Epigenetic Age Acceleration in Mothers and Offspring 4–10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Measurements
2.3. DNA Methylation Measurements
2.4. Epigenetic Age and Age Acceleration
2.5. Statistical Analysis
3. Results
3.1. Demographics
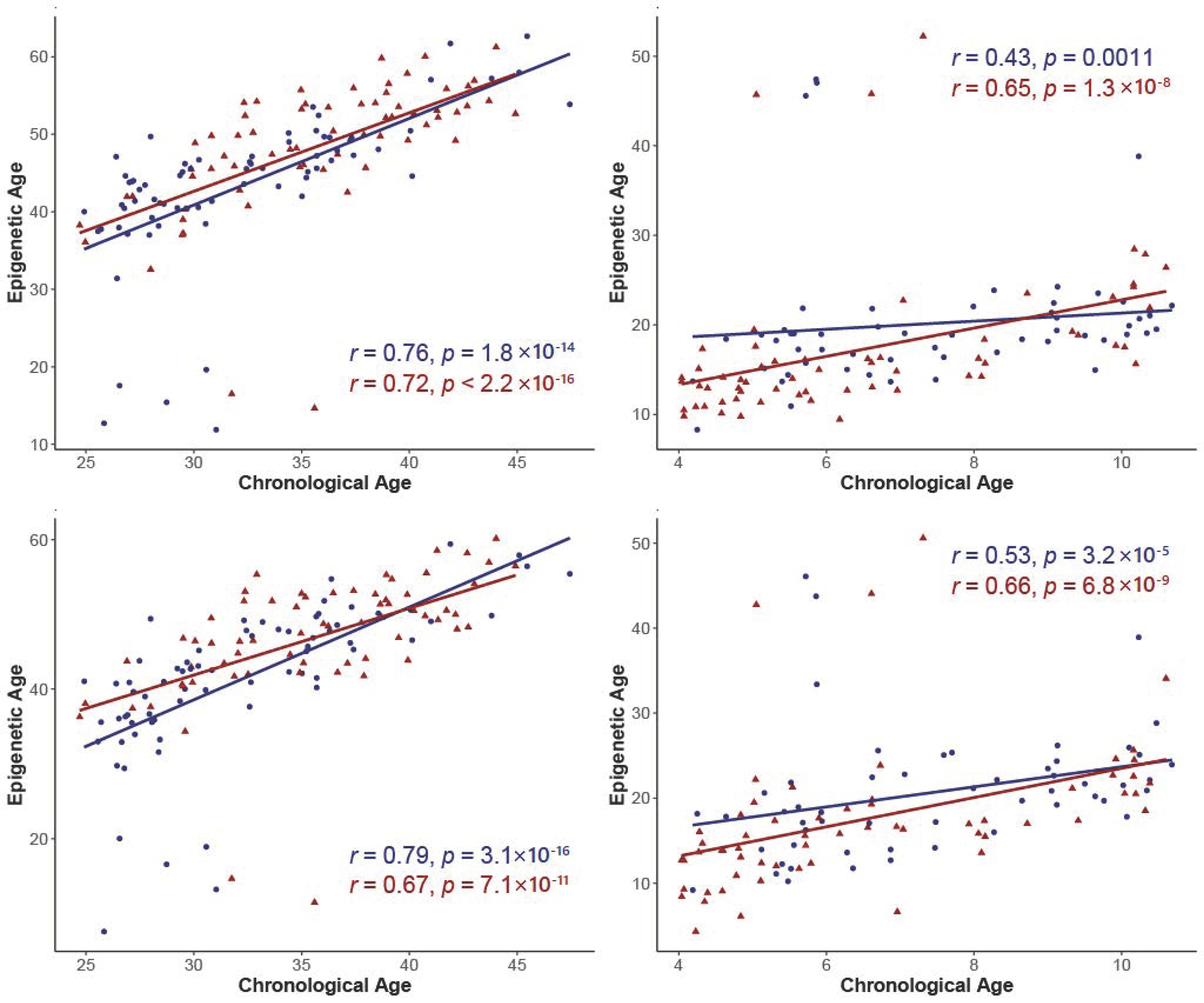
3.2. Correlations between Epigenetic Age and Chronological Age
3.3. Age Acceleration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSisto, C.L.; Kim, S.Y.; Sharma, A.J. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS). Prev. Chronic Dis. 2014, 11, 2007–2010. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, S.S.; Linder, B.; Cowie, C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018, 141, 200–208. [Google Scholar] [CrossRef]
- Buchanan, A.T.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, B.; Toulis, K.A.; Thomas, N.; Gokhale, K.; Martin, J.; Webber, J.; Keerthy, D.; Jolly, K.; Saravanan, P.; Nirantharakumar, K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018, 15, e1002488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, B.R.; Retnakaran, R.; Booth, G.L. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008, 31, 1668–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Pan, L.; Liu, E.; Liu, H.; Liu, J.; Wang, S.; Guo, J.; Li, N.; Zhang, C.; Hu, G. Gestational diabetes and offspring’s growth from birth to 6 years old. Int. J. Obes. 2019, 43, 663–672. [Google Scholar] [CrossRef]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sørensen, H.T.; Qin, G.; Li, J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sørensen, H.T.; Qin, G.; Li, J. Maternal Gestational Diabetes Is Associated with Offspring’s Hypertension. Am. J. Hypertens. 2019, 32, 335–342. [Google Scholar]
- Gillman, M.W.; Rifas-Shiman, S.; Berkey, C.S.; Field, A.E.; Colditz, G.A. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003, 111, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Lowe, W.L.J.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liu, F.; Zhang, H.; Kan, M.; Wang, T.; Dong, M.; Liu, Y. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Awamleh, Z.; Butcher, D.T.; Hanley, A.; Retnakaran, R.; Haertle, L.; Haaf, T.; Hamilton, J.; Weksberg, R. Exposure to Gestational Diabetes Mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Res. Clin. Pract. 2021, 174, 108690. [Google Scholar] [CrossRef] [PubMed]
- Finer, S.; Mathews, C.; Lowe, R.; Smart, M.; Hillman, S.; Foo, L.; Sinha, A.; Williams, D.; Rakyan, V.K.; Hitman, G.A. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum. Mol. Genet. 2015, 24, 3021–3029. [Google Scholar] [CrossRef]
- Kim, C.; Harrall, K.K.; Glueck, D.H.; Needham, B.L.; Dabelea, D. Gestational diabetes mellitus, epigenetic age and offspring metabolism-Kim-2022-Diabetic Medicine-Wiley Online Library. Diabet. Med. 2022, 39, e14925. [Google Scholar]
