Metabolomics—A Tool to Find Metabolism of Endocrine Cancer
Abstract
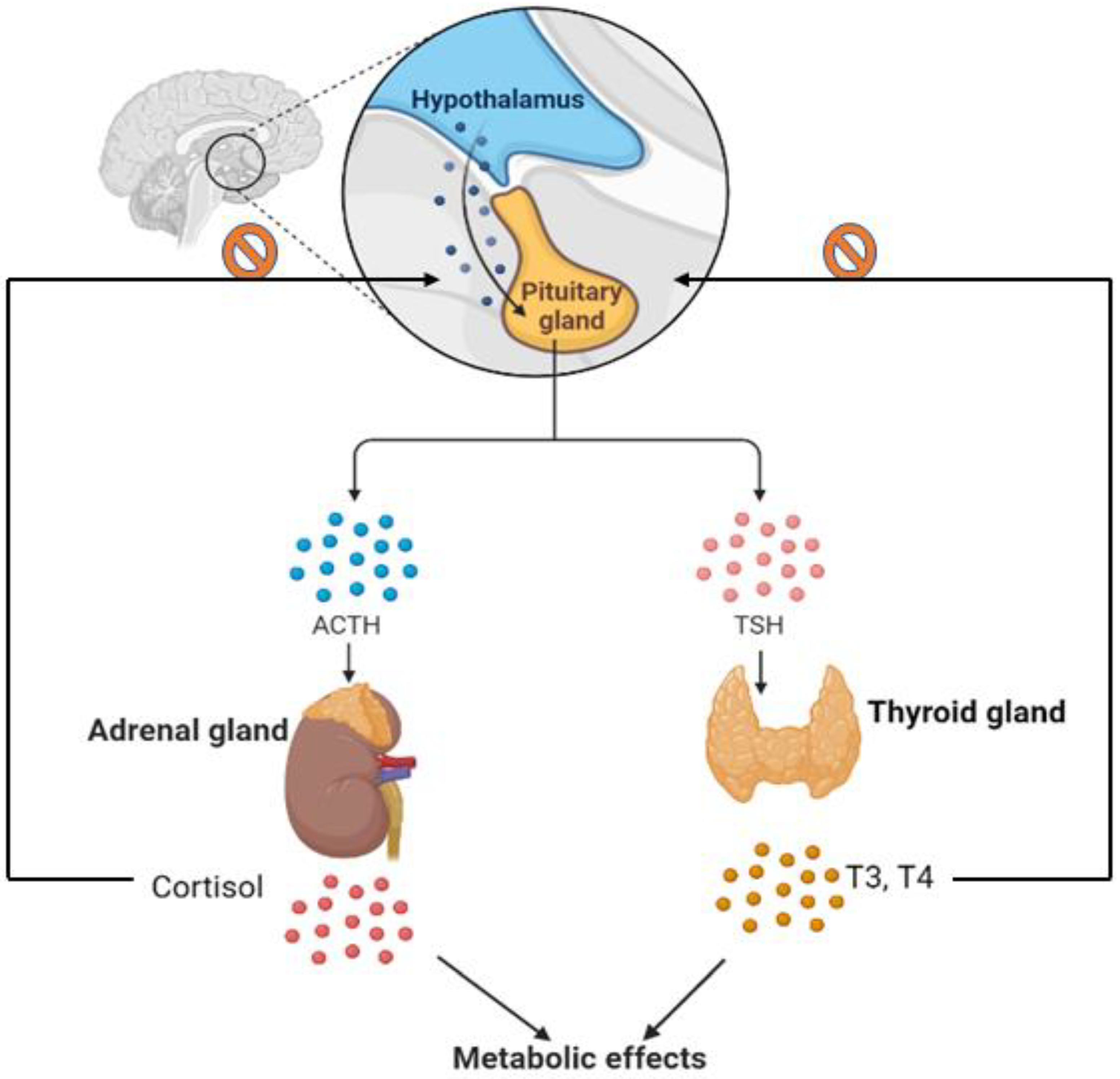
1. Introduction
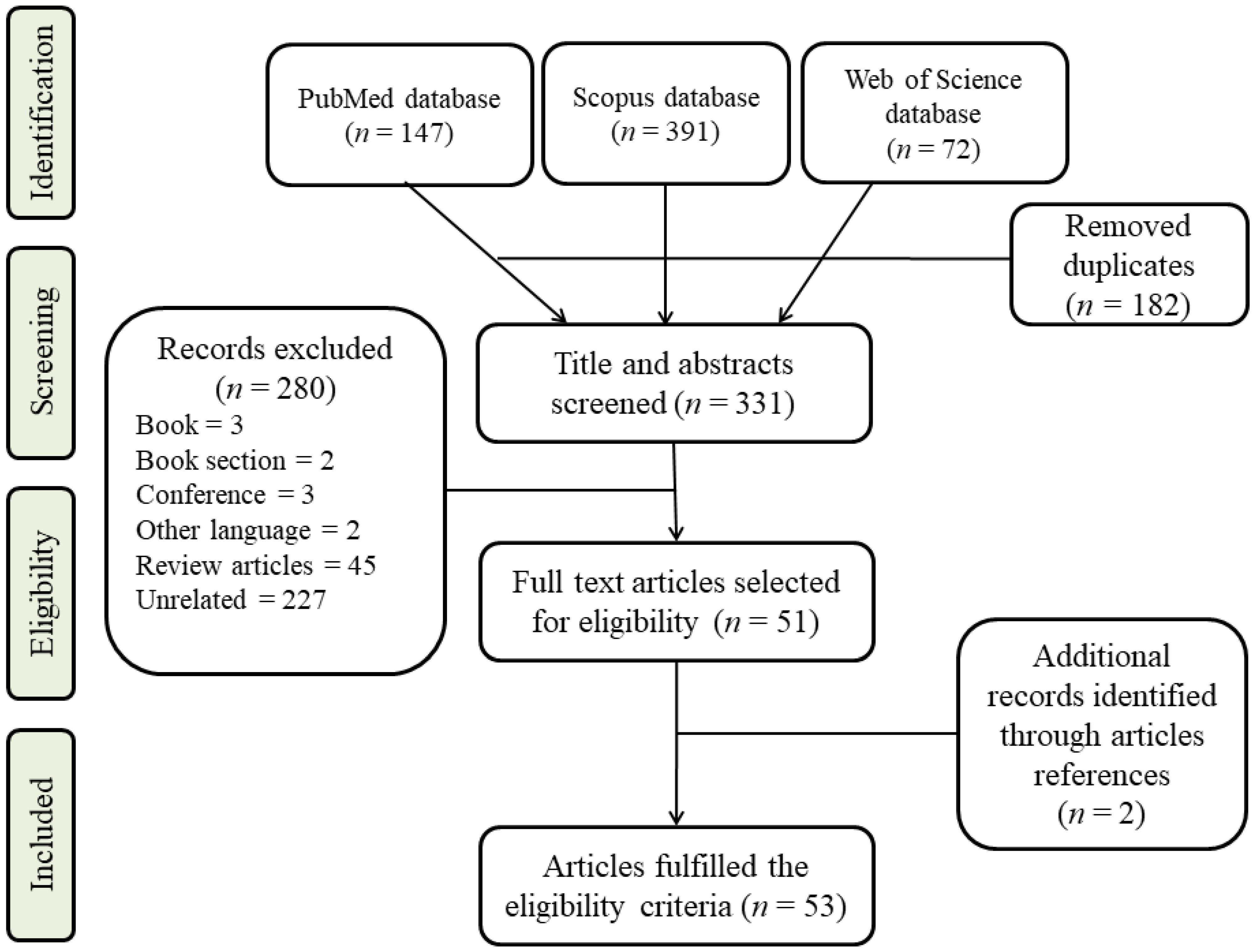
2. Search Strategy and Study Inclusion Criteria
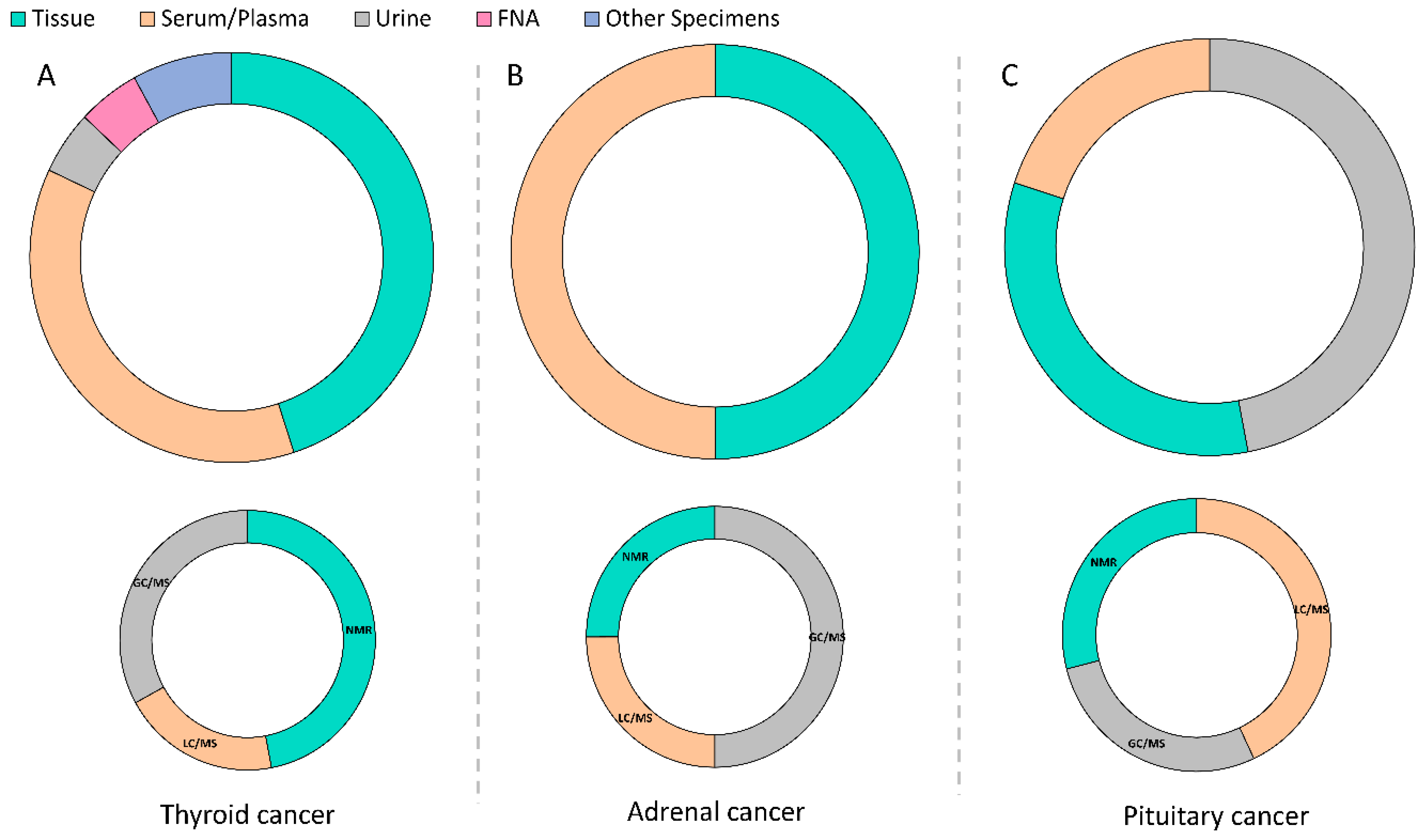
2.1. Thyroid Cancer
2.2. Adrenal Cancer
2.3. Pituitary Adenoma
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Cancer. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Correct, P.; Chen, V.W. Endocrine gland cancer. Cancer 1995, 75 (Suppl. S1), 338–352. [Google Scholar] [CrossRef]
- Geurts, J.L.; Strong, E.A.; Wang, T.S.; Evans, D.B.; Clarke, C.N. Screening guidelines and recommendations for patients at high risk of developing endocrine cancers. J. Surg. Oncol. 2020, 121, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Perks, C.M. Grand Challenges in Cancer Endocrinology: Endocrine Related Cancers, an Expanding Concept. Front. Endocrinol. 2013, 4, 141. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.E.; Lamy, F.; Roger, P.; Maenhaut, C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol. Rev. 1992, 72, 667–697. [Google Scholar] [CrossRef]
- Kim, H.K.; Yoon, J.H.; Kim, S.J.; Cho, J.S.; Kweon, S.-S.; Kang, H.-C. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin. Endocrinol. 2013, 78, 472–477. [Google Scholar] [CrossRef]
- Joehlin-Price, A.S.; Hardesty, D.A.; Arnold, C.A.; Kirschner, L.S.; Prevedello, D.M.; Lehman, N.L. Case report: ACTH-secreting pituitary carcinoma metastatic to the liver in a patient with a history of atypical pituitary adenoma and Cushing’s disease. Diagn. Pathol. 2017, 12, 34. [Google Scholar] [CrossRef]
- Raff, H.; Carroll, T. Cushing’s syndrome: From physiological principles to diagnosis and clinical care. J. Physiol. 2015, 593, 493–506. [Google Scholar] [CrossRef]
- Kosuda, A.; Shirahata, T.; Kudo, N.; Uehara, Y.; Miyawaki, M.; Hagiwara, A.; Murakami, R.; Shimizu, K. Long-term survival of a patient with small cell lung cancer secreting ADH and ACTH simultaneously, following the prolonged use of amrubicin. Intern. Med. 2020, 59, 107–112. [Google Scholar] [CrossRef]
- Menyhárt, O.; Győrffy, B. Multi-omics approaches in cancer research with applications in tumor subtyping, prognosis, and diagnosis. Comput. Struct. Biotechnol. J. 2021, 19, 949–960. [Google Scholar] [CrossRef]
