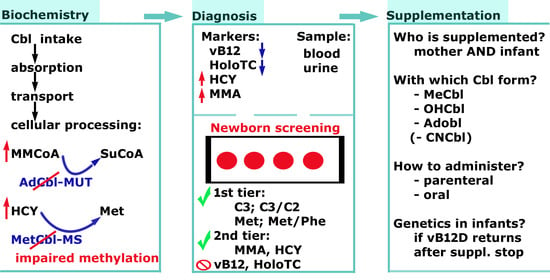
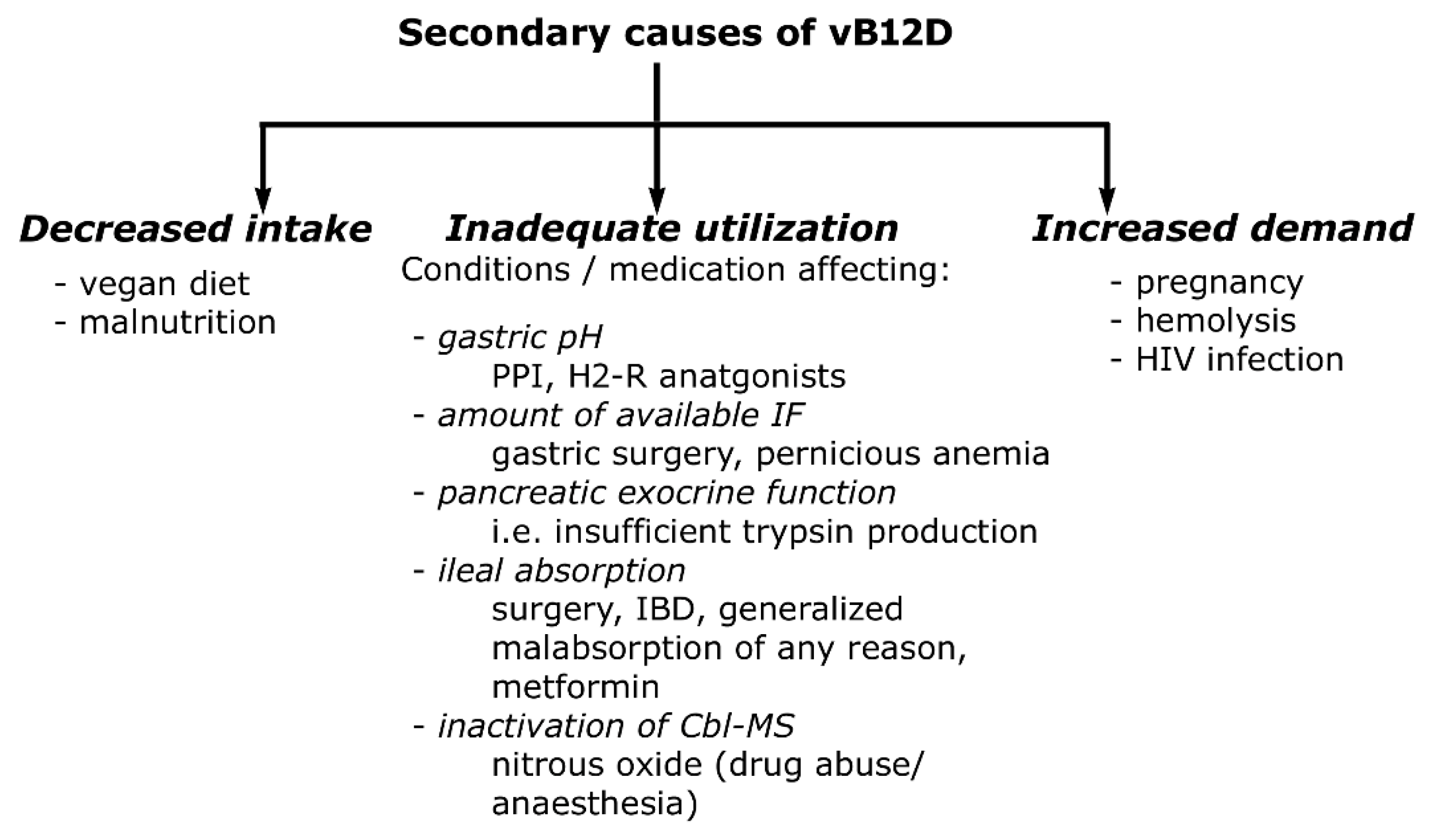
Vitamin B12 (Cobalamin): Its Fate from Ingestion to Metabolism with Particular Emphasis on Diagnostic Approaches of Acquired Neonatal/Infantile Deficiency Detected by Newborn Screening
Abstract
1. Introduction
2. From Uptake to Utilization
2.1. Chemistry and Dietary Source
2.2. Fate of Cbl in the Gastrointestinal Trackt
2.3. Intracellular Fate, Transport in Blood and Storage
3. MUT and MS Catalyzed Reactions
4. Pathobiochemical Consequences of vB12D
5. Diagnostic Criteria of Vitamin B12 Deficiency
5.1. VB12 Assays and Result Interpretation
5.2. HoloTC Assays and Result Interpretation
5.3. MMA Assays and Result Interpretation
5.4. HCY Assays and Result Interpretation
5.5. General Considerations
6. Vitamin B12 Deficiency among Pregnant Women
7. Vitamin B12 Deficiency among Newborn
8. NBS Protocols and New Candidate Markers
9. Treatment and Follow-Up
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef]
- Huemer, M.; Baumgartner, M.R. The Clinical Presentation of Cobalamin-related Disorders: From Acquired Deficiencies to Inborn Errors of Absorption and Intracellular Pathways. J. Inherit. Metab. Dis. 2019, 42, 686–705. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.J. Methods for Assessment of Vitamin B12. In Laboratory Assessment of Vitamin Status; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–299. [Google Scholar]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.; Van den Heuvel, E.G.; De Groot, L.C.; Eussen, S.J. Vitamin B12 Intake from Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.-K.; Hannibal, L.; Hettich, M.; Behringer, S.; Spiekerkoetter, U.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Simmet, T. Vitamin B12 Status upon Short-Term Intervention with a Vegan Diet—A Randomized Controlled Trial in Healthy Participants. Nutrients 2019, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Denissen, K.F.; Heil, S.G.; Eussen, S.J.; Heeskens, J.P.; Thijs, C.; Mommers, M.; Smits, L.J.; van Dongen, M.C.; Dagnelie, P.C. Intakes of Vitamin B-12 from Dairy Food, Meat, and Fish and Shellfish Are Independently and Positively Associated with Vitamin b-12 Biomarker Status in Pregnant Dutch Women. J. Nutr. 2019, 149, 131–138. [Google Scholar] [CrossRef]
- Uniprot. Available online: https://www.uniprot.org/ (accessed on 21 October 2022).
