Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo
Abstract
:1. Introduction
2. Results
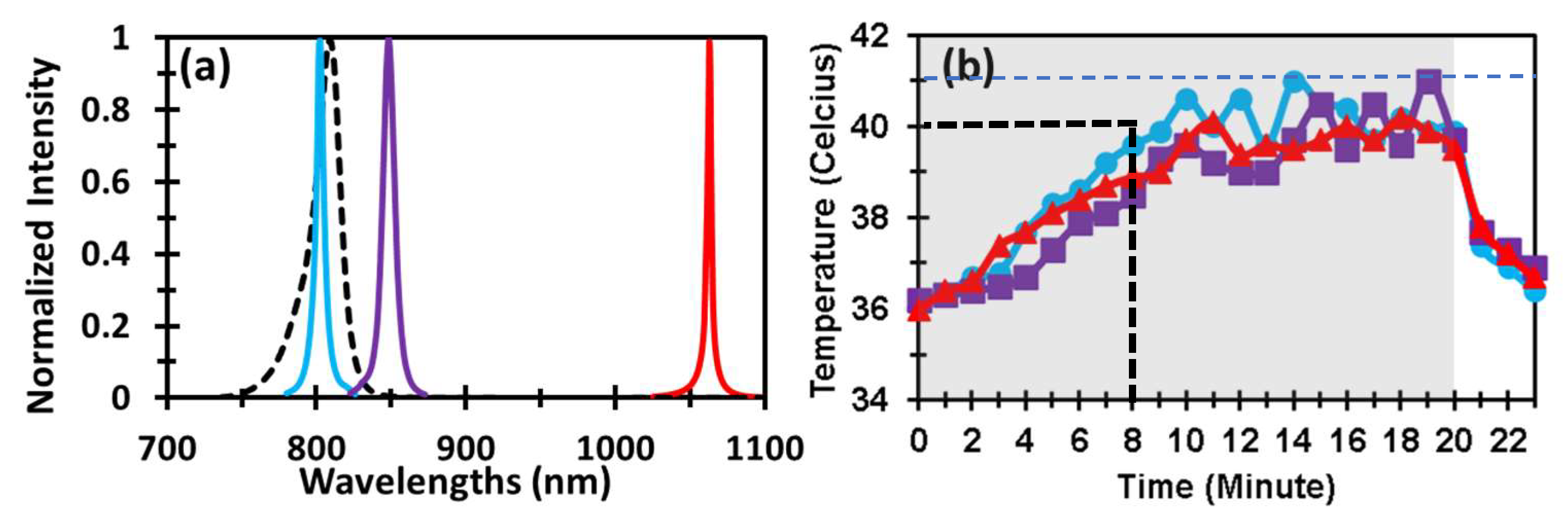
2.1. Optical Spectra of Lasers and LED and Laser-Induced Increases of Skin Temperature
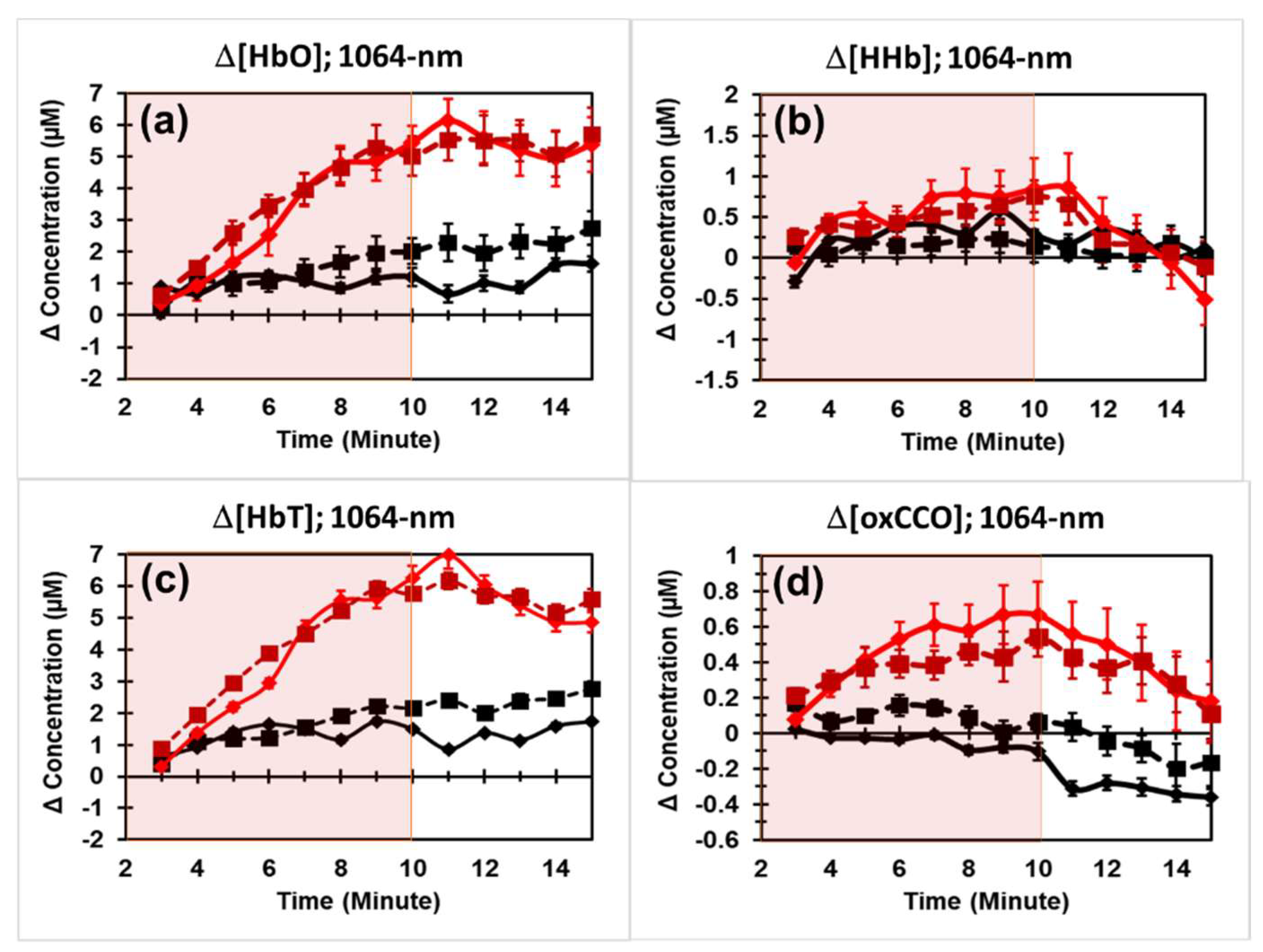
2.2. PBM Effects by 1064 nm Laser on the Human Forearm
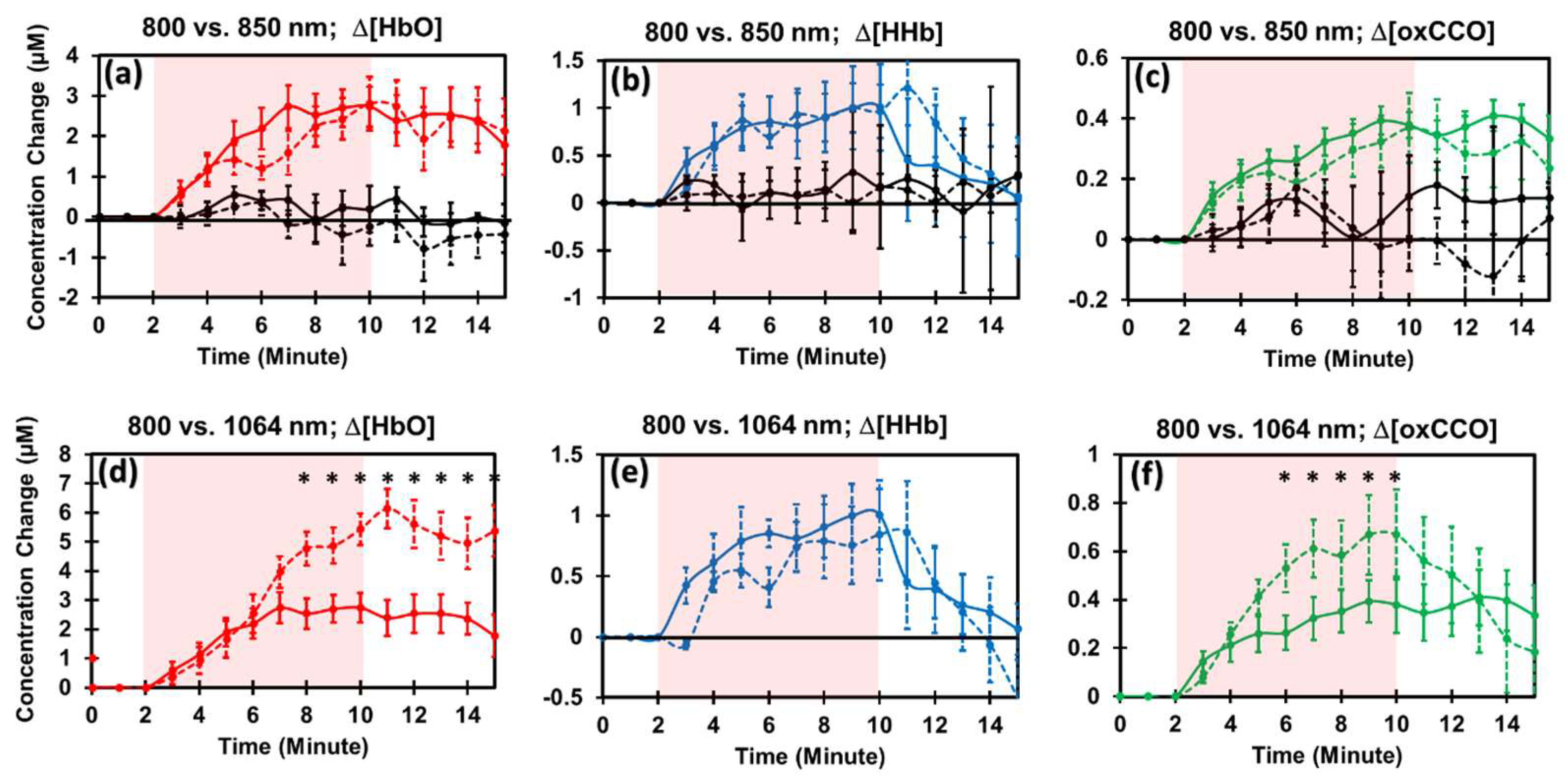
2.3. PBM Effects by 800 nm and 850 nm Lasers on the Human Forearm
2.4. Comparison of PBM Effects by 1064 nm vs. 800 nm Lasers
2.5. PBM Effects by 810 nm LED and Comparison with Those by 800 nm Laser
3. Discussion
3.1. High Reproducibility of 1064 nm PBM on the Human Forearm
3.2. Experimental Evidence for Proval of Hypothesis 1
3.3. Experimental Confirmation for Hypothesis 2
3.4. Experimental Confirmation for Hypothesis 3
3.5. Tool to Guide Light Selection and Dosage for Effective Clinical Applications of PBM
3.6. Limitations of the Study and Future Work
4. Materials and Methods
4.1. Participants
4.2. Instrumentation for PBM and bbNIRS
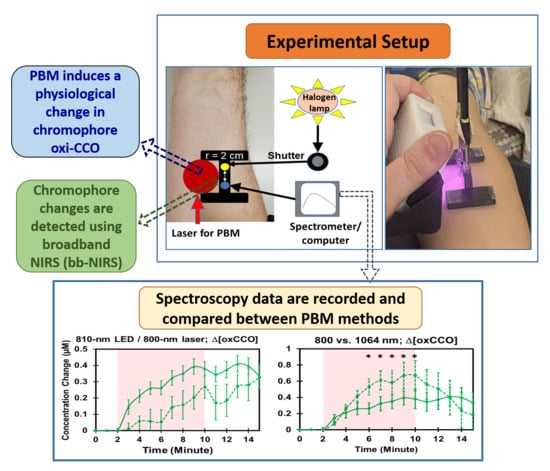
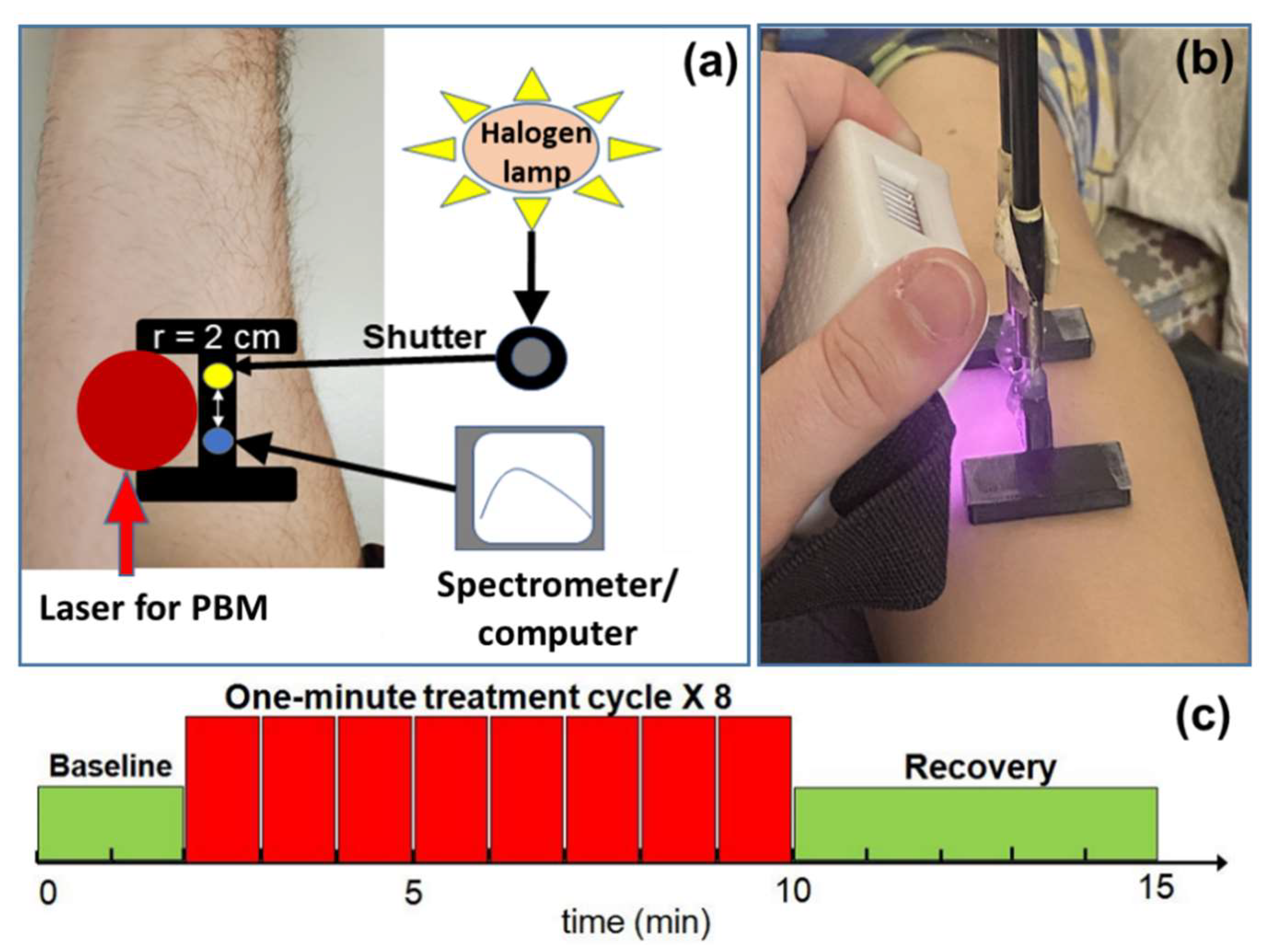
4.3. Experimental Setup and Protocol
4.4. Spectra of Lasers/LED and PBM-Induced Temperature Changes
4.5. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arany, P. Craniofacial wound healing with photobiomodulation therapy: New insights and current challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Barna, L.; Chenault, V.M.; Waynant, R.W.; Ilev, I.K.; Longo, L.; Miracco, C.; Johnson, B.; Anders, J.J. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed. Laser Ther. 2004, 22, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Medrado, A.P.; Soares, A.P.; Santos, E.T.; Reis, S.R.A.; Andrade, Z.A. Influence of laser photobiomodulation upon connective tissue remodeling during wound healing. J. Photochem. Photobiol. B Biol. 2008, 92, 144–152. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Albertini, R.; de Carvalho, P.D.T.C.; Leal-Junior, E.C.P.; Bussadori, S.K.; Vieira, S.S.; Bocalini, D.S.; de Oliveira, L.V.F.; Grandinetti, V.; Silva, J.A. Randomized, blinded, controlled trial on effectiveness of photobiomodulation therapy and exercise training in the fibromyalgia treatment. Lasers Med. Sci. 2018, 33, 343–351. [Google Scholar] [CrossRef]
- De Andrade, A.L.M.; Bossini, P.S.; do Canto De, A.L.M.; Sanchez, A.D.; Parizotto, N.A. Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med. Sci. 2017, 32, 865–872. [Google Scholar] [CrossRef] [PubMed]
- De Paula Gomes, C.A.; Leal-Junior, E.C.; Dibai-Filho, A.V.; de Oliveira, A.R.; Bley, A.S.; Biasotto-Gonzalez, D.A.; de Tarso Camillo de Carvalho, P. Incorporation of photobiomodulation therapy into a therapeutic exercise program for knee osteoarthritis: A placebo-controlled, randomized, clinical trial. Lasers Surg. Med. 2018, 50, 819–828. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.V.P.; Kawakubo, M.; Ferraresi, C.; Kaippert, B.; Yoshimura, E.M.; Hamblin, M.R. Pain management using photobiomodulation: Mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice. J. Biophotonics 2018, 11, e201700370. [Google Scholar] [CrossRef]
- Argibay, B.; Campos, F.; Perez-Mato, M.; Vieites-Prado, A.; Correa-Paz, C.; López-Arias, E.; Silva-Candal, D.; Moreno, V.; Montero, C.; Sobrino, T. Light-emitting diode photobiomodulation after cerebral ischemia. Front. Neurol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Y.; Lu, Y.; Zhang, W.; Brann, D.W.; Zhang, Q. Photobiomodulation for global cerebral ischemia: Targeting mitochondrial dynamics and functions. Mol. Neurobiol. 2019, 56, 1852–1869. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.-Y.; Zhang, Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp. Neurol. 2018, 299, 86–96. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Cassano, P.; Sadigh-Eteghad, S.; Erfani, M.; Hamblin, M.R.; Salimi, M.M.