- Shiau, S.; Wang, L.; Liu, H.; Zheng, Y.; Drong, A.; Joyce, B.T.; Baccarelli, A.A. Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics 2021, 16, 186–195. [Google Scholar] [CrossRef]
- Martin, S.L.; Zhang, L.; Callahan, M.L.; Bahorski, J.; Lewis, C.E.; Hidalgo, B.A.; Durant, N.; Harper, L.M.; Battarbee, A.N.; Habegger, K.; et al. Mother-child cardiometabolic health 4–10 years after pregnancy complicated by obesity with and without gestational diabetes. Obes. Sci. Pract. 2022, 8, 627–640. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report; Obesity Research; National Library of Medicine: Bethesda, MD, USA, 1998; Volume 6. [Google Scholar]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. CDC Growth Charts: United States; DHHS Publication: Hyattsville, AR, USA, 2000. Available online: https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf (accessed on 1 November 2022).
- Dezenberg, C.V.; Nagy, T.R.; Gower, B.A.; Johnson, R.; Goran, M.I. Predicting body composition from anthropometry in pre-adolescent children. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1999, 23, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, L.; Li, L.; Taylor, J.A. ENmix: A novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acid. Res. 2016, 44, e20. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvin, M.R.; Aslibekyan, S.; Do, A.; Zhi, D.; Hidalgo, B.; Claas, S.A.; Srinivasasainagendra, V.; Horvath, S.; Tiwari, H.K.; Absher, D.M.; et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clin. Epigenetics 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.I.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Lee, C.N.; Li, H.Y.; Hsu, K.H.; Lin, S.Y. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res. Clin. Pract. 2017, 132, 127–136. [Google Scholar] [CrossRef]
- Ross, K.M.; Carroll, J.; Horvath, S.; Hobel, C.J.; Coussons-Read, M.E.; Dunkel Schetter, C. Immune epigenetic age in pregnancy and 1 year after birth: Associations with weight change. Am. J. Reprod. Immunol. 2020, 83, e13229. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Lin, L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine 2019, 98, e15320. [Google Scholar] [CrossRef]
- Ross, K.M.; Carroll, J.; Horvath, S.; Hobel, C.J.; Coussons-Read, M.E.; Dunkel Schetter, C. Gestational diabetes and long-term risk for dyslipidemia: A population-based historical cohort study. BMJ Open Diabetes Res. Care 2020, 8, e000870. [Google Scholar]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–67. [Google Scholar] [CrossRef] [PubMed]
- PPeuchant, E.; Brun, J.L.; Rigalleau, V.; Dubourg, L.; Thomas, M.J.; Daniel, J.Y.; Leng, J.J.; Gin, H. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin. Biochem. 2004, 37, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Peuchant, E.; Brun, J.L.; Rigalleau, V.; Dubourg, L.; Thomas, M.J.; Daniel, J.Y.; Leng, J.J.; Gin, H. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta 2004, 37, 293–298. [Google Scholar]