- Brito, A.; Habeych, E.; Silva-Zolezzi, I.; Galaffu, N.; Allen, L.H. Methods to Assess Vitamin B12 Bioavailability and Technologies to Enhance Its Absorption. Nutr. Rev. 2018, 76, 778–792. [Google Scholar] [CrossRef]
- Kitai, K.; Kawaguchi, K.; Tomohiro, T.; Morita, M.; So, T.; Imanaka, T. The Lysosomal Protein ABCD4 Can Transport Vitamin B12 across Liposomal Membranes in Vitro. J. Biol. Chem. 2021, 296, 100654. [Google Scholar] [CrossRef]
- Wang, D.; Eraslan, B.; Wieland, T.; Hallström, B.; Hopf, T.; Zolg, D.P.; Zecha, J.; Asplund, A.; Li, L.; Meng, C. A Deep Proteome and Transcriptome Abundance Atlas of 29 Healthy Human Tissues. Mol. Syst. Biol. 2019, 15, e8503. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.; Nexø, E.; Moestrup, S.K. Vitamin B12 Transport from Food to the Body’s Cells—A Sophisticated, Multistep Pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Bolisetty, K.; Axhemi, A.; DiBello, P.M.; Quadros, E.V.; Fedosov, S.; Jacobsen, D.W. Transcellular Transport of Cobalamin in Aortic Endothelial Cells. FASEB J. 2018, 32, 5506–5519. [Google Scholar] [CrossRef] [PubMed]
- Rowley, C.A.; Kendall, M.M. To B12 or Not to B12: Five Questions on the Role of Cobalamin in Host-Microbial Interactions. PLoS Pathog. 2019, 15, e1007479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, X.; Zhang, Y.; Zhang, X.; Zhang, C.; Liu, M.; Sun, S.; Dong, N.; Wu, Q. CD320 Expression and Apical Membrane Targeting in Renal and Intestinal Epithelial Cells. Int. J. Biol. Macromol. 2022, 201, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Birn, H.; Willnow, T.E.; Nielsen, R.; Norden, A.G.; Bönsch, C.; Moestrup, S.K.; Nexø, E.; Christensen, E.I. Megalin Is Essential for Renal Proximal Tubule Reabsorption and Accumulation of Transcobalamin-B12. Am. J. Physiol.-Ren. Physiol. 2002, 282, F408–F416. [Google Scholar] [CrossRef]
- Silva, B.; Velosa, A.; Barahona-Corrêa, J.B. Reversible Dementia, Psychotic Symptoms and Epilepsy in a Patient with Vitamin B12 Deficiency. BMJ Case Rep. CP 2019, 12, e229044. [Google Scholar] [CrossRef]
- Serin, H.M.; Arslan, E.A. Neurological Symptoms of Vitamin B12 Deficiency: Analysis of Pediatric Patients. Acta Clin. Croat. 2019, 58, 295. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.; Wouters, H.J.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 200–214. [Google Scholar] [CrossRef]
- Htut, T.W.; Thein, K.Z.; Oo, T.H. Pernicious Anemia: Pathophysiology and Diagnostic Difficulties. J. Evid.-Based Med. 2021, 14, 161–169. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. The Effectiveness of Cobalamin (B12) Treatment for Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 784. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined Indicator of Vitamin B12 Status: Modification for Missing Biomarkers and Folate Status and Recommendations for Revised Cut-Points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.J.; Risch, L.; Nydegger, U.; Wiesner, J.; Dyck, M.V.V.; Seger, C.; Stanga, Z.; Renz, H.; Risch, M. Diagnostic Characteristics of 3-Parameter and 2-Parameter Equations for the Calculation of a Combined Indicator of Vitamin B12 Status to Predict Cobalamin Deficiency in a Large Mixed Patient Population. Clin. Lab. 2020, 66, 10. [Google Scholar] [CrossRef] [PubMed]
- Forny, P.; Hörster, F.; Ballhausen, D.; Chakrapani, A.; Chapman, K.A.; Dionisi-Vici, C.; Dixon, M.; Grünert, S.C.; Grunewald, S.; Haliloglu, G. Guidelines for the Diagnosis and Management of Methylmalonic Acidaemia and Propionic Acidaemia: First Revision. J. Inherit. Metab. Dis. 2021, 44, 566–592. [Google Scholar] [CrossRef]