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 2019, 144, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Petrie, S.R.; Mischoulon, D.; Cusin, C.; Katnani, H.; Yeung, A.; De Taboada, L.; Archibald, A.; Bui, E.; Baer, L. Transcranial photobiomodulation for the treatment of major depressive disorder. The ELATED-2 pilot trial. Photomed. Laser Surg. 2018, 36, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Caldieraro, M.A.; Cassano, P. Transcranial and systemic photobiomodulation for major depressive disorder: A systematic review of efficacy, tolerability and biological mechanisms. J. Affect. Disord. 2019, 243, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.H.; Halper, J.P.; Nichols, T.W.; Jarrett, H.; Lundy, A.; Huang, J.H. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J. Neurol. Neurosci. 2017, 8, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.S.; Lee, T.L.; Yeung, M.K.; Hamblin, M.R. Photobiomodulation improves the frontal cognitive function of older adults. Int. J. Geriatr. Psychiatry 2019, 34, 369–377. [Google Scholar] [CrossRef]
- Mitrofanis, J.; Henderson, L.A. How and why does photobiomodulation change brain activity? Neural Regen. Res. 2020, 15, 2243. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.W.; Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013, 230, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.J.; Maddox, W.T.; Gonzalez-Lima, F. Improving executive function using transcranial infrared laser stimulation. J. Neuropsychol 2017, 11, 14–25. [Google Scholar] [CrossRef]
- Karu, T.I. Cellular and molecular mechanisms of photobiomodulation (low-power laser therapy). IEEE J. Sel. Top. Quantum Electron. 2013, 20, 143–148. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.-Y.; Lee, M.Y.; Chung, P.-S.; Kim, S.; Choi, B.; Suh, M.-W.; Rhee, C.-K.; Jung, J.Y. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.M.; Gonçalves, E.C.; Cavalli, J.; Vieira, G.; Laurindo, L.R.; Simões, R.R.; Coelho, I.S.; Santos, A.R.; Marcolino, A.M.; Cola, M. Photobiomodulation therapy improves acute inflammatory response in mice: The role of cannabinoid receptors/ATP-sensitive K+ channel/p38-MAPK signalling pathway. Mol. Neurobiol. 2018, 55, 5580–5593. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Moon, J.H.; Jung, J.Y.; Oh, C.; Ahn, J.-C.; Chung, P.-S. Effect of photobiomodulation therapy on neuronal injuries by ouabain: The regulation of Na, K-ATPase; Src; and mitogen-activated protein kinase signaling pathway. BMC Neurosci. 2019, 20, 19. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337. [Google Scholar] [CrossRef]
- Pruitt, T.; Wang, X.; Wu, A.; Kallioniemi, E.; Husain, M.M.; Liu, H. Transcranial Photobiomodulation (tPBM) With 1064-nm Laser to Improve Cerebral Metabolism of the Human Brain In Vivo. Lasers Surg. Med. 2020, 52, 807–813. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain photobiomodulation therapy: A narrative review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Wang, X.; Dmochowski, J.P.; Zeng, L.; Kallioniemi, E.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. Neurophotonics 2019, 6, 025013. [Google Scholar] [CrossRef]
- Zomorrodi, R.; Saltmarche, A.E.; Loheswaran, G.; Ho, K.F.; Lim, L. Complementary EEG evidence for a significantly improved Alzheimer’s disease case after photobiomodulation treatment. In Proceedings of the 26th Annual Scientific Conference, Canadian Academy of Geriatric Psychiatry, Toronto, ON, Canada, 4–5 November 2017. [Google Scholar]
- Bainbridge, A.; Tachtsidis, I.; Faulkner, S.D.; Price, D.; Zhu, T.; Baer, E.; Broad, K.D.; Thomas, D.L.; Cady, E.B.; Robertson, N.J.; et al. Brain mitochondrial oxidative metabolism during and after cerebral hypoxia-ischemia studied by simultaneous phosphorus magnetic-resonance and broadband near-infrared spectroscopy. NeuroImage 2014, 102 Pt 1, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachtsidis, I.; Koh, P.H.; Stubbs, C.; Elwell, C.E. Functional optical topography analysis using statistical parametric mapping (SPM) methodology with and without physiological confounds. Adv. Exp. Med. Biol. 2010, 662, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolyva, C.; Tachtsidis, I.; Ghosh, A.; Moroz, T.; Cooper, C.E.; Smith, M.; Elwell, C.E. Systematic investigation of changes in oxidized cerebral cytochrome c oxidase concentration during frontal lobe activation in healthy adults. Biomed. Opt. Express 2012, 3, 2550–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolyva, C.; Ghosh, A.; Tachtsidis, I.; Highton, D.; Cooper, C.E.; Smith, M.; Elwell, C.E. Cytochrome c oxidase response to changes in cerebral oxygen delivery in the adult brain shows higher brain-specificity than haemoglobin. NeuroImage 2014, 85 Pt 1, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Bale, G.; Mitra, S.; Meek, J.; Robertson, N.; Tachtsidis, I. A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed. Opt. Express 2014, 5, 3450–3466. [Google Scholar] [CrossRef] [Green Version]
- Bale, G.; Elwell, C.E.; Tachtsidis, I. From Jobsis to the present day: A review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 2016, 21, 091307. [Google Scholar] [CrossRef]
- Rajaram, A.; Bale, G.; Kewin, M.; Morrison, L.B.; Tachtsidis, I.; St Lawrence, K.; Diop, M. Simultaneous monitoring of cerebral perfusion and cytochrome c oxidase by combining broadband near-infrared spectroscopy and diffuse correlation spectroscopy. Biomed. Opt. Express 2018, 9, 2588–2603. [Google Scholar] [CrossRef]
- Anderson, P.G.; Kainerstorfer, J.M.; Sassaroli, A.; Krishnamurthy, N.; Homer, M.J.; Graham, R.A.; Fantini, S. Broadband optical mammography: Chromophore concentration and hemoglobin saturation contrast in breast cancer. PLoS ONE 2015, 10, e0117322. [Google Scholar] [CrossRef]