- Chen, X.; Scholl, T.O. Oxidative stress: Changes in pregnancy and with gestational diabetes mellitus. Curr. Diabetes Rep. 2005, 5, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Tam, W.H.; Ma, R.C.W.; Yang, X.; Li, A.M.; Ko, G.T.C.K.; Kong, A.P.S.K.; Lao, T.T.H.; Chan, M.H.M.; Lam, C.W.K.; Chan, J.C.N. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: A 15-year follow-up study. Diabetes Care 2010, 33, 1382–1384. [Google Scholar] [CrossRef] [Green Version]
- Tam, W.H.; Ma, R.C.; Yang, X.; Li, A.M.; Ko, G.T.; Kong, A.P.; Lao, T.T.; Chan, M.H.; Lam, C.W.; Chan, J.C. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar]
- Simpkin, A.J.; Hemani, G.; Suderman, M.; Gaunt, T.R.; Lyttleton, O.; Mcardle, W.L.; Ring, S.M.; Sharp, G.C.; Tilling, K.; Horvath, S.; et al. Prenatal and early life influences on epigenetic age in children: A study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet. 2016, 25, 191–201. [Google Scholar] [CrossRef]
| Non-GDM | GDM | P-Value | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mothers N = 71 | Offspring N = 55 | Mothers N = 66 | Offspring N = 61 | Mothers | Offspring |
| Age (years) | 0.0002 | 0.0161 | ||||
| Mean (SD) | 32.2 (5.4) | 7.5 (2.0) | 35.7 (5.0) | 6.5 (2.1) | ||
| Maternal Race, n (%) | 0.0824 | -- | ||||
| Black | 56 (78.9) | 42 (76.4) | 59 (89.4) | 54 (88.5) | ||
| White | 14 (19.7) | 12 (21.8) | 6 (9.1) | 6 (9.8) | ||
| Asian/AI | 1 (1.4) | 1 (1.8) | 1 (1.5) | 1 (1.6) | ||
| Maternal Ethnicity, n (%) | 0.3518 | -- | ||||
| Not Hispanic | 69 (97.2) | 53 (96.4) | 63 (95.5) | 58 (95.1) | ||
| Hispanic | 1 (1.4) | 1 (1.8) | 3 (4.6) | 3 (4.9) | ||
| Missing | 1 (1.4) | 1 (1.8) | 0 (0) | 0 (0) | ||
| Mom Current BMI (kg/m2) | <0.0001 | -- | ||||
| Mean (SD) | 24.9 (4.1) | -- | 37.9 (9.7) | -- | ||
| Body Fat Percent a | <0.0001 | 0.3057 | ||||
| Mean (SD) | 31.8 (7.3) | 23.6 (8.9) | 42.1 (6.3) | 25.3 (9.6) | ||
| Waist-to-hip ratio | <0.0001 | 0.0245 | ||||
| Mean (SD) | 0.8 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | ||
| HOMA-IR | <0.0001 | 0.9978 | ||||
| Mean (SD) | 2.0 (1.6) | 1.8 (2.7) | 5.0 (4.5) | 1.8 (1.9) | ||
| Leptin | <0.0001 | 0.2259 | ||||
| Mean (SD) | 38.2 (35.6) | 12.9 (17.2) | 73.8 (34.3) | 16.8 (16.9) | ||
| HDL-C (mg/dL) | <0.0001 | 0.8316 | ||||
| Mean (SD) | 68.2 (13.0) | 63.8 (11.8) | 56.0 (11.9) | 64.2 (10.8) | ||
| Fasting Glucose (mg/dL) | 0.0008 | 0.7887 | ||||
| Mean (SD) | 89.9 (8.4) | 88.2 (8.6) | 118.3 (65.3) | 88.6 (6.9) | ||
| Fasting Insulin (μU/mL) | <0.0001 | 0.9314 | ||||
| Mean (SD) | 8.7 (6.2) | 7.7 (10.8) | 17.1 (11.8) | 7.9 (8.0) | ||
| Child Sex, n (%) | -- | 0.9459 | ||||
| Female | -- | 31 (56.4) | -- | 34 (55.7) | ||
| Male | -- | 24 (43.6) | -- | 27 (44.3) | ||
| Tanner Stage, n (%) | -- | 0.8902 | ||||
| Stage 1 | -- | 46 (83.6) | -- | 51 (83.6) | ||
| Stage 2 | -- | 7 (12.7) | -- | 8 (13.1) | ||
| Stage 3 | -- | 1 (1.8) | -- | 2 (3.3) | ||
