Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle–yak and Yak)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparation of mRNA and Proteins
2.3. Molecular Cloning of Hsp90
2.4. Physical and Chemical Properties Analysis of Hsp90
2.5. Expression of Hsp70 and Hsp90 Gene in Different Non-Reproductive Organs
2.6. Protein Expression of Hsp70 and Hsp90 in Testicular Tissues
2.7. Positive Reaction Analysis of Hsp70 and Hsp90 Protein
2.8. Measurement and Statistical Analyses
3. Results
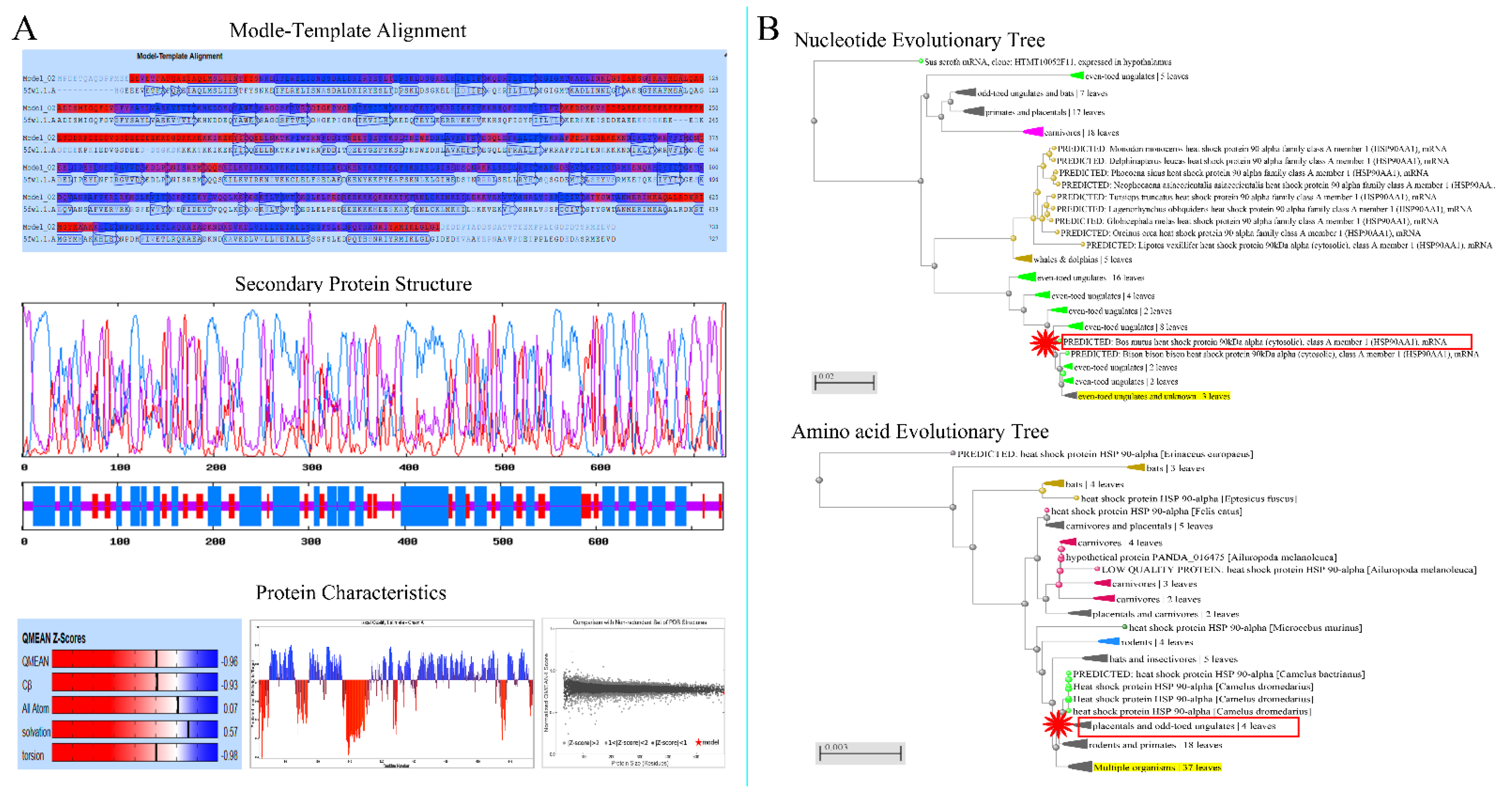
3.1. Analysis of Physical and Chemical Properties of Hsp90
3.2. Analysis of Structure Specificity of Hsp90 Protein
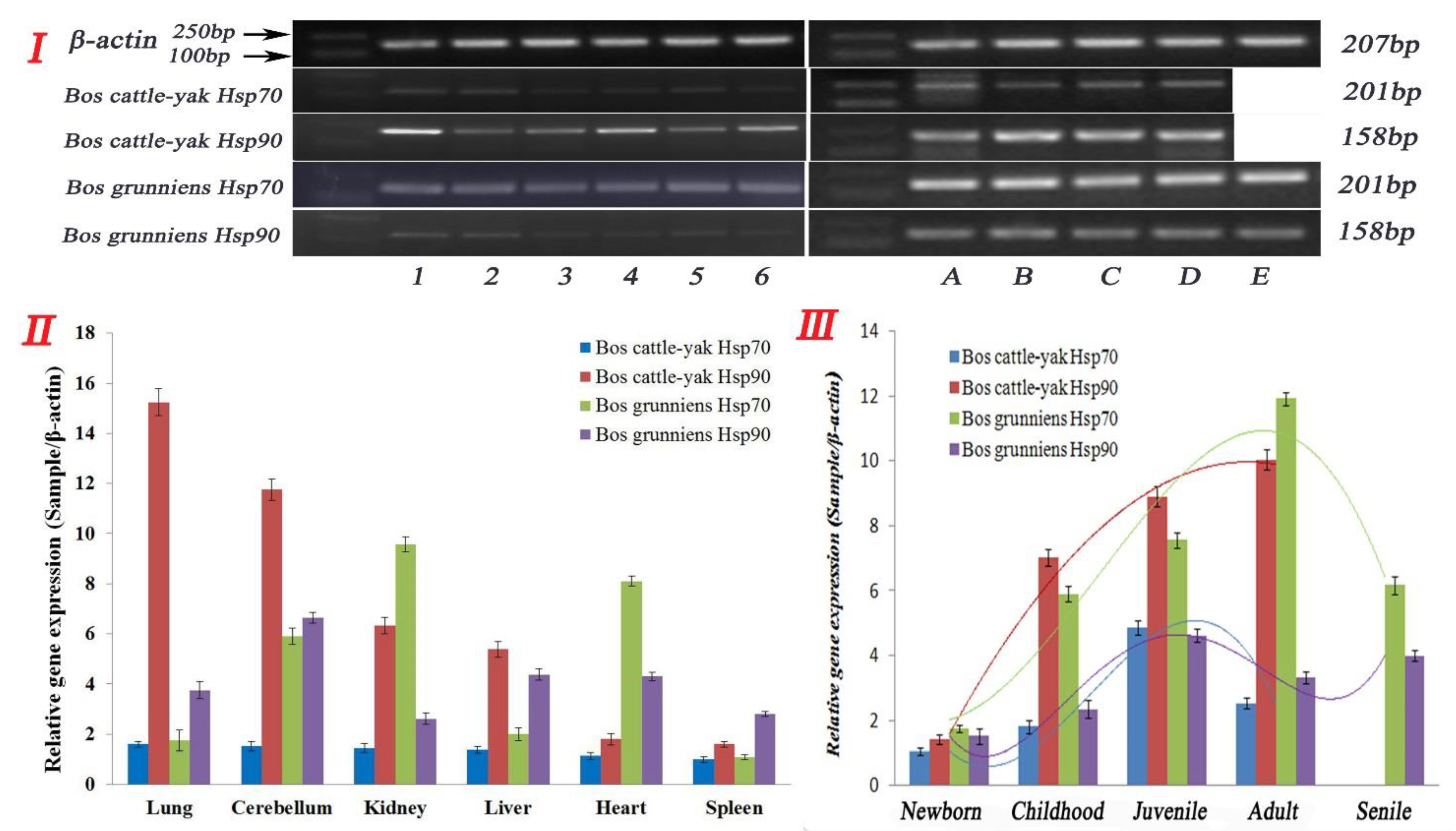
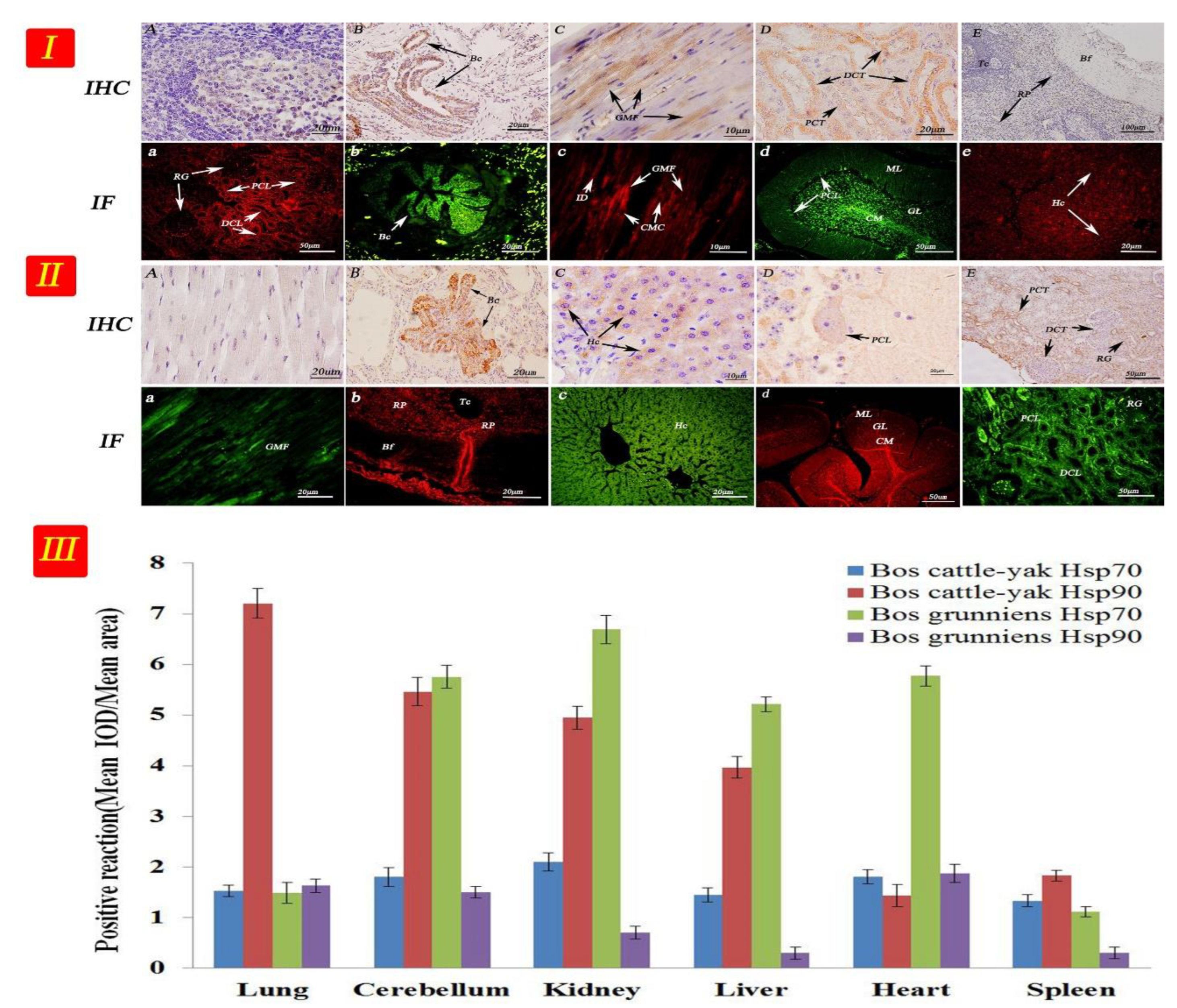
3.3. Expression and Distribution of Hsp70 and Hsp90 in Non-Reproductive Organs
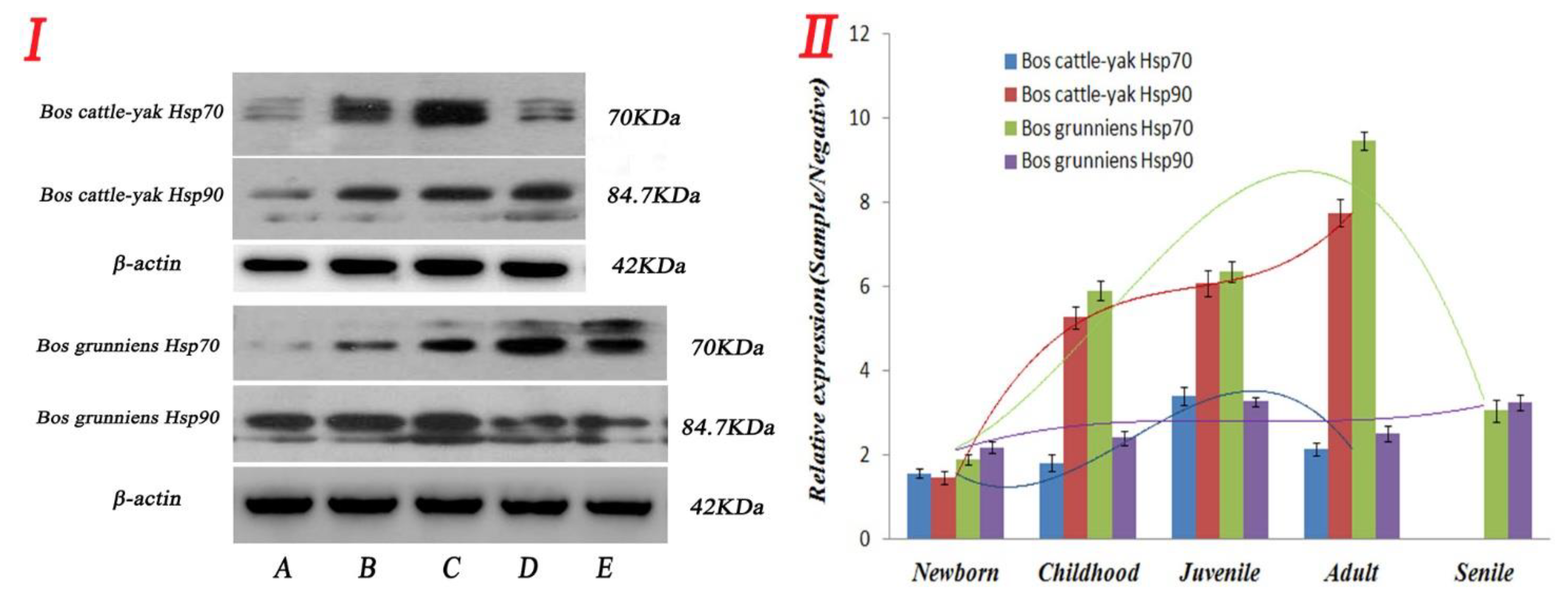
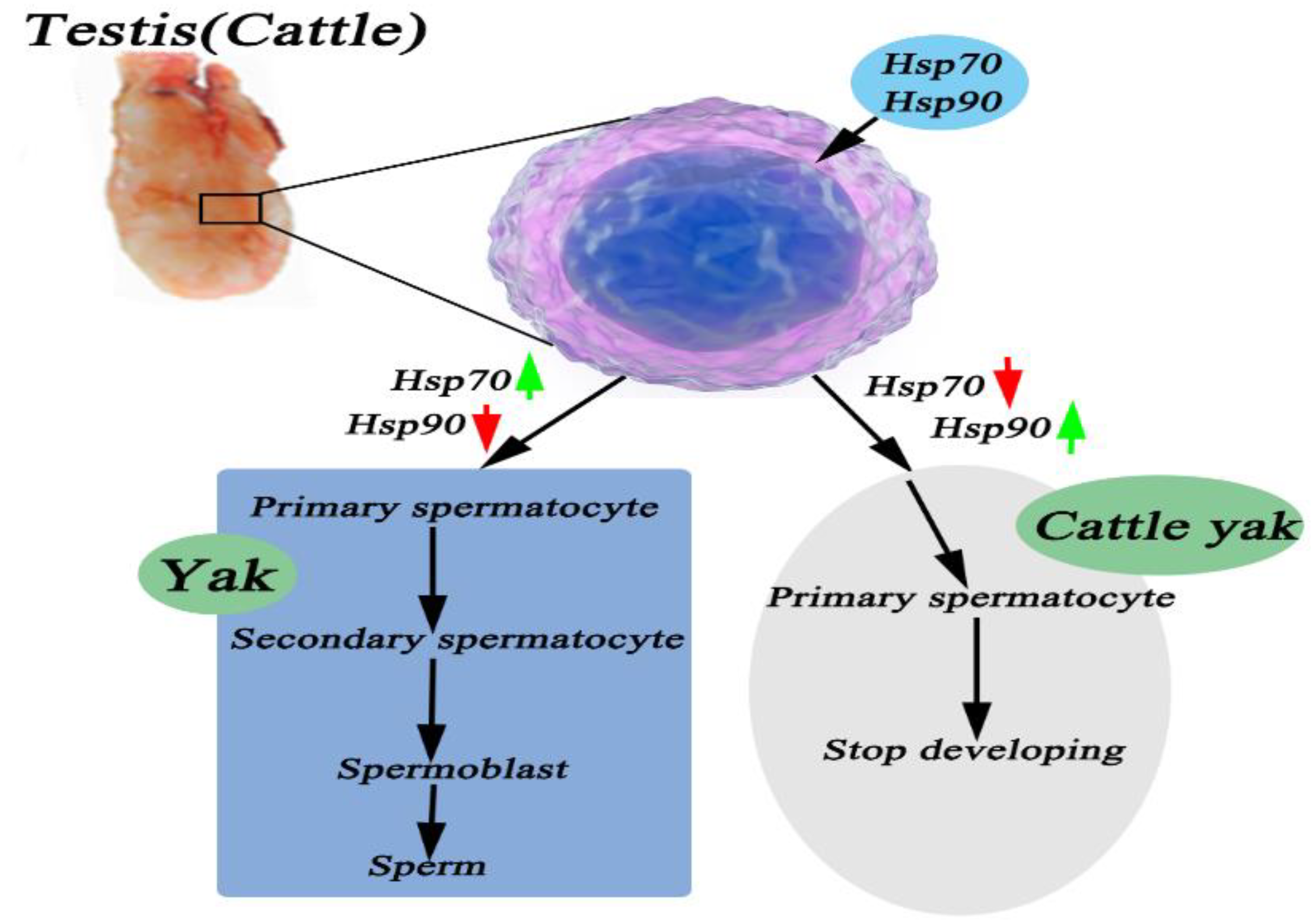
3.4. Expression and Distribution of Hsp70 and Hsp90 in Testicular Tissues at Different Developmental Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiener, G.; Jianlin, H.; Ruijun, L. The Yak; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2003. [Google Scholar]
- Zou, S.N.; Liu, P.G.; Yu, S.J.; Cui, Y.; He, J.F.; Afedo, S.Y.; Zhang, H.Z.; Niayale, R.; Zhao, K. Hsp60 expression and localization in different tissues and testis development of male cattle (Cattle-yak and Yak). Folia Morphol. 2021, 80, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.G.; Yu, S.J.; Cui, Y.; He, J.F.; Zhang, Q.; Sun, J.; Huang, Y.F.; Yang, X.Q.; Cao, M.X.; Liao, B.; et al. Regulation by Hsp27/P53 in testis development and sperm apoptosis of male cattle (cattle-yak and yak). J. Cell. Physiol. 2018, 234, 650–660. [Google Scholar] [PubMed] [Green Version]
- Shen, H.; Fan, X.R.; Zhang, Z.; Xi, H.M.; Ji, R.; Liu, Y.H.; Yue, M.S.; Li, Q.H.; He, J.P. Effects of elevated ambient temperature and local testicular heating on the expressions of heat shock protein 70 and androgen receptor in boar testes. Acta Histochem. 2019, 121, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.D.; Malkovsky, M. Heat shock proteins, tumor immunogenicity and antigen presentation: An integrated view. Immunol. Today 2000, 21, 129–132. [Google Scholar] [CrossRef]