- Boxer, G.E.; Richards, J.C. Chemical Determination of Vitamin B12. 2. The Quantitative Isolation and Colorimetric Determination of Millimicrogram Quantities of Cyanide. Arch Biochem 1951, 30, 372–381. [Google Scholar] [PubMed]
- Peeler, H.T.; Yacowitz, H.; Norris, L.C. A Microbiological Assay for Vitamin B12 Using Lactobacillus Leichmannii. Proc. Soc. Exp. Biol. Med. 1949, 72, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Ekins, R. Assay of Vitamin B12 in Blood: A Simple Method. Lancet 1961, 278, 25–26. [Google Scholar] [CrossRef]
- İspir, E.; Serdar, M.A.; Ozgurtas, T.; Gulbahar, O.; Akın, K.O.; Yesildal, F.; Kurt, İ. Comparison of Four Automated Serum Vitamin B12 Assays. Clin. Chem. Lab. Med. 2015, 53, 1205–1213. [Google Scholar] [CrossRef]
- Thorpe, S.J.; Heath, A.; Blackmore, S.; Lee, A.; Hamilton, M.; O’broin, S.; Nelson, B.C.; Pfeiffer, C. International Standard for Serum Vitamin B12 and Serum Folate: International Collaborative Study to Evaluate a Batch of Lyophilised Serum for B12 and Folate Content. Clin Chem Lab Med. 2007, 45, 380–386. [Google Scholar] [CrossRef]
- Duim, S.N.; Vlasveld, L.T.; Mezger, S.T.; Mingels, A.M.; Ramakers, C.R.; de Boer, D.; Heil, S.G.; Nexo, E.; van Rossum, A.P. Macro Transcobalamin Causing Raised Vitamin B12: Case-Based Laboratory Investigation. Ann. Clin. Biochem. 2022, 59, 302–307. [Google Scholar] [CrossRef]
- Scarpa, E.; Candiotto, L.; Sartori, R.; Radossi, P.; Maschio, N.; Tagariello, G. Undetected Vitamin B12 Deficiency Due to False Normal Assay Results. Blood Transfus. 2013, 11, 627. [Google Scholar]
- Wainwright, P.; Narayanan, S.; Cook, P. False-Normal Vitamin B12 Results in a Patient with Pernicious Anaemia. Clin. Biochem. 2015, 48, 1366–1367. [Google Scholar] [CrossRef] [PubMed]
- Nexo, E.; Christensen, A.-L.; Hvas, A.-M.; Petersen, T.E.; Fedosov, S.N. Quantification of Holo-Transcobalamin, a Marker of Vitamin B12 Deficiency. Clin. Chem. 2002, 48, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Knoepfel, C.; Blanco, M.M.; Nydegger, U.; Risch, L.; Renz, H.; Risch, M. Failure of the Holotranscobalamin Assay in Vitamin B12-Deficient Patients. J. Lab. Med. 2018, 42, 141–147. [Google Scholar] [CrossRef]
- Alam, S.F.; Kumar, S.; Ganguly, P. Measurement of Homocysteine: A Historical Perspective. J. Clin. Biochem. Nutr. 2019, 65, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chernecky, C.C.; Berger, B.J. Laboratory Tests and Diagnostic Procedures; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; ISBN 1-4557-4502-2. [Google Scholar]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of Hyperhomocysteinemia and Its Pathological Significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Ljungblad, U.W.; Paulsen, H.; Mørkrid, L.; Pettersen, R.D.; Hager, H.B.; Lindberg, M.; Astrup, H.; Eklund, E.A.; Bjørke-Monsen, A.-L.; Rootwelt, T. The Prevalence and Clinical Relevance of Hyperhomocysteinemia Suggesting Vitamin B12 Deficiency in Presumed Healthy Infants. Eur. J. Paediatr. Neurol. 2021, 35, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.C.; Aguilera, M.N.L.; Marziali, N.R.; Moritz, L.; Wingert, V.; Klotz, K.; Schumann, A.; Grünert, S.C.; Spiekerkoetter, U.; Berger, U. Analysis of S-Adenosylmethionine and S-Adenosylhomocysteine: Method Optimisation and Profiling in Healthy Adults upon Short-Term Dietary Intervention. Metabolites 2022, 12, 373. [Google Scholar] [CrossRef]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status during Pregnancy and Its Association with Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Rashid, S.; Meier, V.; Patrick, H. Review of Vitamin B12 Deficiency in Pregnancy: A Diagnosis Not to Miss as Veganism and Vegetarianism Become More Prevalent. Eur. J. Haematol. 2021, 106, 450–455. [Google Scholar] [CrossRef]
