Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimisation of SPME Extraction Conditions
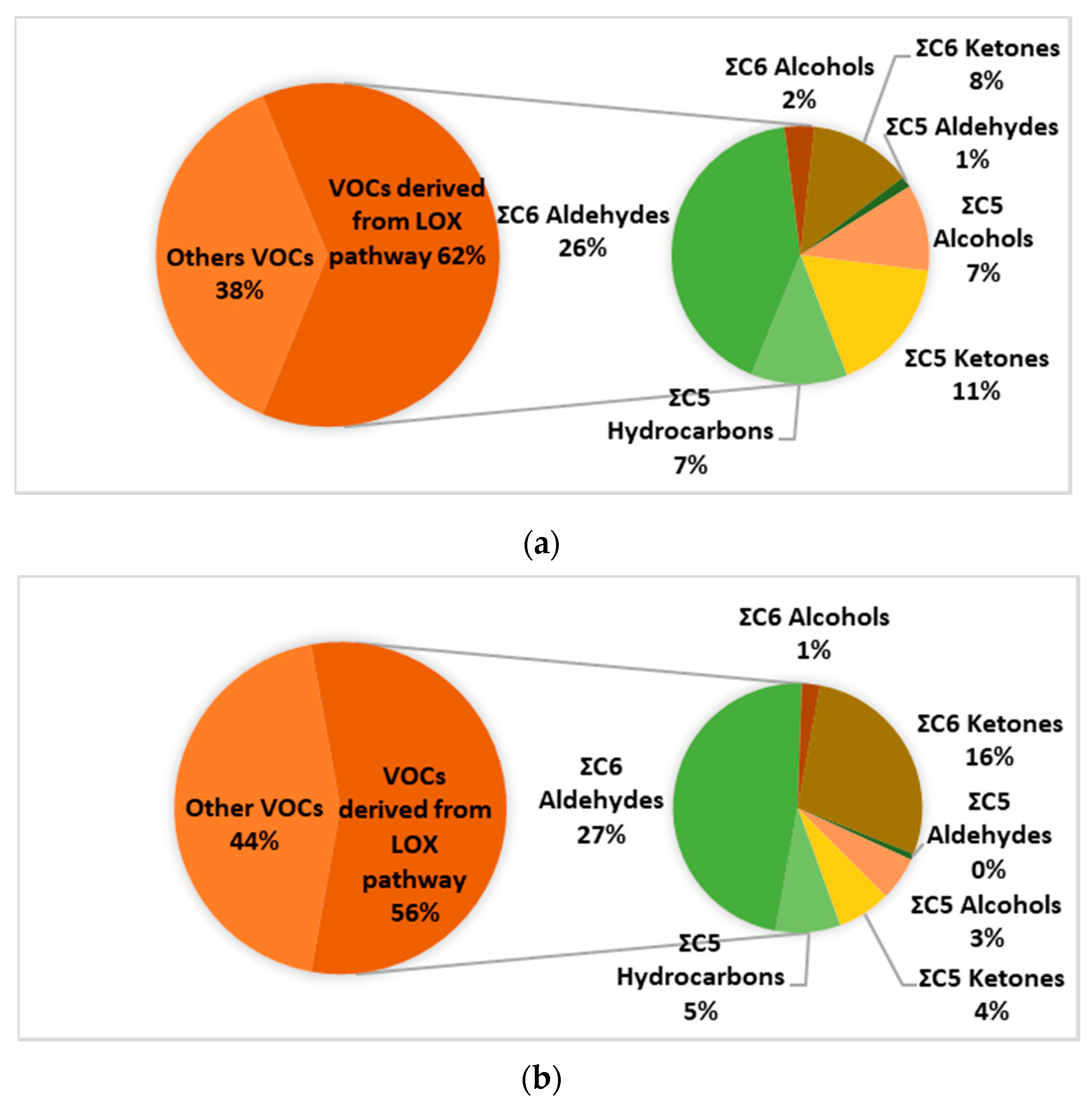
2.2. Identification of VOCs by HS-SPME/GC-MS Analysis
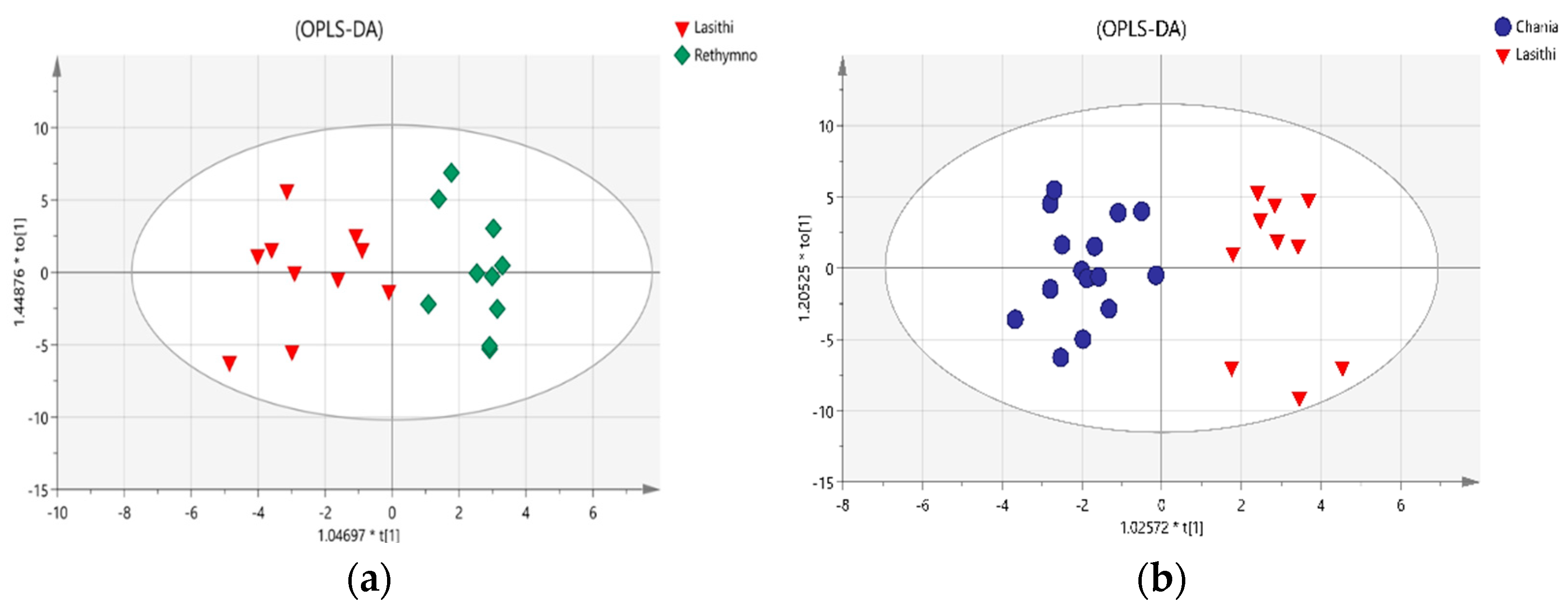
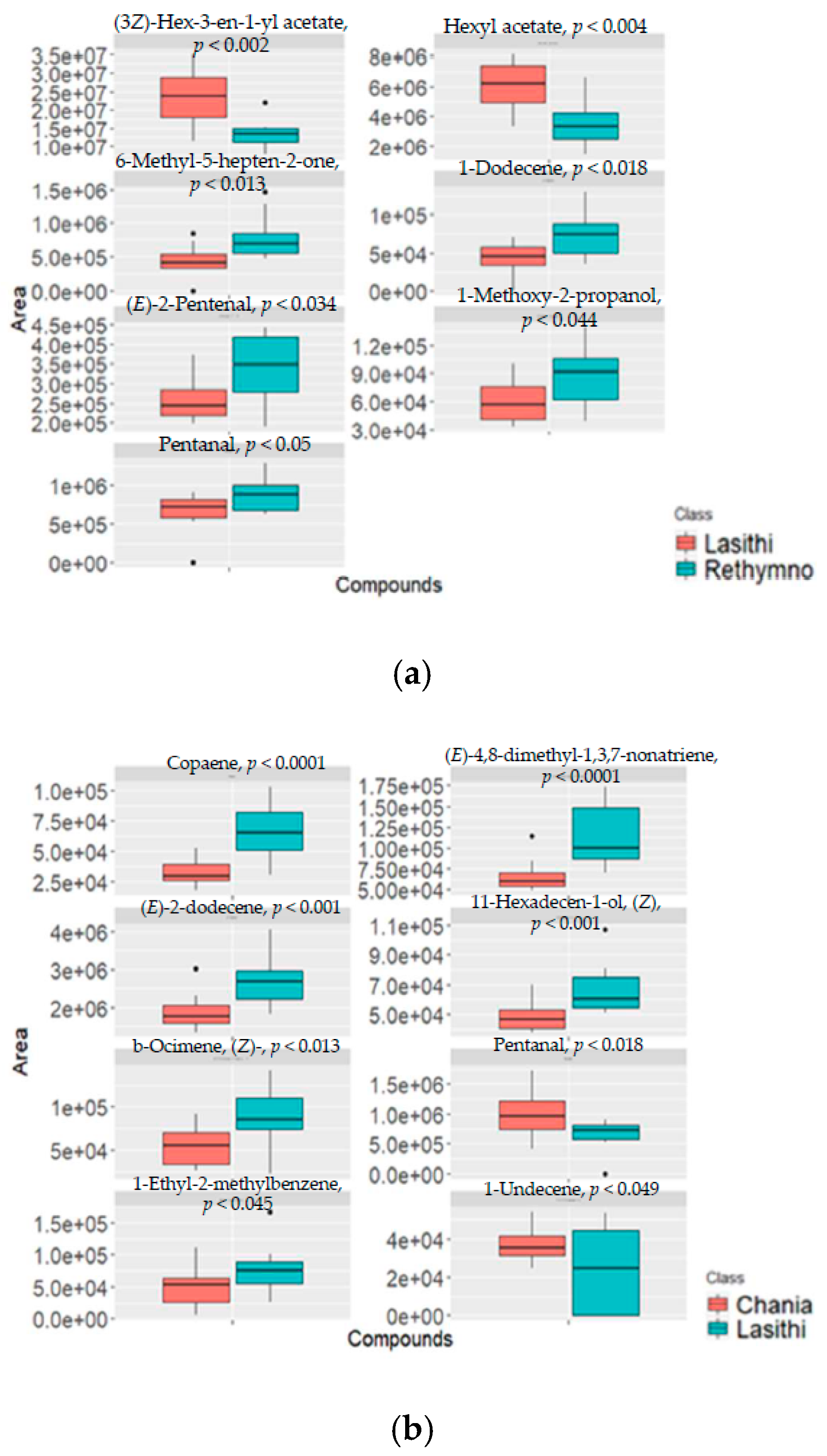
2.3. Classification of Cretan VOO Samples
3. Materials and Methods
3.1. Samples
3.2. Chemicals
3.3. Optimisation of SPME Extraction Conditions
3.4. Sample Preparation
3.5. Gas Chromatography-Mass Spectrometry
3.6. Data Processing and Chemometrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo-Manzanares, N.; Gabriel, F.; Carpio, A.; Arce, L. Use of Whole Electrophoretic Profile and Chemometric Tools for the Differentiation of Three Olive Oil Qualities. Talanta 2019, 197, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive Oil Quality and Authenticity: A Review of Current EU Legislation, Standards, Relevant Methods of Analyses, Their Drawbacks and Recommendations for the Future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Da Silva, M.D.; Freitas, A.M.; Cabrita, M.J.; Garcia, R. Olive Oil Composition: Volatile Compounds; IntechOpen: London, UK, 2012; ISBN 978-953-307-921-9. [Google Scholar]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; European Union: Luxembourg, 1991; Volume 248.