- Weng, Z.X.; Yu, S.J.; Zhai, Y.J.; Yang, K.; Liu, P.G.; He, J.F.; Cui, Y. Differences in Expression of HSPA2 in Different Tissues and Organs of Yak. Sci. Agric. Sin. 2014, 47, 3475–3482. [Google Scholar]
- Liu, Z.; Xiong, J.; Gao, S.; Zhu, M.X.; Sun, K.; Li, M.; Zhang, G.H.; Li, Y.P. Ameliorating cancer cachexia by inhibiting cancer cell release of Hsp70 and Hsp90 with omeprazole. J. Cachexia Sarcopenia Muscle 2021, 11, 636–647. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Lateef, O.M.; Adebayo, O.I.; Aet, A. Hsps70 and 90 protect the heart of hyperthyroid rats via nitric oxide production and VEGF inhibition of apoptosis. Endocr. Metab. Sci. 2021, 4, 100097. [Google Scholar]
- Kausar, S.; Abbas, M.N.; Yang, L.; Cui, H.J. Biotic and abiotic stress induces the expression of Hsp70/90 organizing protein gene in silkworm, Bombyx mori. Int. J. Biol. Macromol. 2020, 143, 610–618. [Google Scholar]
- Liu, P.; Yu, S.; Cui, Y.; He, J.F.; Yu, C.; Wen, Z.X.; Pan, Y.Y.; Yang, K.; Song, L.L.; Yang, X. Cloning of HSP90, expression and localization of HSP70/90 in different tissues including lactating/non-lactating yak (Bos grunniens) breast tissue. PLoS ONE 2017, 12, e0179321. [Google Scholar] [CrossRef] [Green Version]
- Tekayev, M.; Bostancieri, N.; Saadat, K.A.; Turker, M.; Yuncu, M.; Ulusal, H.; Cicek, H.; Arman, K. Effects of Moringa oleifera Lam Extract (MOLE) in the heat shock protein 70 expression and germ cell apoptosis on experimentally induced cryptorchid testes of rats. Gene 2019, 688, 140–150. [Google Scholar] [CrossRef]
- Lu, J.Y.; Zi, X.D. Anatomical structure in testicular of F1 cattle-yak. Anim. Husb. Vet. Med. 2014, 12, 61–63. [Google Scholar]
