Postmortem Metabolomics Reveal Acylcarnitines as Potential Biomarkers for Fatal Oxycodone-Related Intoxication
Abstract
:1. Introduction
2. Results
2.1. Study Cohort
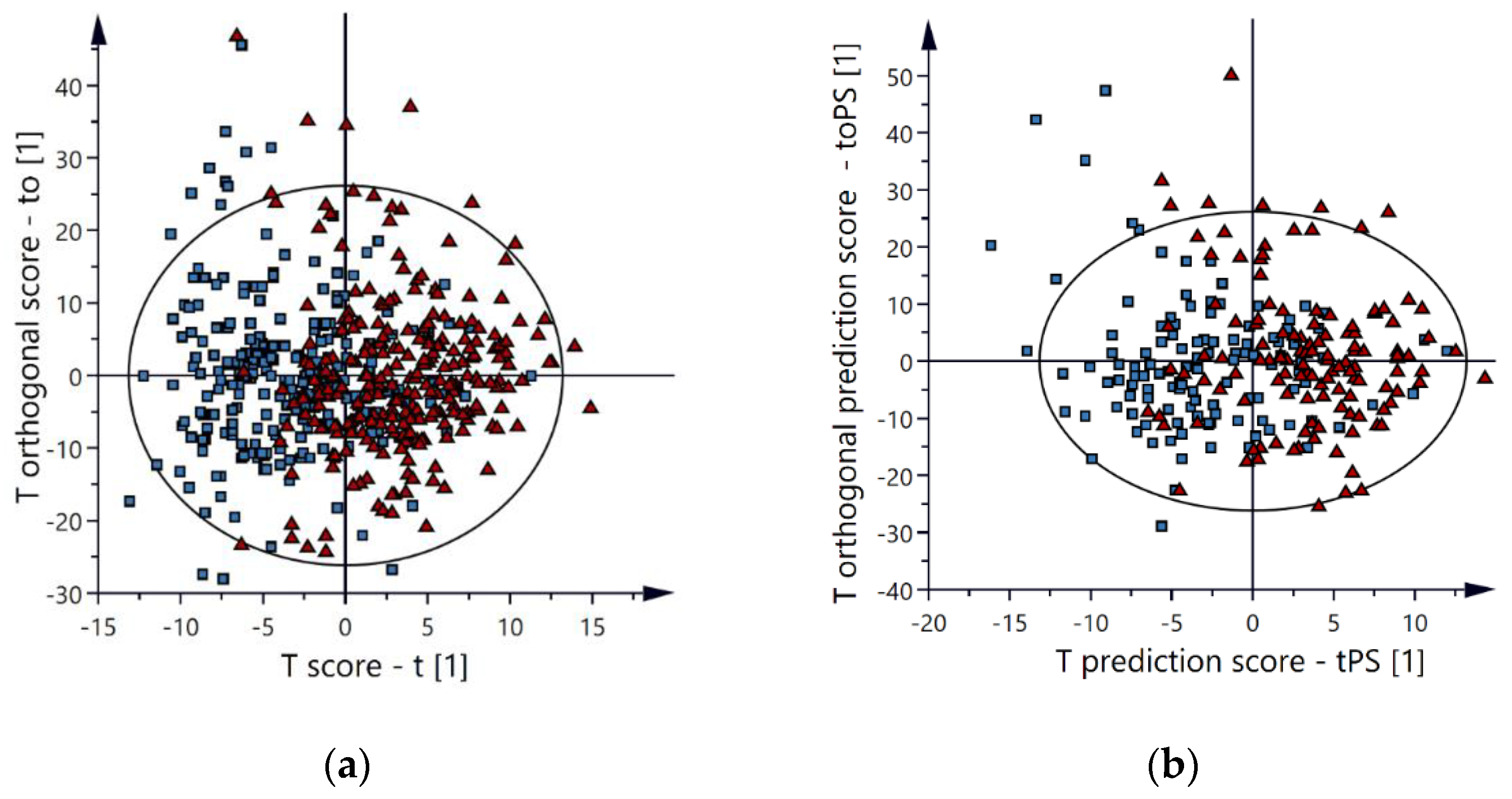
2.2. Multivariate Models
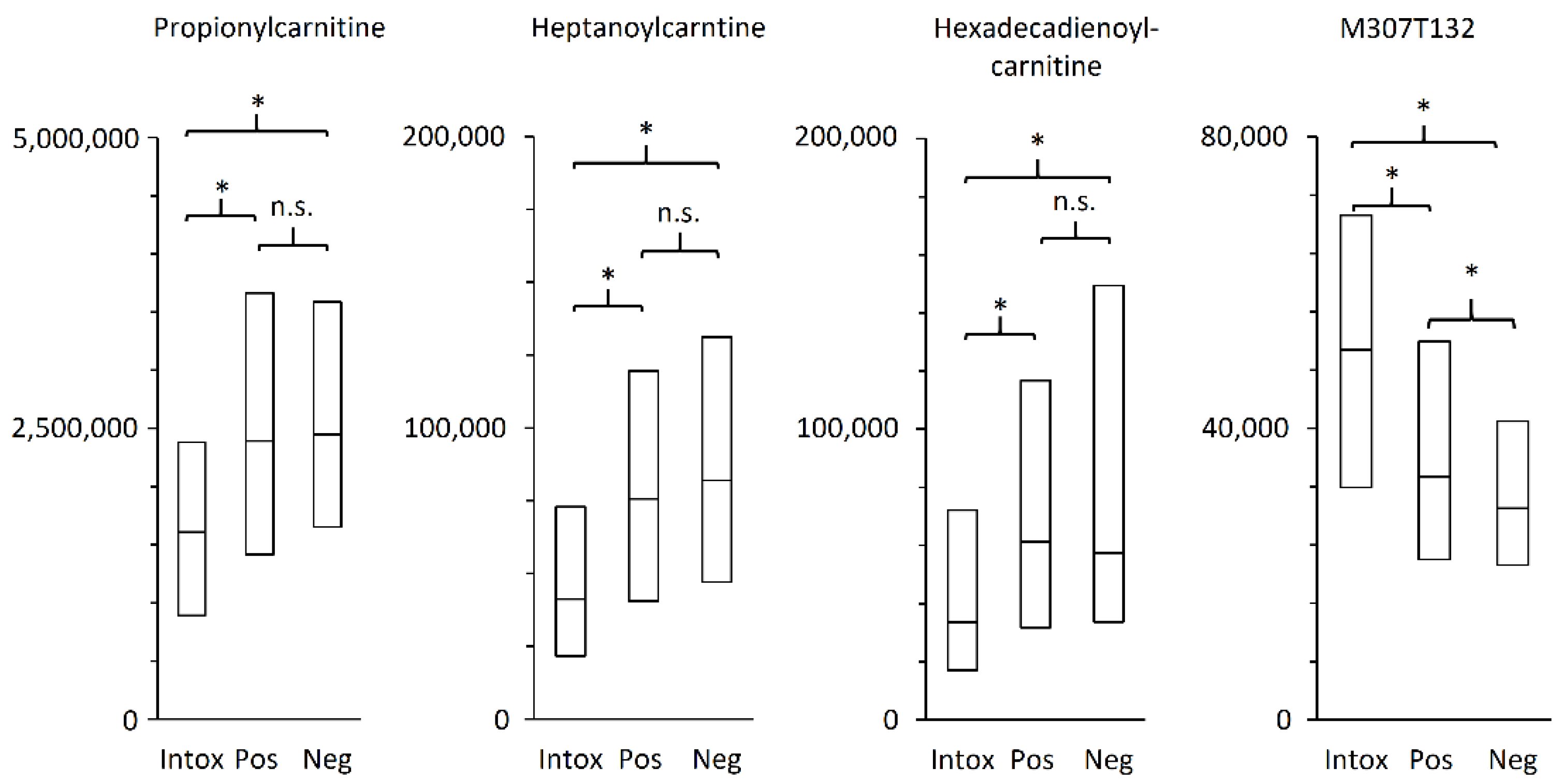
2.3. Metabolites of Importance
3. Discussion
3.1. Study Strengths and Limitations
3.2. Acylcarnitines Potential as Biomarker for Oxycodone-Related Intoxications
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawidowska, J.; Zanowska, M.K.; Markuszewski, M.J.; Kaliszan, M. The Application of Metabolomics in Forensic Science with Focus on Forensic Toxicology and Time-of-Death Estimation. Metabolites 2021, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Elmsjö, A.; Vikingsson, S.; Söderberg, C.; Kugelberg, F.C.; Green, H. Post-Mortem Metabolomics: A Novel Approach in Clinical Biomarker Discovery and a Potential Tool in Death Investigations. Chem. Res. Toxicol. 2021, 34, 1496–1502. [Google Scholar] [CrossRef]
- Pattinson, K.T.S. Opioids and the control of respiration. Br. J. Anaesth. 2008, 100, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Van Der Schrier, R.; Roozekrans, M.; Olofsen, E.; Aarts, L.; Van Velzen, M.; De Jong, M.; Dahan, A.; Niesters, M. Influence of Ethanol on Oxycodone-induced Respiratory DepressionA Dose-escalating Study in Young and Elderly Individuals. Anesthesiology 2017, 126, 534–542. [Google Scholar] [CrossRef]
- Pirnay, S.O.; Mégarbane, B.; Borron, S.W.; Risède, P.; Monier, C.; Ricordel, I.; Baud, F.J. Effects of Various Combinations of Benzodiazepines with Buprenorphine on Arterial Blood Gases in Rats. Basic Clin. Pharmacol. Toxicol. 2008, 103, 228–239. [Google Scholar] [CrossRef]
- Rookey, B.D. Drugged Driving in the Opioid Era: Spatial Patterns of Oxycodone Use in Fatal Traffic Crashes, 2001–2016. Subst. Use Misuse 2018, 53, 1288–1298. [Google Scholar] [CrossRef]
- Simonsen, K.W.; Kriikku, P.; Thelander, G.; Edvardsen, H.M.E.; Thordardottir, S.; Andersen, C.U.; Jönsson, A.K.; Frost, J.; Christoffersen, D.J.; Delaveris, G.J.M.; et al. Fatal poisoning in drug addicts in the Nordic countries in 2017. Forensic Sci. Int. 2020, 313, 110343. [Google Scholar] [CrossRef]