- Kashyap, D. Development of a Broadband Multi-Channel NIRS System for Quantifying Absolute Concentrations of Hemoglobin Derivatives and Reduced Scattering Coefficients. Ph.D. Thesis, The University of Texas, Arlington, TX, USA, 2008. [Google Scholar]
- Pinti, P.; Siddiqui, M.F.; Levy, A.D.; Jones, E.J.H.; Tachtsidis, I. An analysis framework for the integration of broadband NIRS and EEG to assess neurovascular and neurometabolic coupling. Sci. Rep. 2021, 11, 3977. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Reddy, D.D.; Nalawade, S.S.; Barrett, D.W.; Gonzalez-Lima, F.; Liu, H. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 2017, 37, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Bragin, A.; Liu, H.; Li, L. Preclinical studies of transcranial photobiomodulation in the neurological diseases. Transl. Biophotonics 2021, 3, e202000024. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y.Y. (Eds.) Photobiomodulation in the Brain; Acamemic Press: San Diago, CA, USA, 2019. [Google Scholar]
- Blanco, N.J.; Saucedo, C.L.; Gonzalez-Lima, F. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol. Learn. Mem. 2017, 139, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, D.; Qi, X.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J.H. Transcranial Near Infrared Light Stimulations Improve Cognition in Patients with Dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef]
- Tedford, C.E.; DeLapp, S.; Jacques, S.; Anders, J. Re: “Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue” Lasers in Surgery and Medicine, 2015;47(4):312–322. Lasers Surg. Med. 2015, 47, 466. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Pigatto, G.R.; Silva, C.S.; Parizotto, N.A. Photobiomodulation therapy reduces acute pain and inflammation in mice. J. Photochem. Photobiol. B Biol. 2019, 196, 111513. [Google Scholar] [CrossRef]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflügers Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Shuba, Y.M. Beyond neuronal heat sensing: Diversity of TRPV1 heat-capsaicin receptor-channel functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef] [PubMed]