- Rothen, J.-P.; Walter, P.N.; Tsakiris, D.A.; Infanti, L.; Hersberger, K.E.; Arnet, I. Identification of Patients with Cobalamin Deficiency Crucially Depends on the Diagnostic Strategy. Clin Lab 2021, 67, 1229–1235. [Google Scholar] [CrossRef]
- Gramer, G.; Hoffmann, G.F. Vitamin B12 Deficiency in Newborns and Their Mothers—Novel Approaches to Early Detection, Treatment and Prevention of a Global Health Issue. Curr. Med. Sci. 2020, 40, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Sayar, E.H.; Orhaner, B.B.; Sayar, E.; NesrinTuran, F.; Küçük, M. The Frequency of Vitamin B12, Iron, and Folic Acid Deficiency in the Neonatal Period and Infancy, and the Relationship with Maternal Levels. Turk. Arch. Pediatr. Pediatri. Arş. 2020, 55, 139. [Google Scholar] [CrossRef] [PubMed]
- Scolamiero, E.; Villani, G.R.D.; Ingenito, L.; Pecce, R.; Albano, L.; Caterino, M.; di Girolamo, M.G.; Di Stefano, C.; Franzese, I.; Gallo, G. Maternal Vitamin B12 Deficiency Detected in Expanded Newborn Screening. Clin. Biochem. 2014, 47, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Thayabaran, D.; Burrage, D. Nitrous Oxide-Induced Neurotoxicity: A Case Report and Literature Review. Br. J. Clin. Pharmacol. 2021, 87, 3622–3626. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Bergholt, T.; Byg, K.-E.; Eriksen, L.; Hvas, A.-M. Reference Intervals for Haematological Variables during Normal Pregnancy and Postpartum in 434 Healthy Danish Women. Eur. J. Haematol. 2007, 79, 39–46. [Google Scholar] [CrossRef]
- Schroder, T.H.; Tan, A.; Mattman, A.; Sinclair, G.; Barr, S.I.; Vallance, H.D.; Lamers, Y. Reference Intervals for Serum Total Vitamin B12 and Holotranscobalamin Concentrations and Their Change Points with Methylmalonic Acid Concentration to Assess Vitamin B12 Status during Early and Mid-Pregnancy. Clin. Chem. Lab. Med. 2019, 57, 1790–1798. [Google Scholar] [CrossRef]
- Schroder, T.H.; Sinclair, G.; Mattman, A.; Jung, B.; Barr, S.I.; Vallance, H.D.; Lamers, Y. Pregnant Women of South Asian Ethnicity in Canada Have Substantially Lower Vitamin B12 Status Compared with Pregnant Women of European Ethnicity. Br. J. Nutr. 2017, 118, 454–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teasdale, S.; Morton, A. Changes in Biochemical Tests in Pregnancy and Their Clinical Significance. Obstet. Med. 2018, 11, 160–170. [Google Scholar] [CrossRef]
- Dib, M.-J.; Gumban-Marasigan, M.; Yoxall, R.; Andrew, T.; Harrington, D.J.; Sobczyńska-Malefora, A.; Ahmadi, K.R. Evaluating the Diagnostic Value of a Combined Indicator of Vitamin B12 Status (CB12) Throughout Pregnancy. Front. Nutr. 2022, 8, 789357. [Google Scholar] [CrossRef]
- Tan, A.; Sinclair, G.; Mattman, A.; Vallance, H.D.; Lamers, Y. Maternal Vitamin B12 Status in Early Pregnancy and Its Association with Birth Outcomes in Canadian Mother–Newborn Dyads. Br. J. Nutr. 2021, 126, 1823–1831. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Head Zauche, L.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Kurpad, A.V.; Thomas, T.; Srinivasan, K.; Duggan, C. Vitamin B12 Status in Pregnant Women and Their Infants in South India. Eur. J. Clin. Nutr. 2017, 71, 1046–1053. [Google Scholar] [CrossRef]