- Muller, A.E.; Clausen, T.; Sjøgren, P.; Odsbu, I.; Skurtveit, S. Prescribed opioid analgesic use developments in three Nordic countries, 2006–2017. Scand. J. Pain 2019, 19, 345–353. [Google Scholar] [CrossRef]
- Nedahl, M.; Johansen, S.S.; Linnet, K. Postmortem Brain–Blood Ratios of Codeine, Fentanyl, Oxycodone and Tramadol. J. Anal. Toxicol. 2021, 45, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, W.; Liebisch, G.; Audebert, F.-X.; Fröhlich, D.; Glück, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, L.W.; Samuel, T.; Noble, R.; Gmbh, S.D.; Barrett, D.; Beale, M.H.; Hardy, N. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chighine, A.; Locci, E.; Nioi, M.; D’Aloja, E. Looking for Post-Mortem Metabolomic Standardization: Waiting for Godot—The Importance of Post-Mortem Interval in Forensic Metabolomics. Chem. Res. Toxicol. 2021, 34, 1946–1947. [Google Scholar] [CrossRef]
- Graber, M.L. The incidence of diagnostic error in medicine. BMJ Qual. Saf. 2013, 22, ii21–ii27. [Google Scholar] [CrossRef] [Green Version]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A Plasma Long-Chain Acylcarnitine Predicts Cardiovascular Mortality in Incident Dialysis Patients. J. Am. Heart Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.S.; Gao, F.; Liu, J.; Fridianto, K.T.; Ching, J.; Tan, R.S.; Wong, J.-I.; Chua, S.J.; Leng, S.; Zhong, L.; et al. Metabolomic profile of arterial stiffness in aged adults. Diabetes Vasc. Dis. Res. 2018, 15, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Bouchouirab, F.-Z.; Fortin, M.; Noll, C.; Dubé, J.; Carpentier, A.C. Plasma Palmitoyl-Carnitine (AC16:0) Is a Marker of Increased Postprandial Nonesterified Incomplete Fatty Acid Oxidation Rate in Adults with Type 2 Diabetes. Can. J. Diabetes 2018, 42, 382–388. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- Jarrell, Z.R.; Smith, M.R.; Hu, X.; Orr, M.; Liu, K.H.; Quyyumi, A.A.; Jones, D.P.; Go, Y.-M. Plasma acylcarnitine levels increase with healthy aging. Aging 2020, 12, 13555–13570. [Google Scholar] [CrossRef]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef]