| Missing | -- | 1 (1.8) | -- | 0 (0) | ||
| Child BMI z | -- | 0.1996 | ||||
| Mean (SD) | -- | 0.4 (1.1) | -- | 0.7 (1.3) | ||
| Child WHtZ | -- | 0.0376 | ||||
| Mean (SD) | -- | 0.9 (1.1) | -- | 0.4 (1.3) | ||
| Mothers (N = 137) | Offspring (N = 116) | |||||||
|---|---|---|---|---|---|---|---|---|
| Horvath Age Acceleration Residual | Hannum Age Acceleration Residual | Horvath Age Acceleration Residual | Hannum Age Acceleration Residual | |||||
| r | P | r | P | r | P | r | P | |
| BMI * | 0.02713 | 0.7530 | 0.08014 | 0.3519 | 0.02134 | 0.8202 | −0.00857 | 0.9273 |
| WHR | 0.07456 | 0.3865 | 0.04489 | 0.6025 | −0.15946 | 0.0873 | −0.03823 | 0.6837 |
| Leptin | −0.02402 | 0.7805 | 0.05452 | 0.5269 | −0.08996 | 0.3369 | 0.01168 | 0.9009 |
| HOMA-IR | 0.06392 | 0.4580 | 0.10197 | 0.2358 | 0.06193 | 0.5090 | 0.06326 | 0.4999 |
| HDL-C | −0.07286 | 0.3975 | −0.23223 | 0.0063 | −0.04985 | 0.5951 | −0.10348 | 0.2690 |
| Fasting Glucose | −0.03274 | 0.7041 | 0.07880 | 0.3600 | 0.14334 | 0.1248 | 0.07058 | 0.4515 |
| Fasting Insulin | 0.07759 | 0.3675 | 0.11728 | 0.1723 | 0.04552 | 0.6275 | 0.05466 | 0.5601 |
| Horvath Age Acceleration | Hannum Age Acceleration | |||
|---|---|---|---|---|
| Variable | β (SE) | P-Value | β (SE) | P-Value |
| HDL-C (mg/dL) | −0.073(0.041) | 0.0751 | −0.088(0.038) | 0.0244 |
| Leptin | −0.028(0.022) | 0.2038 | −0.021(0.020) | 0.3155 |
| HOMA-IR | 0.090(0.164) | 0.5833 | 0.058(0.154) | 0.7056 |
| Fasting Glucose (mg/dL) | −0.005(0.013) | 0.7206 | −0.008(0.012) | 0.4901 |
| Fasting Insulin (μU/mL) | 0.053(0.057) | 0.3605 | 0.035(0.054) | 0.5145 |
| Horvath | Hannum | |||
|---|---|---|---|---|
| Variable | β (SE) | P-Value | β (SE) | P-Value |
| HDL-C (mg/dL) | 0.030(0.061) | 0.6226 | 0.017(0.051) | 0.7488 |
| Leptin | −0.037(0.058) | 0.5226 | −0.0098(0.049) | 0.8420 |
| HOMA-IR | 0.461(0.342) | 0.1807 | 0.533(0.286) | 0.0648 |
| Fasting Glucose (mg/dL) | 0.087(0.088) | 0.3259 | 0.062(0.074) | 0.4014 |
| Fasting Insulin (μU/mL) | 0.128(0.084) | 0.1320 | 0.143(0.070) | 0.0488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanney, N.; Patki, A.; Chandler-Laney, P.; Garvey, W.T.; Hidalgo, B.A. Epigenetic Age Acceleration in Mothers and Offspring 4–10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity. Metabolites 2022, 12, 1226. https://doi.org/10.3390/metabo12121226
Kanney N, Patki A, Chandler-Laney P, Garvey WT, Hidalgo BA. Epigenetic Age Acceleration in Mothers and Offspring 4–10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity. Metabolites. 2022; 12(12):1226. https://doi.org/10.3390/metabo12121226
Chicago/Turabian StyleKanney, Nita, Amit Patki, Paula Chandler-Laney, W. Timothy Garvey, and Bertha A. Hidalgo. 2022. "Epigenetic Age Acceleration in Mothers and Offspring 4–10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity" Metabolites 12, no. 12: 1226. https://doi.org/10.3390/metabo12121226