- Hay, G.; Clausen, T.; Whitelaw, A.; Trygg, K.; Johnston, C.; Henriksen, T.; Refsum, H. Maternal Folate and Cobalamin Status Predicts Vitamin Status in Newborns and 6-Month-Old Infants. J. Nutr. 2010, 140, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Na’im Mohamad Ayob, M.; Cai, S.; Quah, P.L.; Gluckman, P.D.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; Godfrey, K.M. Maternal Plasma Vitamin B12 Concentrations during Pregnancy and Infant Cognitive Outcomes at 2 Years of Age. Br. J. Nutr. 2019, 121, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Thomas, T.; Bosch, R.J.; Ramthal, A.; Bellinger, D.C.; Kurpad, A.V.; Duggan, C.P.; Srinivasan, K. Effect of Maternal Vitamin B12 Supplementation on Cognitive Outcomes in South Indian Children: A Randomized Controlled Clinical Trial. Matern. Child Health J. 2019, 23, 155–163. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Guillet, R.; Pressman, E.K.; Fothergill, A.; Guetterman, H.M.; Kent, T.R.; O’Brien, K.O. Vitamin B12 Status in Pregnant Adolescents and Their Infants. Nutrients 2019, 11, 397. [Google Scholar] [CrossRef]
- Drummond, J.T.; Matthews, R.G. Nitrous Oxide Degradation by Cobalamin-Dependent Methionine Synthase: Characterization of the Reactants and Products in the Inactivation Reaction. Biochemistry 1994, 33, 3732–3741. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Zakowski, M.I. Pro-Con Debate: Nitrous Oxide for Labor Analgesia. BioMed Res. Int. 2019, 4618798, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ljungblad, U.W.; Lindberg, M.; Eklund, E.A.; Saeves, I.; Bjørke-Monsen, A.-L.; Tangeraas, T. Nitrous Oxide in Labour Predicted Newborn Screening Total Homocysteine and Is a Potential Risk Factor for Infant Vitamin B12 Deficiency. Acta Paediatr. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Yesildal, F.; Koc, E.; Ozturk, H.; Ozgurtas, T. Change in Vitamin B12 Level in Breast Milk According to the Week of Delivery in Turkish Women. Int. J. Med. Biochem. 2020, 3, 96–100. [Google Scholar]
- Ozben, T. Expanded Newborn Screening and Confirmatory Follow-up Testing for Inborn Errors of Metabolism Detected by Tandem Mass Spectrometry. Clin. Chem. Lab. Med. 2013, 51, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The Use of Mass Spectrometry to Analyze Dried Blood Spots. Mass Spectrom. Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef] [PubMed]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current Status of Newborn Screening Worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef]
- Gramer, G.; Fang-Hoffmann, J.; Feyh, P.; Klinke, G.; Monostori, P.; Okun, J.G.; Hoffmann, G.F. High Incidence of Maternal Vitamin B12 Deficiency Detected by Newborn Screening: First Results from a Study for the Evaluation of 26 Additional Target Disorders for the German Newborn Screening Panel. World J. Pediatr. 2018, 14, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Fang-Hoffmann, J.; Feyh, P.; Klinke, G.; Monostori, P.; Mütze, U.; Posset, R.; Weiss, K.H.; Hoffmann, G.F.; Okun, J.G. Newborn Screening for Vitamin B12 Deficiency in Germany—Strategies, Results, and Public Health Implications. J. Pediatr. 2020, 216, 165–172.e4. [Google Scholar] [CrossRef]