- Beger, R.D. A review of applications of metabolomics in cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.E.; Barbas, C. Metabolomics in cancer biomarker discovery: Current trends and future perspectives. J. Pharm. Biomed. Anal. 2014, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Bowne, J. What is metabolomics all about? Biotechniques 2009, 46, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Trethewey, R.N.; Krotzky, A.J.; Willmitzer, L. Metabolite profiling: From diagnostics to systems biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Villas-Boas, G.S.; Nielsen, J.; Smedsgaard, J.; Hansen, M.A.E.; Roessner-Tunali, U. Metabolome Analysis: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Abooshahab, R.; Hooshmand, K.; Razavi, F.; Dass, C.R.; Hedayati, M. A glance at the actual role of glutamine metabolism in thyroid tumorigenesis. EXCLI J. 2021, 20, 1170. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Cooper, D.S. American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Lemoine, N.R.; Mayall, E.S.; Wyllie, F.S.; Farr, C.J.; Hughes, D.; A Padua, R.; Thurston, V.; Williams, E.D.; Wynford-Thomas, D. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988, 48, 4459–4463. [Google Scholar]
- Cibas, S.E.; Ali, S.Z. The Bethesda system for reporting thyroid cytopathology. Thyroid 2009, 19, 1159–1165. [Google Scholar] [CrossRef]
- Yang, J.; Schnadig, V.; Logrono, R.; Wasserman, P.G. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer Cytopathol. 2007, 111, 306–315. [Google Scholar] [CrossRef]
- Zarkesh, M.; Zadeh-Vakili, A.; Akbarzadeh, M.; Nozhat, Z.; Fanaei, S.A.; Hedayati, M.; Azizi, F. BRAF V600E mutation and microRNAs are helpful in distinguishing papillary thyroid malignant lesions: Tissues and fine needle aspiration cytology cases. Life Sci. 2019, 223, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.W.; Adkins, C.B.; Cheng, L.L.; Faquin, W.C. Application of Magnetic-Resonance-Spectroscopy-Based Metabolomics to the Fine-Needle Aspiration Diagnosis of Papillary Thyroid Carcinoma. Acta Cytol. 2011, 55, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Torregrossa, L.; Shintu, L.; Magalhaes, A.; Chandran, J.; Tintaru, A.; Ugolini, C.; Minuto, M.N.; Miccoli, M.; Basolo, F.; et al. Metabolomics approach to thyroid nodules: A high-resolution magic-angle spinning nuclear magnetic resonance-based study. Surgery 2012, 152, 1118–1124. [Google Scholar] [CrossRef]
- Torregrossa, L.; Shintu, L.; Chandran, J.N.; Tintaru, A.; Ugolini, C.; Magalhães, A.; Basolo, F.; Miccoli, P.; Caldarelli, S. Toward the Reliable Diagnosis of Indeterminate Thyroid Lesions: A HRMAS NMR-Based Metabolomics Case of Study. J. Proteome Res. 2012, 11, 3317–3325. [Google Scholar] [CrossRef]