- Xu, X.; Luo, D.; Xuan, Q.; Lu, P.; Yu, C.; Guan, Q. Atlas of metabolism reveals palmitic acid results in mitochondrial dysfunction and cell apoptosis by inhibiting fatty acid b-oxidation in Sertoli cells. Anim. Husb. Vet. Med. 2022, 9, 1021263. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, J.; Fürbass, R. The bovine genome contains three differentially methylated paralogous copies of the P450c17 encoding gene (CYP17A1). Gen. Comp. Endocrinol. 2011, 170, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Afedo, S.Y.; Cui, Y.; Yu, S.; Liao, B.; Zhao, Z.H.; Li, H.; Zhang, H.Z.; Zou, S.N.; Li, D.H.; Zhang, P. Histological Analysis, Bioinformatics Profile, and Expression of Methylenetetrahydrofolate Reductase (MTHFR) in Bovine Testes. Animals 2020, 10, 1731–1748. [Google Scholar]
- Sun, J.; Beger, R.; Sloper, D.; Nakamura, N. Metabolomics-based pathway changes in testis fragments treated with ethinylestradiol in vitro. Birth Defects Res. 2019, 111, 1643–1654. [Google Scholar] [CrossRef]
- Motiei, M.; Tavalaee, M.; Nasr-Esfahani, M.H. Evaluation of HSPA2 in fertile and infertile individuals. Andrologia 2013, 45, 66–72. [Google Scholar] [CrossRef]
- Silva, J.V.; Santiago, J.; Matos, B.; Henriques, M.C.; Patricio, D.; Martins, A.D.; Duarte, J.A.; Ferreira, R.; Alves, M.G.; Oliveira, P.; et al. Effects of Age and Lifelong Moderate-Intensity Exercise Training on Rats’ Testicular Function. Int. J. Mol. Sci. 2022, 23, 11619. [Google Scholar] [CrossRef]
- Mikulski, A.; Bernatowicz, P.; Grzesiuk, M.; Kloc, M.; Pijanowska, J. Differential levels of stress proteins (HSPs) in male and female Daphnia magna in response to thermal stress: A consequence of sex-related behavioral differences. J. Chem. Ecol. 2011, 37, 670–676. [Google Scholar] [CrossRef] [Green Version]
- He, Y.Y.; Yu, S.J.; Hu, J.W.; Cui, Y.; Liu, P.G. Changes in the Anatomic and Microscopic Structure and the Expression of HIF-1α and VEGF of the Yak Heart with Aging and Hypoxia. PLoS ONE 2016, 11, e0149947. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, A.; Miao, Z.; Zhang, X.; Wang, T.; Kang, A.; Shan, J.; Zhu, D.; Li, W. Fluorescent ligand fishing combination with in-situ imaging and characterizing to screen Hsp 90 inhibitors from Curcuma longa L. based on InP/ZnS quantum dots embedded mesoporous nanoparticles. Talanta 2018, 178, 258–267. [Google Scholar] [CrossRef]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamal, L.T.; Khan, A.W.; Thudi, M.; Suk-Ha, L.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelham, H.R.; Bienz, M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982, 1, 1473. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Aston, K.I.; Meyer, T.D.; Hotaling, J.M.; Shamsi, M.B.; Johnstone, E.B.; Cox, K.J.; Stanford, J.B.; Porucznik, C.A.; Carrell, D.T. Decreased fecundity and sperm DNA methylation patterns. Fertil. Steril. 2016, 105, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.Y.; Gu, Y.J.; An, T.; Liu, J.X.; Pan, Y.Y.; Mo, F.F.; Miao, J.N.; Zhao, D.D.; Zhang, D.W.; Gao, S.H.; et al. Proteomics Analysis of Testis of Rats Fed a High-Fat Diet. Celluar Ghysiology Biochem. 2018, 47, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Paroo, Z.; Tiidus, P.M.; Noble, E.G. Estrogen attenuates HSP 72 expression in acutely exercised male rodents. Eur. J. Appl. Physiol. 1999, 80, 180–184. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Zhang, Z.W.; Qu, J.P.; Yao, H.D.; Li, M.; Li, S.; Xu, S.W. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones 2014, 19, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, E.; Liang, W.; Lin, Y.; Huang, L.; Xin, H.; Cai, S.; Chen, J.; Ning, Z.; Li, J.; Zhang, Q. HSP70/HSP90-Organizing Protein Contributes to Gastric Cancer Progression in an Autocrine Fashion and Predicts Poor Survival in Gastric Cancer. Cell Stress Chaperones 2018, 47, 879–892. [Google Scholar] [CrossRef]
- Haseeb, K.; Wang, J.; Xiao, K.; Yang, K.L.; Peng, K.M. Effects of Boron Supplementation on Expression of Hsp70 in the Spleen of African Ostrich. Biol. Trace Elem. Res. 2018, 182, 317–327. [Google Scholar] [CrossRef]
- Krüger, K.; Reichel, T.; Zeilinger, C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J. Appl. Physiol. 2019, 126, 916–927. [Google Scholar] [CrossRef]