- Bjørndal, B.; Alterås, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Çam, H.; Yildirim, B.; Aydin, A.; Say, A. Carnitine Levels in Neonatal Hypoxia. J. Trop. Pediatr. 2005, 51, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Bruder, E.D.; Raff, H. Cardiac and plasma lipid profiles in response to acute hypoxia in neonatal and young adult rats. Lipids Health Dis. 2010, 9, 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitmer, J.T.; Idell-Wenger, J.A.; Rovetto, M.J.; Neely, J.R. Control of fatty acid metabolism in ischemic and hypoxic hearts. J. Biol. Chem. 1978, 253, 4305–4309. [Google Scholar] [CrossRef]
- Friolet, R.; Hoppeler, H.; Krähenbühl, S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J. Clin. Investig. 1994, 94, 1490–1495. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. l-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Bagetta, V.; Barone, I.; Ghiglieri, V.; Di Filippo, M.; Sgobio, C.; Bernardi, G.; Calabresi, P.; Picconi, B. Acetyl-l-Carnitine selectively prevents post-ischemic LTP via a possible action on mitochondrial energy metabolism. Neuropharmacology 2008, 55, 223–229. [Google Scholar] [CrossRef]
- Zanelli, S.A.; Solenski, N.J.; Rosenthal, R.E.; Fiskum, G. Mechanisms of Ischemic Neuroprotection by Acetyl-L-carnitine. Ann. N. Y. Acad. Sci. 2005, 1053, 153. [Google Scholar] [CrossRef] [Green Version]
- Barhwal, K.; Hota, S.K.; Prasad, D.; Singh, S.B.; Ilavazhagan, G. Hypoxia-induced deactivation of NGF-mediated ERK1/2 signaling in hippocampal cells: Neuroprotection by acetyl-L-carnitine. J. Neurosci. Res. 2008, 86, 2705–2721. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Sayed-Ahmed, M.M.; Al-Omar, F.A.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Shabanah, O.A. Carnitine esters prevent oxidative stress damage and energy depletion following transient forebrain ischaemia in the rat hippocampus. Clin. Exp. Pharmacol. Physiol. 