- Deja, S.; Dawiskiba, T.; Balcerzak, W.; Orczyk-Pawiłowicz, M.; Głód, M.; Pawełka, D.; Młynarz, P. Follicular adenomas exhibit a unique metabolic profile. ¹H NMR studies of thyroid lesions. PLoS ONE 2013, 8, e84637. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Nie, X.; Xu, S.; Li, Y.; Huang, T.; Tang, H.; Wang, Y. Integrative metabonomics as potential method for diagnosis of thyroid malignancy. Sci. Rep. 2015, 5, 14869. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Wang, B.; Zhang, L.-Y.; Yan, S.-Y.; Yang, Y.-H. Analysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonance. Int. J. Clin. Exp. Med. 2015, 8, 18013–18022. [Google Scholar]
- Lu, J.; Hu, S.; Miccoli, P.; Zeng, Q.; Liu, S.; Ran, L.; Hu, C. Non-invasive diagnosis of papillary thyroid microcarcinoma: A NMR-based metabolomics approach. Oncotarget 2016, 7, 81768–81777. [Google Scholar] [CrossRef]
- Ryoo, I.; Kwon, H.N.; Kim, S.C.; Jung, S.C.; A Yeom, J.; Shin, H.S.; Cho, H.R.; Yun, T.J.; Choi, S.H.; Sohn, C.-H.; et al. Metabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: Potential application for the preoperative diagnosis of thyroid cancer. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef]
- Wojtowicz, W.; Zabek, A.; Deja, S.; Dawiskiba, T.; Pawelka, D.; Glod, M.; Balcerzak, W.; Mlynarz, P. Serum and urine H-1 NMR-based metabolomics in the diagnosis of selected thyroid diseases. Sci. Rep. 2017, 7, 13. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, C.; Xia, Y.; Xu, B.; Hu, Y.; Chen, T.; Shen, M.; Tang, W. Metabolic changes associated with papillary thyroid carcinoma: A nuclear magnetic resonance-based metabolomics study. Int. J. Mol. Med. 2018, 41, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Servadio, A.; Torregrossa, L.; Miccoli, P.; Basolo, F.; Shintu, L.; Caldarelli, S. Diagnosis of post-surgical fine-needle aspiration biopsies of thyroid lesions with indeterminate cytology using HRMAS NMR-based metabolomics. Metabolomics 2018, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Han, K.; Lee, J.; Kim, E.-K.; Moon, H.J.; Yoon, J.H.; Park, V.; Baek, H.-M.; Kwak, J.Y. Application of metabolomics in prediction of lymph node metastasis in papillary thyroid carcinoma. PLoS ONE 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Yekta, R.F.; Tavirani, M.R.; Oskuie, A.A.; Tehrani, M.R.M.; Soroush, A.R.; Baghban, A.A. Serum-based metabolic alterations in patients with papillary thyroid carcinoma unveiled by non-targeted 1H-NMR metabolomics approach. Iran. J. Basic Med. Sci. 2018, 21, 1140–1147. [Google Scholar]
- Metere, A.; Graves, C.E.; Chirico, M.; Caramujo, M.J.; Pisanu, M.E.; Iorio, E. Metabolomic Reprogramming Detected by (1)H-NMR Spectroscopy in Human Thyroid Cancer Tissues. Biology 2020, 9, 112. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Z.; Wang, Y.; Li, F.; Zhou, X.; Tian, X.; Wang, S. Diagnosis of papillary thyroid carcinoma by H-1 NMR spectroscopy-based metabolomic analysis of whole blood. Drug Discov. Ther. 2020, 14, 187–196. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.-Y.; Xie, C.; Zhang, M.-L.; Wang, Y.-J.; Liu, G.-H. Metabolomics as a potential method for predicting thyroid malignancy in children and adolescents. Pediatric Surg. Int. 2020, 36, 145–153. [Google Scholar] [CrossRef]