- Li, W.D.; Huang, M.; Lu, W.G.; Chen, X.; Shen, M.H.; Li, X.M.; Wang, R.X.; Ke, C.H. Involvement of Antizyme Characterized from the Small Abalone Haliotis diversicolor in Gonadal Development. PLoS ONE 2015, 10, e0135251. [Google Scholar] [CrossRef]
- Mo, Q.; Kulyar, M.F.; Ding, Y.; Zhang, Y.; Pan, H.; Li, J. Thiram induces myocardial oxidative damage and apoptosis in broilers via interfering their cardiac metabolism. Ecotoxicol. Environ. Saf. 2022, 23, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Liu, C.L.; Ma, H.; Fu, B.; Wang, L.; Peng, F.G.; Ma, J.Z.; Liu, D. Cold stress of northeastern wild boar fibroblasts HSP90 mRNA transcription. J. Anim. Husb. Vet. 2012, 43, 1978–1983. [Google Scholar]
- Shao, Y.; Zhao, H.; Wang, Y.; Liu, J.J.; Xing, M.W. Arsenic and/or copper caused inflammatory response via activation of inducible nitric oxide synthase pathway and triggered heat shock protein responses in testis tissues of chicken. Environ. Sci. Pollut. Res. 2018, 25, 7719–7729. [Google Scholar] [CrossRef] [PubMed]
- Stygar, D.; Skrzep-Poloczek, B.; Romuk, E.; Chelmecka, E.; Poloczek, T.; Sawczyn, T.; Maciarz, J.; Kukla, M.; Karcz, K.W.; Jochem, J. The influence of high-fat, high-sugar diet and bariatric surgery on HSP70 and HSP90 plasma and liver concentrations in diet-induced obese rats. Cell Stress Chaperones 2019, 24, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, T.E.; Roach, C.M.; Baumgard, L.H.; Ross, J.W.; Keating, A.F.; Selsby, J.T. The impact of Zearalenone on heat-stressed skeletal muscle in pigs. J. Anim. Sci. 2022, 100, skac215. [Google Scholar] [CrossRef]
- An, X.; Li, Q.; Chen, N.; Li, T.; Wang, H.; Su, M.; Shi, H.; Ma, Y. Effects of Pgam1-mediated glycolysis pathway in Sertoli cells on Spermatogonial stem cells based on transcriptomics and energy metabolomics. Front. Vet. Sci. 2022, 9, 992877. [Google Scholar] [CrossRef]
- Spinaci, M.; Volpe, S.; Bernardini, C.; Ambrogi, M.; Tamanini, C.; Seren, E.; Galeati, G. Sperm Sorting Procedure Induces a Redistribution of Hsp70 but Not Hsp60 and Hsp90 in Boar Spermatozoa. J. Androl. 2006, 27, 899–907. [Google Scholar] [CrossRef]