- Smutzer, G.; Devassy, R.K. Integrating TRPV1 receptor function with capsaicin psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 1512457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.C.; Gonzalez-Lima, F. Low-level light therapy of the eye and brain. Eye Brain 2011, 3, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, E.; Barrett, D.W.; Saucedo, C.L.; O’Connor, P.; Liu, H.; Gonzalez-Lima, F. Cognitive enhancement by transcranial photobiomodulation is associated with cerebrovascular oxygenation of the prefrontal cortex. Front. Neurosci. 2019, 13, 1129. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Liu, H.; Zeng, L. Learning Hemodynamic Effect of Transcranial Infrared Laser Stimulation Using Longitudinal Data Analysis. IEEE J. Biomed. Health Inform. 2019, 24, 1772–1779. [Google Scholar] [CrossRef]
- Hedeker, D.; Gibbons, R.D. Longitudinal Data Analysis; Wiley-Interscience: Newark, NJ, USA, 2006. [Google Scholar]
- Saucedo, C.L.; Courtois, E.C.; Wade, Z.S.; Kelley, M.N.; Kheradbin, N.; Barrett, D.W.; Gonzalez-Lima, F. Transcranial laser stimulation: Mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimul. 2021, 14, 440–449. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruitt, T.; Carter, C.; Wang, X.; Wu, A.; Liu, H. Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo. Metabolites 2022, 12, 103. https://doi.org/10.3390/metabo12020103
Pruitt T, Carter C, Wang X, Wu A, Liu H. Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo. Metabolites. 2022; 12(2):103. https://doi.org/10.3390/metabo12020103
Chicago/Turabian StylePruitt, Tyrell, Caroline Carter, Xinlong Wang, Anqi Wu, and Hanli Liu. 2022. "Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo" Metabolites 12, no. 2: 103. https://doi.org/10.3390/metabo12020103