- Abooshahab, R.; Gholami, M.; Sanoie, M.; Azizi, F.; Hedayati, M. Advances in metabolomics of thyroid cancer diagnosis and metabolic regulation. Endocrine 2019, 65, 1–14. [Google Scholar] [CrossRef]
- Chen, M.; Shen, M.; Li, Y.; Liu, C.; Zhou, K.; Hu, W.; Xu, B.; Xia, Y.; Tang, W. GC-MS-based metabolomic analysis of human papillary thyroid carcinoma tissue. Int. J. Mol. Med. 2015, 36, 1607–1614. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Chi, C.; Wang, X.; Liu, S.; Zhao, W.; Ke, C.; Xu, G.; Li, E. Exhaled breath volatile biomarker analysis for thyroid cancer. Transl. Res. 2015, 166, 188–195. [Google Scholar] [CrossRef]
- Wojakowska, A.; Chekan, M.; Marczak, Ł.; Polanski, K.; Lange, D.; Pietrowska, M.; Widlak, P. Detection of metabolites discriminating subtypes of thyroid cancer: Molecular profiling of FFPE samples using the GC/MS approach. Mol. Cell Endocrinol. 2015, 417, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhong, X.; Tian, X. Metabolomics of papillary thyroid carcinoma tissues: Potential biomarkers for diagnosis and promising targets for therapy. Tumour Biol. 2016, 37, 11163–11175. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-T.; Zhang, Y.; Liu, Y.-M.; Yin, S.; Zhang, X.-Y.; Wei, W.-J.; Sun, Z.-K.; Song, H.-J.; Qiu, Z.-L.; Wang, C.-R.; et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin. Endocrinol. 2017, 87, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Abooshahab, R.; Hooshmand, K.; Razavi, S.A.; Gholami, M.; Sanoie, M.; Hedayati, M. Plasma Metabolic Profiling of Human Thyroid Nodules by Gas Chromatography-Mass Spectrometry (GC-MS)-Based Untargeted Metabolomics. Front. Cell Dev. Biol. 2020, 8, 13. [Google Scholar] [CrossRef]
- Jajin, M.G.; Abooshahab, R.; Hooshmand, K.; Moradi, A.; Siadat, S.D.; Mirzazadeh, R.; Chegini, K.G.; Hedayati, M. Gas chromatography-mass spectrometry-based untargeted metabolomics reveals metabolic perturbations in medullary thyroid carcinoma. Sci. Rep. 2022, 12, 8397. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.; Qiu, Y.; Jia, W.; Wang, J.; Yin, S. Distinct Metabolomic Profiles of Papillary Thyroid Carcinoma and Benign Thyroid Adenoma. J. Proteome Res. 2015, 14, 3315–3321. [Google Scholar] [CrossRef]