- Elfattah, A.A.; Fahim, A.T.; Sadik, N.A.; Ali, B.M. Resveratrol and curcumin ameliorate di-(2-ethylhexyl) phthalate induced testicular injury in rats. Gen. Comp. Endocrinol. 2016, 225, 45–54. [Google Scholar]
- Chai, Z.; Wang, X.; Ji, Q.M.; Zhang, C.F.; Xin, J.W.; Zhong, J.C. Expression Study on HAFY2, DAZAP2 Gene of Yak, Cattle and Pianniu. Southwest China J. Agric. Sci. 2014, 27, 1278–1283. [Google Scholar]
- Erukainure, O.L.; Atolani, O.; Banerjee, P.; Abel, R.; Pooe, Q.J.; Adeyemi, O.S.; Preissner, R.; Chukwuma, C.L.; Koorbanaiiy, N.A.; Islam, M.S. Oxidative testicular injury: Effect of l-leucine on redox, cholinergic and purinergic dysfunctions, and dysregulated metabolic pathways. Amino Acids 2021, 53, 359–380. [Google Scholar] [CrossRef]
- Zannoni, A.; Bernardini, C.; Zaniboni, A.; Ferlizza, E.; Forni, M. Relative abundance of heat shock proteins and clusterin transcripts in spermatozoa collected from boar routinely utilised in an artificial insemination centre: Preliminary results. Vet. Res. Commun. 2017, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Erata, G.O.; Toker, N.K.; Durlanik, O.; Kadiogli, A.; Toker, G.A. The role of heat shock protein 70 (Hsp 70) in male infertility: Is it a line of defense against sperm DNA fragmentation. Fertil. Steril. 2008, 90, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Yl, L.X.; Li, X.; Chen, J.; Xia, S.; University, H.A. Creation of Male Sterile Line in Tobacco with HSP70 Anti-sense Fragment. Agric. Sci. Technol. 2016, 17, 10. [Google Scholar]
- Gujar, G.; Choudhary, V.K.; Vivek, P.; Sodhi, M.; Choudlhary, M.; Tiwari, M.; Masharing, N.; Mukesh, M. Characterization of thermo-physiological, hematological, and molecular changes in response to seasonal variations in two tropically adapted native cattle breeds of Bos indicus lineage in hot arid ambience of Thar Desert. Int. J. Biometeorol. 2022, 66, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Dix, D.J.; Allen, J.W.; Collins, B.W.; Mori, C.; Nakamura, N.; Poorman-Allen, P.; Gouiding, E.H.; Eddy, E.M. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc. Natl. Acad. Sci. USA 1996, 93, 3264–3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, F.; Liu, Y.; Lv, Z.; Zhang, J.; Xiong, S.; Zha, L.; Liu, Z.; Shu, J. Regulatory effects of dierential dietary energy levels on spermatogenesis and sperm motility of yellow-feathered breeder cocks. Front. Vet. Sci. 2022, 10, 1241. [Google Scholar]
- Wu, Y.; Pei, Y.; Qin, Y. Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res. 2011, 344, 355–363. [Google Scholar] [CrossRef]
- Cedenho, A.P.; Lima, S.B.; Cenedeze, M.A.; Spaine, D.M.; Ortiz, V.; Oehninger, S. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum. Reprod. 2006, 21, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Economou, K.; Kotsiliti, E.; Mintzas, A.C. Stage and cell-specific expression and intracellular localization of the small heat shock protein Hsp27 during oogenesis and spermatogenesis in the Mediterranean fruit fly, Ceratitis capitata. J. Insect Physiol. 2017, 96, 64–72. [Google Scholar] [CrossRef]
- Cardozo, C.; Michaud, C.; Ost, M.C.; Fliss, A.E.; Yang, E.; Patterson, C.; Hall, S.J.; Caplan, A.J. C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch. Biochem. Biophys. 2003, 410, 134–140. [Google Scholar] [CrossRef]
- Parmar, K.H.; Singh, V.; Savsani, H.H.; Kavani, F.S.; Rajoriya, J.S.; Lone, S.A. Seasonal variation in heat shock proteins (hsp70 and hsp90) and their association with frozen semen quality and fertility in buffaloes. Cryo Lett. 2021, 42, 1515–1529. [Google Scholar]
- Steiner, C.C.; Ryder, O.A. Characterization of Prdm9 in Equids and Sterility in Mules. PLoS ONE 2013, 8, e61746. [Google Scholar] [CrossRef] [PubMed]


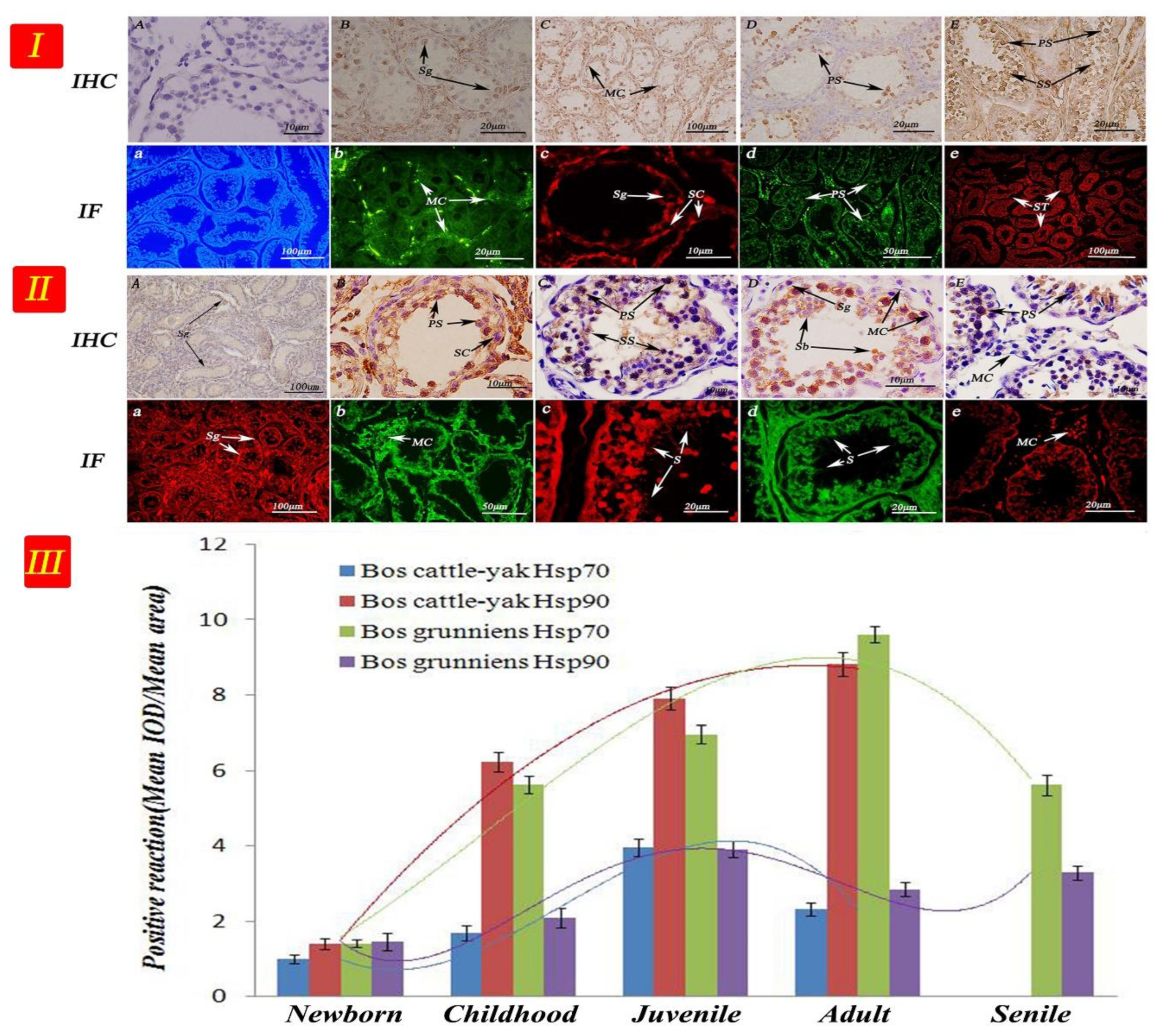
| Number Cattle–yak + Yaks | Group | Age | Source | Application |
|---|---|---|---|---|
| 6 + 6 | Newborn | 1~7 days | Xi’ning City Qinghai Province | Molecular biology Histology Measurement |
| 6 + 6 | Calf | 5~6 months | ||
| 6 + 6 | Juvenile | 1~1.5 years | ||
