.80–39.92) | (23.99–29.62) | (23.48–29.43) | ||
| A | A | B | B | ||
| Gestational weight gain (kg) | 7.25 ± 6.73 | 8.00 ± 5.60 | 10.38 ± 6.84 | 10.98 ± 5.29 | 0.095 * |
| Daily toothbrushing | 3 | 3 | 3 | 3 | 0.585 † |
| (2–3) | (2–3) | (2–3) | (2–3) | ||
| Daily flossing | 0 | 1 | 0 | 0 | 0.073 † |
| (0–0) | (0–1) | (0–1) | (0–1) | ||
| Dental plaque (%) | 76.59 ± 20.56 | 55.74 ± 25.25 | 77.62 ± 18.19 | 62.71 ± 21.33 | 0.001 * |
| A | B | A | AB | ||
| BOP (%) | 61.31 | 34.22 | 78.66 | 35.18 | <0.001 † |
| (48.62–75.99) | (26.19–46.42) | (65.03–87.22) | (29.76–47.02) | ||
| A | B | A | B | ||
| PPD (mm) | 2.45 | 2.03 | 2.54 | 2.06 | < 0.001 † |
| (2.23–2.64) | (1.98–2.10) | (2.42–2.78) | (2.00–2.16) | ||
| A | B | A | B | ||
| CAL (mm) | 2.45 | 2.06 | 2.54 | 2.08 | <0.001 † |
| (2.25–2.65) | (2.02–2.11) | (2.43–2.80) | (2.01–2.16) | ||
| A | B | A | B | ||
| Periodontitis stages (PS)–n (%) | - | - | - | ||
| I | 11 (55%) | 9 (42.9%) | |||
| II | 8 (40%) | 10 (47.6%) | |||
| III | 1 (5%) | 2 (9.5%) | |||
| IV | 0 | 0 |
| HMDB Card | Metabolites | CS (ppm) | OP (n = 20) Mean ± SD | OWP (n = 27) Mean ± SD | NP (n = 21) Mean ± SD | NWP (n = 30) Mean ± SD | p |
|---|---|---|---|---|---|---|---|
| HMDB0000687 | Leucine 2 | 0.96 | 0.41 ± 0.04 | 0.42 ± 0.05 | 0.39 ± 0.03 | 0.41 ± 0.05 | 0.192 |
| HMDB0000172 | Isoleucine 1,3 | 1.01 | 0.15 ± 0.01 A | 0.16 ± 0.02 AB | 0.16 ± 0.02 AB | 0.17 ± 0.03 B | 0.047 |
| HMDB0000108 | Ethanol 1,2 | 1.17 | 1.30 ± 0.83 | 2.45 ± 4.00 | 1.09 ± 0.51 | 1.89 ± 3.02 | 0.313 |
| - | Fatty acids 1 | 1.17 | 32.47 ± 5.50 | 32.11 ± 8.34 | 33.05 ± 8.07 | 33.01 ± 9.97 | 0.973 |
| HMDB0000011 | 3-Hydroxybutyric acid 1 | 1.20 | 0.47 ± 0.07 | 0.51 ± 0.14 | 0.48 ± 0.08 | 0.55 ± 0.23 | 0.267 |
| HMDB0000161 | Alanine 1 | 1.48 | 0.77 ± 0.09 | 0.74 ± 0.11 | 0.73 ± 0.11 | 0.73 ± 0.10 | 0.676 |
| HMDB0000042 | Acetic acid 1 | 1.92 | 0.66 ± 0.15 | 0.68 ± 0.14 | 0.69 ± 0.15 | 0.77 ± 0.27 | 0.223 |
| HMDB0001659 | Acetone 1 | 2.22 | 0.17 ± 0.04 | 0.18 ± 0.05 | 0.19 ± 0.06 | 0.20 ± 0.07 | 0.406 |
| HMDB0000060 | Acetoacetic acid 1 | 2.27 | 0.13 ± 0.03 | 0.14 ± 0.05 | 0.12 ± 0.02 | 0.16 ± 0.09 | 0.083 |
| HMDB0000168 | Asparagine 2 | 2.87 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.084 |
| HMDB0000214 | Ornithine 2 | 3.05 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.282 |
| HMDB0000097 | Choline 1 | 3.10 | 2.40 ± 0.19 | 2.33 ± 0.29 | 2.33 ± 0.31 | 2.36 ± 0.30 | 0.823 |