- Yao, Z.; Yin, P.; Su, D.; Peng, Z.; Zhou, L.; Ma, L.; Guo, W.; Ma, L.; Xu, G.; Shi, J.; et al. Serum metabolic profiling and features of papillary thyroid carcinoma and nodular goiter. Mol. BioSyst. 2011, 7, 2608–2614. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Q.; Hou, H.; Wang, S.; Zhang, Y.; Luo, Y.; Chen, H.; Deng, H.; Zhu, H.; Zhang, L.; et al. Metabolite analysis-aided diagnosis of papillary thyroid cancer. Endocr.-Relat. Cancer 2019, 26, 829–841. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2019, 144, 2728–2745. [Google Scholar] [CrossRef]
- Huang, F.-Q.; Li, J.; Jiang, L.; Wang, F.-X.; Alolga, R.N.; Wang, M.-J.; Min, W.-J.; Ma, G.; Zhao, Y.-J.; Wang, S.-L.; et al. Serum-plasma matched metabolomics for comprehensive characterization of benign thyroid nodule and papillary thyroid carcinoma. Int. J. Cancer 2019, 144, 868–876. [Google Scholar] [CrossRef]
- Du, Y.; Zou, L.; Fan, P. Research of tissue metabolomics in papillary thyroid carcinoma based on HPLC/Q-TOF-MS. Chin. J. Cancer Biother. 2020, 27, 1264–1271. [Google Scholar]
- Du, Y.; Fan, P.; Zou, L.; Jiang, Y.; Gu, X.; Yu, J.; Zhang, C. Serum Metabolomics Study of Papillary Thyroid Carcinoma Based on HPLC-Q-TOF-MS/MS. Front. Cell Dev. Biol. 2021, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, Z.; Chen, X.; Zhang, G.; Wang, Y.; Pan, L.; Yan, C.; Yang, G.; Zhao, L.; Han, J.; et al. Plasma Lipidomics Profiling Reveals Biomarkers for Papillary Thyroid Cancer Diagnosis. Front. Cell Dev. Biol. 2021, 9, 682269. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Sun, M.; Ding, C.; Zhang, L.; Kong, Y.; Cai, M.; Miccoli, P.; Ma, C.; Yue, X. Multi-Omics Analysis of Fatty Acid Metabolism in Thyroid Carcinoma. Front. Oncol. 2021, 11, 737127. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, H.; Liu, Z.; Wang, F.; He, Q.; Xiu, C.; Guo, L.; Tian, Q.; Fan, L.; Sun, J.; et al. Identifying potential metabolic tissue biomarkers for papillary thyroid cancer in different iodine nutrient regions. Endocrine 2021, 74, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, X.; Li, Y.; Li, X.; Qian, C.; Tian, Y.; Ling, R.; Duan, Y. Diagnostic approach to thyroid cancer based on amino acid metabolomics in saliva by ultra-performance liquid chromatography with high resolution mass spectrometry. Talanta 2021, 235, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, C.; Hou, Y.; Li, J.; Guo, Z.; Chen, X.; Zhang, L.; Peng, S.; Hong, S.; Xu, L.; et al. Integrative metabolomic characterization identifies plasma metabolomic signature in the diagnosis of papillary thyroid cancer. Oncogene 2022, 41, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.-y. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef]