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Theodosi, S.; Kosma, I.S.; Badeka, A.V. Quality Characteristics of Koroneiki Olive Oil from Zakynthos Island (Greece) and Differentiation Depending on the Altitude Level. Eur. Food Res. Technol. 2021, 247, 1235–1248. [Google Scholar] [CrossRef]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers. Foods 2020, 9, 155. [Google Scholar] [CrossRef]
- Revelou, P.-K.; Pappa, C.; Kakouri, E.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Discrimination of Botanical Origin of Olive Oil from Selected Greek Cultivars by SPME-GC-MS and ATR-FTIR Spectroscopy Combined with Chemometrics. J. Sci. Food Agric. 2021, 101, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Pousinis, P.; Pechlivanis, A.; Lioupi, A.; Gika, H.; Virgiliou, C.; Kodra, D.; Marinaki, M.; Theodoridis, G. Innovation in Nutrition and Food Analysis: The FoodOmicsGR_Research Infrastructure. Column 2021, 17, 12–19. [Google Scholar]
- Theodoridis, G.; Pechlivanis, A.; Thomaidis, N.S.; Spyros, A.; Georgiou, C.A.; Albanis, T.; Skoufos, I.; Kalogiannis, S.; Tsangaris, G.T.; Stasinakis, A.S.; et al. FoodOmicsGR_RI: A Consortium for Comprehensive Molecular Characterisation of Food Products. Metabolites 2021, 11, 74. [Google Scholar] [CrossRef]
- Lioupi, A.; Nenadis, N.; Theodoridis, G. Virgin Olive Oil Metabolomics: A Review. J. Chromatogr. B 2020, 1150, 122161. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Míguez, R.; Montero, L.; Herrero, M. Reprint of: Application of Mass Spectrometry-Based Metabolomics Approaches for Food Safety, Quality and Traceability. TrAC Trends Anal. Chem. 2017, 96, 62–78. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Dynamic Headspace/GC–MS to Control the Aroma Fingerprint of Extra-Virgin Olive Oil from the Same and Different Olive Varieties. Food Control. 2012, 25, 684–695. [Google Scholar] [CrossRef]
- Aprea, E.; Gasperi, F.; Betta, E.; Sani, G.; Cantini, C. Variability in Volatile Compounds from Lipoxygenase Pathway in Extra Virgin Olive Oils from Tuscan Olive Germoplasm by Quantitative SPME/GC-MS. J. Mass Spectrom. 2018, 53, 824–832. [Google Scholar] [CrossRef]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Validation of SPME-GCMS Method for the Analysis of Virgin Olive Oil Volatiles Responsible for Sensory Defects. Talanta 2015, 134, 394–401. [Google Scholar] [CrossRef]
- Aprea, E.; Gika, H.; Carlin, S.; Theodoridis, G.; Vrhovsek, U.; Mattivi, F. Metabolite Profiling on Apple Volatile Content Based on Solid Phase Microextraction and Gas-Chromatography Time of Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 4517–4524. [Google Scholar] [CrossRef]
- Borges, T.H.; Ramalhosa, E.; Seiquer, I.; Pereira, J.A. Use of Response Surface Methodology (RSM) for the Identification of the Best Extraction Conditions for Headspace Solid-Phase Micro Extraction (HS-SPME) of the Volatile Profile of Cv. Arbequina Extra-Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700356. [Google Scholar] [CrossRef]
- De los Angeles Fernandez, M.; Assof, M.; Jofre, V.; Silva, M.F. Volatile Profile Characterization of Extra Virgin Olive Oils from Argentina by HS-SPME/GC-MS and Multivariate Pattern Recognition Tools. Food Anal. Methods 2014, 7, 2122–2136. [Google Scholar] [CrossRef]
- Wardencki, W.; Michulec, M.; Curyło, J. A Review of Theoretical and Practical Aspects of Solid-Phase Microextraction in Food Analysis. Int. J. Food Sci. Technol. 2004, 39, 703–717. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Flavor Components of Olive Oil—A Review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Nunes, C.A.; Souza, V.R.; de Corrêa, S.C.; Silva, M.; de Cássia da Costa e Silva, M.; Bastos, S.C.; Pinheiro, A.C.M. Heating on the Volatile Composition and Sensory Aspects of Extra-Virgin Olive Oil. Ciênc. Agrotec. 2013, 37, 566–572. [Google Scholar] [CrossRef][Green Version]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer US: Boston, MA, USA, 2013; pp. 479–522. ISBN 978-1-4614-7777-8. [Google Scholar]