| HMDB0000123 | Glycine 1,2 | 3.56 | 0.35 ± 0.06 | 0.37 ± 0.07 | 0.33 ± 0.04 | 0.34 ± 0.07 | 0.231 |
| HMDB0250525 | Creatine phosphate 2 | 3.96 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.407 |
| HMDB0000190 | Lactate 2 | 4.11 | 0.84 ± 0.17 | 0.73 ± 0.17 | 0.76 ± 0.13 | 0.80 ± 0.19 | 0.127 |
| HMDB0000162 | Proline 1 | 4.15 | 0.09 ± 0.04 | 0.10 ± 0.04 | 0.08 ± 0.01 | 0.12 ± 0.09 | 0.127 |
| HMDB0000122 | Glucose 1 | 4.65 | 1.70 ± 0.41 | 1.57 ± 0.23 | 1.63 ± 0.26 | 1.59 ± 0.27 | 0.524 |
| HMDB0000177 | Histidine 2 | 7.08 | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.053 |
| HMDB Card | Metabolites | CS (ppm) | OP (n = 20) Mean ± SD | OWP (n = 27) Mean ± SD | NP (n = 21) Mean ± SD | NWP (n = 30) Mean ± SD | p |
|---|---|---|---|---|---|---|---|
| HMDB0000491 | 3-Methyl-2-ketovaleric acid 2,3 | 0.85 | 0.29 ± 0.08 AB | 0.24 ± 0.04 A | 0.34 ± 0.13 B | 0.27 ± 0.05 A | 0.001 |
| HMDB0000039 | Butyric acid 1,2,3 | 0.89 | 0.51 ± 0.19 AB | 0.37 ± 0.14 A | 0.65 ± 0.33 B | 0.38 ± 0.16 A | <0.001 |
| HMDB0000718 | Isovaleric acid 2,3 | 0.91 | 0.31 ± 0.16 A | 0.20 ± 0.07 B | 0.38 ± 0.19 A | 0.22 ± 0.07 B | <0.001 |
| HMDB0000687 | Leucine 2,3 | 0.97 | 0.10 ± 0.04 A | 0.07 ± 0.01 B | 0.10 ± 0.03 A | 0.08 ± 0.02 B | <0.001 |
| HMDB0000883 | Valine 2,3 | 0.99 | 0.18 ± 0.09 AB | 0.13 ± 0.05 A | 0.20 ± 0.08 B | 0.13 ± 0.05 A | <0.001 |
| HMDB0000172 | Isoleucine 2,3 | 1.01 | 0.10 ± 0.06 A | 0.06 ± 0.02 B | 0.10 ± 0.04 A | 0.07 ± 0.03 AB | 0.008 |
| HMDB0000237 | Propionic acid 1,2,3 | 1.06 | 6.03 ± 3.00 AB | 4.79 ± 2.25 AB | 6.23 ± 3.45 A | 4.10 ± 2.33 B | 0.020 |
| HMDB0003156 | 2,3-Butanediol 1 | 1.14 | 0.31 ± 0.21 | 0.37 ± 0.47 | 0.26 ± 0.09 | 0.25 ± 0.25 | 0.444 |
| HMDB0000108 | Ethanol 1 | 1.18 | 1.51 ± 0.91 | 1.50 ± 1.05 | 1.28 ± 0.60 | 1.23 ± 0.59 | 0.489 |
| HMDB0000161 | Alanine 1 | 1.48 | 0.53 ± 0.16 | 0.49 ± 0.13 | 0.60 ± 0.24 | 0.56 ± 0.17 | 0.210 |
| HMDB0000042 | Acetic acid 1 | 1.92 | 21.08 ± 9.94 | 17.96 ± 6.90 | 21.65 ± 9.50 | 16.30 ± 6.65 | 0.071 |
| HMDB0006029 | N-Acetylglutamine 1 | 2.33 | 1.23 ± 0.32 | 1.25 ± 0.37 | 1.26 ± 0.55 | 1.48 ± 0.55 | 0.166 |
| HMDB0000243 | Pyruvic acid 1 | 2.37 | 1.10 ± 0.29 | 1.19 ± 0.36 | 1.11 ± 0.31 | 1.21 ± 0.36 | 0.610 |
| HMDB0000254 | Succinic acid 1 | 2.41 | 0.91 ± 0.40 | 1.08 ± 1.16 | 0.93 ± 0.66 | 0.86 ± 0.40 | 0.723 |