- Mete, O.; Erickson, L.A.; Juhlin, C.C.; de Krijger, R.R.; Sasano, H.; Volante, M.; Papotti, M.G. Overview of the 2022 WHO classification of adrenal cortical tumors. Endocr. Pathol. 2022, 33, 155–196. [Google Scholar] [CrossRef]
- de Reyniès, A.; Assié, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; René-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009, 27, 1108–1115. [Google Scholar] [CrossRef]
- Bielinska, M.; Parviainen, H.; Kiiveri, S.; Heikinheimo, M.; Wilson, D.B. Origin and molecular pathology of adrenocortical neoplasms. Vet. Pathol. 2009, 46, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Medeiros, L.J.; Vickery, A.L., Jr. Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 1989, 13, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, A.; Elbayed, K.; Moussallieh, F.-M.; Reix, N.; Piotto, M.; Bellocq, J.-P.; Goichot, B.; Bachellier, P.; Namer, I.-J. Metabolomic profile of the adrenal gland: From physiology to pathological conditions. Endocr.-Relat. Cancer 2013, 20, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libé, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef]
- Kotłowska, A.; Sworczak, K.; Stepnowski, P. Urine metabolomics analysis for adrenal incidentaloma activity detection and biomarker discovery. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 359–363. [Google Scholar] [CrossRef]
- Patel, D.; Thompson, M.D.; Manna, S.K.; Krausz, K.W.; Zhang, L.; Nilubol, N.; Gonzalez, F.J.; Kebebew, E. Unique and Novel Urinary Metabolomic Features in Malignant versus Benign Adrenal Neoplasms. Clin. Cancer Res. 2017, 23, 5302–5310. [Google Scholar] [CrossRef]
- Martins, R.G.; Gonçalves, L.G.; Cunha, N.; Bugalho, M.J. Metabolomic Urine Profile: Searching for New Biomarkers of SDHx-Associated Pheochromocytomas and Paragangliomas. J. Clin. Endocrinol. Metab. 2019, 104, 5467–5477. [Google Scholar] [CrossRef]
- Velikanova, L.I.; Shafigullina, Z.R.; Vorokhobina, N.V.; Malevanaya, E.V. Gas Chromatography–Mass Spectrometry Analysis of Urinary Steroid Metabolomics for Detection of Early Signs of Adrenal Neoplasm Malignancy in Patients with Cushing’s Syndrome. Bull. Exp. Biol. Med. 2019, 167, 676–680. [Google Scholar] [CrossRef]
- Bancos, I.; E Taylor, A.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; E Taylor, A.; Ronchi, C.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine Steroid Metabolomics as a Novel Tool for Detection of Recurrent Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2020, 105, e307–e318. [Google Scholar] [CrossRef]