2006, 33, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Ström, L.; Tell, H.; Josefsson, M. Liquid chromatography/time-of-flight mass spectrometry analysis of postmortem blood samples for targeted toxicological screening. Anal. Bioanal. Chem. 2013, 405, 4107–4125. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Pocessing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, an Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]

| Oxycodone Intoxications | Positive Controls | Negative Controls | Statistics | |
|---|---|---|---|---|
| n | 375 | 364 | 195 | |
| Females/males | 160/215 | 123/241 | 44/151 | p < 0.001 1 |
| Age (yrs) | 48 (35–60) | 65 (53–74) | 47 (30–59) | p < 0.001 2 |
| Body weight (kg) | 86 (72–100) | 76 (62–92) | 74(65–83) | p < 0.001 2 |
| Body Height (cm) | 173 (165–181) | 173 (165–179) | 176 (170–182) | p < 0.001 2 |
| Body Mass Index (kg/m2) | 29 (25–33) | 26 (22–30) | 24 (22–26) | p < 0.001 2 |
| Metabolites 1 | Chain Length 2 | Identifier 3 | Mean m/z 4 | Exact m/z 5 | Δ 6 | % 7 | p-Value 8 |
|---|---|---|---|---|---|---|---|
| Acetylcarnitine | C2 | M204T119 | 204.123 | 204.1230 | 0.0 | 0.70 | 4.6 × 10−4 |
| Propionylcarnitine | C3 | M218T126 | 218.139 | 218.1387 | −1.4 | 0.67 | 3.5 × 10−12 |
| Butyrylcarnitine | C4 | M232T167_1 | 232.155 | 232.1543 | −3.0 | 0.64 | 2.8 × 10−7 |
| (Iso)valerylcarnitine | C5 | M246T226 | 246.170 | 246.1700 | 0.0 | 0.51 | 1.6 × 10−19 |
| Hexanoylcarnitine | C6 | M260T289 | 260.186 | 260.1856 | −1.5 | 0.69 | 8.7 × 10−8 |
| Heptanoylcarnitine | C7 | M274T349 | 274.201 | 274.2013 | 1.1 | 0.53 | 2.6 × 10−15 |
| Octanoylcarnitine | C8 | M288T405 | 288.217 | 288.2169 | −0.3 | 0.55 | 7.4 × 10−12 |
| Nonanoylcarnitine | C9 | M302T456_2 | 302.232 | 302.2326 | 2.0 | 0.69 | 3.6 × 10−9 |
| Decenoylcarnitine | C10:1 | M314T459 | 314.232 | 314.2326 | 1.9 | 0.59 | 8.8 × 10−3 |
| Decanoylcarnitine | C10 | M316T504 | 316.248 | 316.2482 | 0.6 | 0.46 | 7.1 × 10−8 |
| Hydroxyhexadecadiencarnitine | C16:2-OH | M412T568 | 412.304 | 412.3057 | 4.1 | 0.64 | 2.7 × 10−4 |