| HMDB0000906 | Trimethylamine 2,3 | 2.89 | 0.07 ± 0.05 A | 0.05 ± 0.02 A | 0.12 ± 0.09 B | 0.05 ± 0.02 A | <0.001 |
| HMDB0000097 | Choline 1,3 | 3.20 | 0.26 ± 0.08 A | 0.28 ± 0.10 A | 0.36 ± 0.12 B | 0.28 ± 0.06 A | 0.009 |
| HMDB0001875 | Methanol 3 | 3.36 | 0.26 ± 0.25 AB | 0.18 ± 0.07 A | 0.19 ± 0.06 A | 0.27 ± 0.12 B | 0.038 |
| HMDB0000123 | Glycine 1 | 3.56 | 0.58 ± 0.54 | 0.46 ± 0.20 | 0.39 ± 0.16 | 0.47 ± 0.20 | 0.247 |
| HMDB0000190 | Lactate 1 | 4.12 | 1.23 ± 1.23 | 1.62 ± 1.94 | 1.03 ± 0.46 | 1.12 ± 0.59 | 0.344 |
| HMDB0000122 | Glucose 1 | 5.24 | 0.30 ± 0.65 | 0.33 ± 0.52 | 0.18 ± 0.22 | 0.24 ± 0.35 | 0.696 |
| HMDB0000163 | Maltose 1 | 5.41 | 0.41 ± 1.63 | 0.26 ± 0.67 | 0.05 ± 0.06 | 0.15 ± 0.32 | 0.542 |
| HMDB0000159 | Phenylalanine 3 | 7.43 | 0.09 ± 0.05 AB | 0.06 ± 0.02 A | 0.09 ± 0.03 B | 0.06 ± 0.02 A | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foratori-Junior, G.A.; Guennec, A.L.; Fidalgo, T.K.d.S.; Cleaver, L.; Buzalaf, M.A.R.; Carpenter, G.H.; Sales-Peres, S.H.d.C. Metabolomic Profiles Associated with Obesity and Periodontitis during Pregnancy: Cross-Sectional Study with Proton Nuclear Magnetic Resonance (1H-NMR)-Based Analysis. Metabolites 2022, 12, 1029. https://doi.org/10.3390/metabo12111029
Foratori-Junior GA, Guennec AL, Fidalgo TKdS, Cleaver L, Buzalaf MAR, Carpenter GH, Sales-Peres SHdC. Metabolomic Profiles Associated with Obesity and Periodontitis during Pregnancy: Cross-Sectional Study with Proton Nuclear Magnetic Resonance (1H-NMR)-Based Analysis. Metabolites. 2022; 12(11):1029. https://doi.org/10.3390/metabo12111029
Chicago/Turabian StyleForatori-Junior, Gerson Aparecido, Adrien Le Guennec, Tatiana Kelly da Silva Fidalgo, Leanne Cleaver, Marília Afonso Rabelo Buzalaf, Guy Howard Carpenter, and Silvia Helena de Carvalho Sales-Peres. 2022. "Metabolomic Profiles Associated with Obesity and Periodontitis during Pregnancy: Cross-Sectional Study with Proton Nuclear Magnetic Resonance (1H-NMR)-Based Analysis" Metabolites 12, no. 11: 1029. https://doi.org/10.3390/metabo12111029
APA StyleForatori-Junior, G. A., Guennec, A. L., Fidalgo, T. K. d. S., Cleaver, L., Buzalaf, M. A. R., Carpenter, G. H., & Sales-Peres, S. H. d. C. (2022). Metabolomic Profiles Associated with Obesity and Periodontitis during Pregnancy: Cross-Sectional Study with Proton Nuclear Magnetic Resonance (1H-NMR)-Based Analysis. Metabolites, 12(11), 1029. https://doi.org/10.3390/metabo12111029