- Rao, J.U.; Engelke, U.F.H.; Sweep, F.C.G.J.; Pacak, K.; Kusters, B.; Goudswaard, A.G.; Hermus, A.R.M.M.; Mensenkamp, A.R.; Eisenhofer, G.; Qin, N.; et al. Genotype-specific differences in the tumor metabolite profile of pheochromocytoma and paraganglioma using untargeted and targeted metabolomics. J. Clin. Endocrinol. Metab. 2015, 100, E214–E222. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, S.; Kunz, M.; Kurlbaum, M.; Vey, J.; Kendl, S.; Deutschbein, T.; Hahner, S.; Fassnacht, M.; Dandekar, T.; Kroiss, M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019, 180, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.W.; Conrad, C.; Brückmann, S.; Pang, Y.; Caleiras, E.; Murakami, M.; Korpershoek, E.; Zhuang, Z.; Rapizzi, E.; Kroiss, M.; et al. Metabolomics, machine learning and immunohistochemistry to predict succinate dehydrogenase mutational status in phaeochromocytomas and paragangliomas. J. Pathol. 2020, 251, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Watts, D.; Steenblock, C.; Wallace, P.W.; Schürmann, A.; Bornstein, S.R.; Wielockx, B.; Eisenhofer, G.; Peitzsch, M. Adrenal Hormone Interactions and Metabolism: A Single Sample Multi-Omics Approach. Horm. Metab. Res. 2021, 53, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Berke, K.; Constantinescu, G.; Masjkur, J.; Kimpel, O.; Dischinger, U.; Peitzsch, M.; Kwapiszewska, A.; Dobrowolski, P.; Nölting, S.; Reincke, M.; et al. Plasma Steroid Profiling in Patients with Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2022, 107, e1181–e1192. [Google Scholar] [CrossRef]
- Bliziotis, N.G.; Kluijtmans, L.A.J.; Soto, S.; Tinnevelt, G.H.; Langton, K.; Robledo, M.; Pamporaki, C.; Engelke, U.F.H.; Erlic, Z.; Engel, J.; et al. Pre-versus post-operative untargeted plasma nuclear magnetic resonance spectroscopy metabolomics of pheochromocytoma and paraganglioma. Endocrine 2022, 75, 254–265. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Bertherat, J.; Giordano, T.J.; Hammer, G.D.; Sasano, H.; Mete, O. What did we learn from the molecular biology of adrenal cortical neoplasia? From histopathology to translational genomics. Endocr. Pathol. 2021, 32, 102–133. [Google Scholar] [CrossRef]
- Rao, J.U.; Engelke, U.F.; Rodenburg, R.J.; Wevers, R.A.; Pacak, K.; Eisenhofer, G.; Qin, N.; Kusters, B.; Goudswaard, A.G.; Lenders, J.W.; et al. Genotype-Specific Abnormalities in Mitochondrial Function Associate with Distinct Profiles of Energy Metabolism and Catecholamine Content in Pheochromocytoma and ParagangliomaEnergy Metabolism and Catecholamine Content in PGL. Clin. Cancer Res. 2013, 19, 3787–3795. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and treatment of pituitary adenomas: A review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef]
- Donovan, E.L.; Corenblum, B. The natural history of the pituitary incidentaloma. Arch. Intern. Med. 1995, 155, 181–183. [Google Scholar] [CrossRef]