- Monostori, P.; Godejohann, M.; Janda, J.; Galla, Z.; Rácz, G.; Klinke, G.; Szatmári, I.; Zsidegh, P.; Kohlmüller, D.; Kölker, S. Identification of Potential Interferents of Methylmalonic Acid: A Previously Unrecognized Pitfall in Clinical Diagnostics and Newborn Screening. Clin. Biochem. 2022, in press. [Google Scholar] [CrossRef]
- Monostori, P.; Klinke, G.; Richter, S.; Baráth, Á.; Fingerhut, R.; Baumgartner, M.R.; Kölker, S.; Hoffmann, G.F.; Gramer, G.; Okun, J.G. Simultaneous Determination of 3-Hydroxypropionic Acid, Methylmalonic Acid and Methylcitric Acid in Dried Blood Spots: Second-Tier LC-MS/MS Assay for Newborn Screening of Propionic Acidemia, Methylmalonic Acidemias and Combined Remethylation Disorders. PLoS ONE 2017, 12, e0184897. [Google Scholar] [CrossRef]
- Dubland, J.A.; Rakić, B.; Vallance, H.; Sinclair, G. Analysis of 2-Methylcitric Acid, Methylmalonic Acid, and Total Homocysteine in Dried Blood Spots by LC-MS/MS for Application in the Newborn Screening Laboratory: A Dual Derivatization Approach. J. Mass Spectrom. Adv. Clin. Lab 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, J.; Lin, Y.; Wang, J.; Hu, L.; Zhang, C.; Zhang, Y.; Huang, X. Determination of Methylmalonic Acid, 2-Methylcitric Acid, and Total Homocysteine in Dried Blood Spots by Liquid Chromatography–Tandem Mass Spectrometry: A Reliable Follow-up Method for Propionylcarnitine-Related Disorders in Newborn Screening. J. Med. Screen. 2021, 28, 93–99. [Google Scholar] [CrossRef]
- Turgeon, C.T.; Magera, M.J.; Cuthbert, C.D.; Loken, P.R.; Gavrilov, D.K.; Tortorelli, S.; Raymond, K.M.; Oglesbee, D.; Rinaldo, P.; Matern, D. Determination of Total Homocysteine, Methylmalonic Acid, and 2-Methylcitric Acid in Dried Blood Spots by Tandem Mass Spectrometry. Clin. Chem. 2010, 56, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Gan-Schreier, H.; Kebbewar, M.; Fang-Hoffmann, J.; Wilrich, J.; Abdoh, G.; Ben-Omran, T.; Shahbek, N.; Bener, A.; Al Rifai, H.; Al Khal, A.L. Newborn Population Screening for Classic Homocystinuria by Determination of Total Homocysteine from Guthrie Cards. J. Pediatr. 2010, 156, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Abdoh, G.; Ben-Omran, T.; Shahbeck, N.; Ali, R.; Mahmoud, L.; Fang-Hoffmann, J.; Hoffmann, G.F.; Al Rifai, H.; Okun, J.G. Newborn Screening for Remethylation Disorders and Vitamin B12 Deficiency-Evaluation of New Strategies in Cohorts from Qatar and Germany. World J. Pediatr. 2017, 13, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, D.K.; Piazza, A.L.; Pino, G.; Turgeon, C.; Matern, D.; Oglesbee, D.; Raymond, K.; Tortorelli, S.; Rinaldo, P. The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism. Int. J. Neonatal Screen. 2020, 6, 33. [Google Scholar] [CrossRef]
- Collaborative Laboratory Integrated Reports (CLIR). Available online: https://clir.mayo.edu (accessed on 21 October 2022).
- Reinson, K.; Künnapas, K.; Kriisa, A.; Vals, M.-A.; Muru, K.; Õunap, K. High Incidence of Low Vitamin B12 Levels in Estonian Newborns. Mol. Genet. Metab. Rep. 2018, 15, 1–5. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Rizzo, C.; Zucchelli, M.; Consalvo, A.; Valentinuzzi, S.; Semeraro, D.; Gasparroni, G.; Brindisino, P.; Gazzolo, D.; et al. A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. Int. J. Environ. Res. Public. Health 2020, 17, 3601. [Google Scholar] [CrossRef]
- Rozmarič, T.; Mitulović, G.; Konstantopoulou, V.; Goeschl, B.; Huemer, M.; Plecko, B.; Spenger, J.; Wortmann, S.B.; Scholl-Bürgi, S.; Karall, D.; et al. Elevated Homocysteine after Elevated Propionylcarnitine or Low Methionine in Newborn Screening Is Highly Predictive for Low Vitamin B12 and Holo-Transcobalamin Levels in Newborns. Diagnostics 2020, 10, 626. [Google Scholar] [CrossRef]