| Tetradecadiencarnitine | C14:2 | M368T572 | 368.279 | 368.2795 | 1.4 | 0.55 | 7.1 × 10−4 |
| Dodecanoylcarnitine | C12 | M344T573 | 344.279 | 344.2795 | 1.5 | 0.59 | 6.4 × 10−6 |
| Hydroxyhexadecenoylcarnitine | C16:1-OH | M414T588 | 414.321 | 414.3214 | 1.0 | 0.64 | 4.0 × 10−14 |
| Tetradecenoylcarnitine | C14:1 | M370T591 | 370.295 | 370.2952 | 0.5 | 0.59 | 1.3 × 10−7 |
| Hexadecadienoylcarnitine | C16:2 | M396T601 | 396.311 | 396.3108 | −0.5 | 0.55 | 5.8 × 10−7 |
| Hydroxyhexadecanoyl carnitine | C16-OH | M416T606 | 416.337 | 416.3371 | 0.2 | 0.79 | 3.7 × 10−8 |
| Tetradecenoylcarnitine | C14 | M372T608 | 372.311 | 372.3108 | −0.5 | 0.64 | 2.9 × 10−8 |
| Linolenylcarnitine | C18:3 | M422T611 | 422.327 | 422.3265 | −1.2 | 0.69 | 1.9 × 10−6 |
| Hydroxyoctadecanoylcarnitine | C18-OH | M442T617 | 442.353 | 442.3527 | −0.7 | 0.65 | 2.3 × 10−10 |
| Linoleylcarnitine | C18:2 | M424T628 | 424.343 | 424.3421 | −2.1 | 0.78 | 1.9 × 10−4 |
| Unidentified | Na | M307T132 | 307.122 | NA | NA | 1.59 | 3.6 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmsjö, A.; Söderberg, C.; Jakobsson, G.; Green, H.; Kronstrand, R. Postmortem Metabolomics Reveal Acylcarnitines as Potential Biomarkers for Fatal Oxycodone-Related Intoxication. Metabolites 2022, 12, 109. https://doi.org/10.3390/metabo12020109
Elmsjö A, Söderberg C, Jakobsson G, Green H, Kronstrand R. Postmortem Metabolomics Reveal Acylcarnitines as Potential Biomarkers for Fatal Oxycodone-Related Intoxication. Metabolites. 2022; 12(2):109. https://doi.org/10.3390/metabo12020109
Chicago/Turabian StyleElmsjö, Albert, Carl Söderberg, Gerd Jakobsson, Henrik Green, and Robert Kronstrand. 2022. "Postmortem Metabolomics Reveal Acylcarnitines as Potential Biomarkers for Fatal Oxycodone-Related Intoxication" Metabolites 12, no. 2: 109. https://doi.org/10.3390/metabo12020109
APA StyleElmsjö, A., Söderberg, C., Jakobsson, G., Green, H., & Kronstrand, R. (2022). Postmortem Metabolomics Reveal Acylcarnitines as Potential Biomarkers for Fatal Oxycodone-Related Intoxication. Metabolites, 12(2), 109. https://doi.org/10.3390/metabo12020109