| 6 + 6 | Adult | 3~6 years | ||
| 0 + 6 | Senior | 8~10 years |
| Primer | Sequence(5′→3′) | Tm (°C) | Note |
|---|---|---|---|
| Name | |||
| Hsp90-1R | GATGAATACTCTGCGAACATACAA | 56.9 | RT-PCR |
| Hsp90-1F | ATGCCCGAGGAGACCCA | 58.6 | 1113bp |
| Hsp90-2R | TGTTTGCTGTCCAGCCGTAT | 58.9 | RT-PCR |
| Hsp90-2F | GGAGGAGCGGAGAATAAAGG | 58.2 | 1238bp |
| Hsp90-3R | CCCGATGTATGGACAATGACTC | 59.2 | RT-PCR |
| Hsp90-3F | GAAAGTTGAAAAGGTGGTTGTG | 56.8 | 979bp |
| β-actin-F | GACCCAGATCATGTTTGAGACC | 58.0 | RT-PCR |
| β-actin-R | ATCTCCTTCTGCATCCTGTCAG | 58.0 | 598bp |
| Hsp70-R | GCCTTGGTCTCCCCTTTGTAG | 58.0 | RT-qPCR |
| Hsp70-F | GCTGAACCCGCAGAACACG | 58.0 | 158bp |
| Hsp90-R | GCTGAATAAAACCCGACACCA | 62.0 | RT-qPCR |
| Hsp90-F | CAAGCAAGATCGAACCCTCAC | 62.0 | 174bp |
| β-actin-R | GCTCGGCTGTGGTGGTAAA | 59.0 | RT-qPCR |
| β-actin-F | AGGCTGTGCTGTCCCTGTATG | 59.0 | 207bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liu, P.; Yu, S.; He, J.; Afedo, S.Y.; Zou, S.; Zhang, Q.; Liu, J.; Song, L.; Xu, Y.; et al. Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle–yak and Yak). Metabolites 2022, 12, 1114. https://doi.org/10.3390/metabo12111114
Cui Y, Liu P, Yu S, He J, Afedo SY, Zou S, Zhang Q, Liu J, Song L, Xu Y, et al. Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle–yak and Yak). Metabolites. 2022; 12(11):1114. https://doi.org/10.3390/metabo12111114
Chicago/Turabian StyleCui, Yan, Penggang Liu, Sijiu Yu, Junfeng He, Seth Y. Afedo, Shengnan Zou, Qian Zhang, Jun Liu, Liangli Song, Yuanfang Xu, and et al. 2022. "Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle–yak and Yak)" Metabolites 12, no. 11: 1114. https://doi.org/10.3390/metabo12111114
APA StyleCui, Y., Liu, P., Yu, S., He, J., Afedo, S. Y., Zou, S., Zhang, Q., Liu, J., Song, L., Xu, Y., Wang, T., & Li, H. (2022). Expression Analysis of Molecular Chaperones Hsp70 and Hsp90 on Development and Metabolism of Different Organs and Testis in Cattle (Cattle–yak and Yak). Metabolites, 12(11), 1114. https://doi.org/10.3390/metabo12111114