- Duggan, C.; Srinivasan, K.; Thomas, T.; Samuel, T.; Rajendran, R.; Muthayya, S.; Finkelstein, J.L.; Lukose, A.; Fawzi, W.; Allen, L.H. Vitamin B-12 Supplementation during Pregnancy and Early Lactation Increases Maternal, Breast Milk, and Infant Measures of Vitamin B-12 Status. J. Nutr. 2014, 144, 758–764. [Google Scholar] [CrossRef]
- Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients 2022, 14, 3767. [Google Scholar] [CrossRef]
- Paul, C.; Brady, D.M. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements with Potential to Mitigate B12-Related Genetic Polymorphisms. Integr. Med. Clin. J. 2017, 16, 42. [Google Scholar]
- Torsvik, I.; Ueland, P.M.; Markestad, T.; Bjørke-Monsen, A.-L. Cobalamin Supplementation Improves Motor Development and Regurgitations in Infants: Results from a Randomized Intervention Study. Am. J. Clin. Nutr. 2013, 98, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, A.; Pundhir, A.; Ghosh, S. Food Fortification: A Nutritional Management Strategy in India. Innovare J. Food Sci. 2018, 6, 1–8. [Google Scholar]



| I.c. Locus | Protein Name | Gene Symbol | Clinical Phenotype | |
|---|---|---|---|---|
| Alternative (Common) | Recommended | |||
| Lysos. membr. | Lysosomal transport escort protein LMBD1 | LMBD1 | LMBDR1 | CblF defect |
| Lysosomal Cbl transporter ABCD4 | ABCD4 | ABCD4 | CblJ defect | |
| Cytosol | CblC, MMACHC, MMAuria and HCYuria type C protein | CNCbl reductase/AlkylCbl dealkylase | MMACHC | CblC defect |
| Cytosol/Mitoch. | CblD, MMADHC, MMAuria and HCYuria type D protein | Cobalamin trafficking protein CblD | MMADHC | CblD defect |
| Mitochondria | Methylmalonic aciduria type B protein | Corrinoid adenosyltransferase MMAB | MMAB | CblB defect |
| MMAA (Methylmalonic aciduria type A protein, mitochondrial) | Methylmalonic aciduria type A protein, mitochondrial | MMAA | CblA defect | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kósa, M.; Galla, Z.; Lénárt, I.; Baráth, Á.; Grecsó, N.; Rácz, G.; Bereczki, C.; Monostori, P. Vitamin B12 (Cobalamin): Its Fate from Ingestion to Metabolism with Particular Emphasis on Diagnostic Approaches of Acquired Neonatal/Infantile Deficiency Detected by Newborn Screening. Metabolites 2022, 12, 1104. https://doi.org/10.3390/metabo12111104
Kósa M, Galla Z, Lénárt I, Baráth Á, Grecsó N, Rácz G, Bereczki C, Monostori P. Vitamin B12 (Cobalamin): Its Fate from Ingestion to Metabolism with Particular Emphasis on Diagnostic Approaches of Acquired Neonatal/Infantile Deficiency Detected by Newborn Screening. Metabolites. 2022; 12(11):1104. https://doi.org/10.3390/metabo12111104
Chicago/Turabian StyleKósa, Magdolna, Zsolt Galla, István Lénárt, Ákos Baráth, Nóra Grecsó, Gábor Rácz, Csaba Bereczki, and Péter Monostori. 2022. "Vitamin B12 (Cobalamin): Its Fate from Ingestion to Metabolism with Particular Emphasis on Diagnostic Approaches of Acquired Neonatal/Infantile Deficiency Detected by Newborn Screening" Metabolites 12, no. 11: 1104. https://doi.org/10.3390/metabo12111104
APA StyleKósa, M., Galla, Z., Lénárt, I., Baráth, Á., Grecsó, N., Rácz, G., Bereczki, C., & Monostori, P. (2022). Vitamin B12 (Cobalamin): Its Fate from Ingestion to Metabolism with Particular Emphasis on Diagnostic Approaches of Acquired Neonatal/Infantile Deficiency Detected by Newborn Screening. Metabolites, 12(11), 1104. https://doi.org/10.3390/metabo12111104