- Aparicio, R.; Morales, M.T. Characterization of Olive Ripeness by Green Aroma Compounds of Virgin Olive Oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Dhifi, W.; Angerosa, F.; Serraiocco, A.; Oumar, I.; Hamrouni, I.; Marzouk, B. Virgin Olive Oil Aroma: Characterization of Some Tunisian Cultivars. Food Chem. 2005, 93, 697–701. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of Volatile Compounds on Virgin Olive Oil Quality Evaluated by Analytical Approaches and Sensor Panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed]
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Lukić, I. Volatile Compounds and Sensory Profiles of Monovarietal Virgin Olive Oils from Buža, Črna and Rosinjola Cultivars in Istria (Croatia). Food Technol. Biotechnol. 2012, 50, 192–198. [Google Scholar]
- Cecchi, T.; Alfei, B. Volatile Profiles of Italian Monovarietal Extra Virgin Olive Oils via HS-SPME–GC–MS: Newly Identified Compounds, Flavors Molecular Markers, and Terpenic Profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef]
- Mansouri, F.; Moumen, A.B.; Richard, G.; Fauconnier, M.-L.; Sindic, M.; Caid, H.S.; Elamrani, A. Flavor Profiles of Monovarietal Virgin Olive Oils Produced in the Oriental Region of Morocco. Oilseeds Fats Crops Lipids 2017, 24, A501. [Google Scholar] [CrossRef]
- Kosma, I.S.; Kontominas, M.G.; Badeka, A.V. The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples. Foods 2020, 9, 1672. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin Olive Oil Odour Notes: Their Relationships with Volatile Compounds from the Lipoxygenase Pathway and Secoiridoid Compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Improvements in the Malaxation Process to Enhance the Aroma Quality of Extra Virgin Olive Oils. Food Chem. 2014, 158, 534–545. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 Varietal Virgin Olive Oils by Their Volatile Compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Pérez, A.G.; de la Rosa, R.; Pascual, M.; Sánchez-Ortiz, A.; Romero-Segura, C.; León, L.; Sanz, C. Assessment of Volatile Compound Profiles and the Deduced Sensory Significance of Virgin Olive Oils from the Progeny of Picual × Arbequina Cultivars. J. Chromatogr. A 2016, 1428, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Cordero, C.; Sgorbini, B.; Bicchi, C.; Liberto, E. Highly Informative Fingerprinting of Extra-Virgin Olive Oil Volatiles: The Role of High Concentration-Capacity Sampling in Combination with Comprehensive Two-Dimensional Gas Chromatography. Separations 2019, 6, 34. [Google Scholar] [CrossRef]
- Youssef, O.; Guido, F.; Manel, I.; Youssef, N.B.; Luigi, C.P.; Mohamed, H.; Daoud, D.; Mokhtar, Z. Volatile Compounds and Compositional Quality of Virgin Olive Oil from Oueslati Variety: Influence of Geographical Origin. Food Chem. 2011, 124, 1770–1776. [Google Scholar] [CrossRef]
- Cerretani, L.; Salvador, M.D.; Bendini, A.; Fregapane, G. Relationship Between Sensory Evaluation Performed by Italian and Spanish Official Panels and Volatile and Phenolic Profiles of Virgin Olive Oils. Chem. Percept. 2008, 1, 258. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Robles-Molina, J.; García-Reyes, J.F.; Molina-Díaz, A. Rapid Determination of BTEXS in Olives and Olive Oil by Headspace-Gas Chromatography/Mass Spectrometry (HS-GC–MS). Talanta 2010, 83, 391–399. [Google Scholar] [CrossRef]
- Bempelou, E.; Anagnostopoulos, C.; Liapis, K. Investigation of Naphthalene Contamination in Olive Oil from Greece. Toxicol. Environ. Chem. 2019, 101, 45–58. [Google Scholar] [CrossRef]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2019, 121, 1800172. [Google Scholar] [CrossRef]