- Freda, P.U.; Beckers, A.M.; Katznelson, L.; Moloch, M.E.; Montori, V.M.; Post, K.D.; Vance, M.L. Pituitary incidentaloma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, E.; Ferraroni, M.; Castrignanò, T.; Menicatti, L.; Anagni, M.; Reimondo, G.M.; Del Monte, P.; Bernasconi, D.; Loli, P.; Faustini-Fustini, M.; et al. Non-functioning pituitary adenoma database: A useful resource to improve the clinical management of pituitary tumors. Eur. J. Endocrinol. 2006, 155, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Deipolyi, A.R.; Wicky, S.; Ergul, E.; A Deik, A.; Chen, J.W.; A Hirsch, J.; Wojtkiewicz, G.R.; Clish, C.B. Identification of small compound biomarkers of pituitary adenoma: A bilateral inferior petrosal sinus sampling study. J. NeuroInterv. Surg. 2014, 6, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Q.; Zhou, Y.; Yu, S.; Hong, L.; Zhao, S.; Yang, J.; Wan, H.; Xu, G.; Zhang, Y.; et al. Integration of Proteomics and Metabolomics Revealed Metabolite-Protein Networks in ACTH-Secreting Pituitary Adenoma. Front. Endocrinol. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, H.; Zhang, Q.; Zhou, Y.; Li, C.; Zhao, S.; Hong, L.; Yang, J.; Hao, S.; Hong, W.; et al. Metabolic profiling reveals distinct metabolic alterations in different subtypes of pituitary adenomas and confers therapeutic targets. J. Transl. Med. 2019, 17, 291. [Google Scholar] [CrossRef] [PubMed]
- Ijare, O.; Holan, C.; Hebert, J.; Sharpe, M.A.; Baskin, D.S.; Pichumani, K. Elevated levels of circulating betahydroxybutyrate in pituitary tumor patients may differentiate prolactinomas from other immunohistochemical subtypes. Sci. Rep. 2020, 10, 1334. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abooshahab, R.; Ardalani, H.; Zarkesh, M.; Hooshmand, K.; Bakhshi, A.; Dass, C.R.; Hedayati, M. Metabolomics—A Tool to Find Metabolism of Endocrine Cancer. Metabolites 2022, 12, 1154. https://doi.org/10.3390/metabo12111154
Abooshahab R, Ardalani H, Zarkesh M, Hooshmand K, Bakhshi A, Dass CR, Hedayati M. Metabolomics—A Tool to Find Metabolism of Endocrine Cancer. Metabolites. 2022; 12(11):1154. https://doi.org/10.3390/metabo12111154
Chicago/Turabian StyleAbooshahab, Raziyeh, Hamidreza Ardalani, Maryam Zarkesh, Koroush Hooshmand, Ali Bakhshi, Crispin R. Dass, and Mehdi Hedayati. 2022. "Metabolomics—A Tool to Find Metabolism of Endocrine Cancer" Metabolites 12, no. 11: 1154. https://doi.org/10.3390/metabo12111154
APA StyleAbooshahab, R., Ardalani, H., Zarkesh, M., Hooshmand, K., Bakhshi, A., Dass, C. R., & Hedayati, M. (2022). Metabolomics—A Tool to Find Metabolism of Endocrine Cancer. Metabolites, 12(11), 1154. https://doi.org/10.3390/metabo12111154