- Kosma, I.; Vatavali, K.; Kontakos, S.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Geographical Differentiation of Greek Extra Virgin Olive Oil from Late-Harvested Koroneiki Cultivar Fruits. J. Am. Oil Chem Soc. 2017, 94, 1373–1384. [Google Scholar] [CrossRef]
- Pouliarekou, E.; Badeka, A.; Tasioula-Margari, M.; Kontakos, S.; Longobardi, F.; Kontominas, M.G. Characterization and Classification of Western Greek Olive Oils According to Cultivar and Geographical Origin Based on Volatile Compounds. J. Chromatogr. A 2011, 1218, 7534–7542. [Google Scholar] [CrossRef]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Brkić Bubola, K. Inter-Varietal Diversity of Typical Volatile and Phenolic Profiles of Croatian Extra Virgin Olive Oils as Revealed by GC-IT-MS and UPLC-DAD Analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Bajoub, A.; Sánchez-Ortiz, A.; Ajal, E.A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Beltrán, G.; Carrasco-Pancorbo, A. First Comprehensive Characterization of Volatile Profile of North Moroccan Olive Oils: A Geographic Discriminant Approach. Food Res. Int. 2015, 76, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Şişik Oğraş, Ş.; Kaban, G.; Kaya, M. Volatile Compounds of Olive Oils from Different Geographic Regions in Turkey. Int. J. Food Prop. 2018, 21, 1833–1843. [Google Scholar] [CrossRef]
- Vichi, S.; Guadayol, J.M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Monoterpene and Sesquiterpene Hydrocarbons of Virgin Olive Oil by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography/Mass Spectrometry. J. Chromatogr. A 2006, 1125, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zunin, P.; Boggia, R.; Salvadeo, P.; Evangelisti, F. Geographical Traceability of West Liguria Extravirgin Olive Oils by the Analysis of Volatile Terpenoid Hydrocarbons. J. Chromatogr. A 2005, 1089, 243–249. [Google Scholar] [CrossRef]
- Mansour, A.B.; Gargouri, B.; Flamini, G.; Bouaziz, M. Effect of Agricultural Sites on Differentiation between Chemlali and Neb Jmel Olive Oils. J. Oleo. Sci. 2015, 64, 381–392. [Google Scholar] [CrossRef]
- Kandylis, P.; Vekiari, A.S.; Kanellaki, M.; Grati Kamoun, N.; Msallem, M.; Kourkoutas, Y. Comparative Study of Extra Virgin Olive Oil Flavor Profile of Koroneiki Variety (Olea Europaea Var. Microcarpa Alba) Cultivated in Greece and Tunisia during One Period of Harvesting. LWT Food Sci. Technol. 2011, 44, 1333–1341. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Ribeiro, L.H.; Costa Freitas, A.M.; Gomes da Silva, M.D.R. The Use of Headspace Solid Phase Microextraction for the Characterization of Volatile Compounds in Olive Oil Matrices. Talanta 2008, 77, 110–117. [Google Scholar] [CrossRef]
- Wenig, P.; Odermatt, J. OpenChrom: A Cross-Platform Open Source Software for the Mass Spectrometric Analysis of Chromatographic Data. BMC Bioinform. 2010, 11, 405. [Google Scholar] [CrossRef]
- Brocks, J.J.; Hope, J.M. Tailing of Chromatographic Peaks in GC–MS Caused by Interaction of Halogenated Solvents with the Ion Source. J. Chromatogr. Sci. 2014, 52, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- RStudio|Open Source & Professional Software for Data Science Teams. Available online: https://rstudio.com/ (accessed on 17 August 2021).
- Grömping, U. R Package FrF2 for Creating and Analyzing Fractional Factorial 2-Level Designs. J. Stat. Softw. 2014, 56, 1–56. [Google Scholar] [CrossRef]
- Grömping, U. R Package DoE.Base for Factorial Experiments. J. Stat. Softw. 2018, 85, 1–41. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R., Using Rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
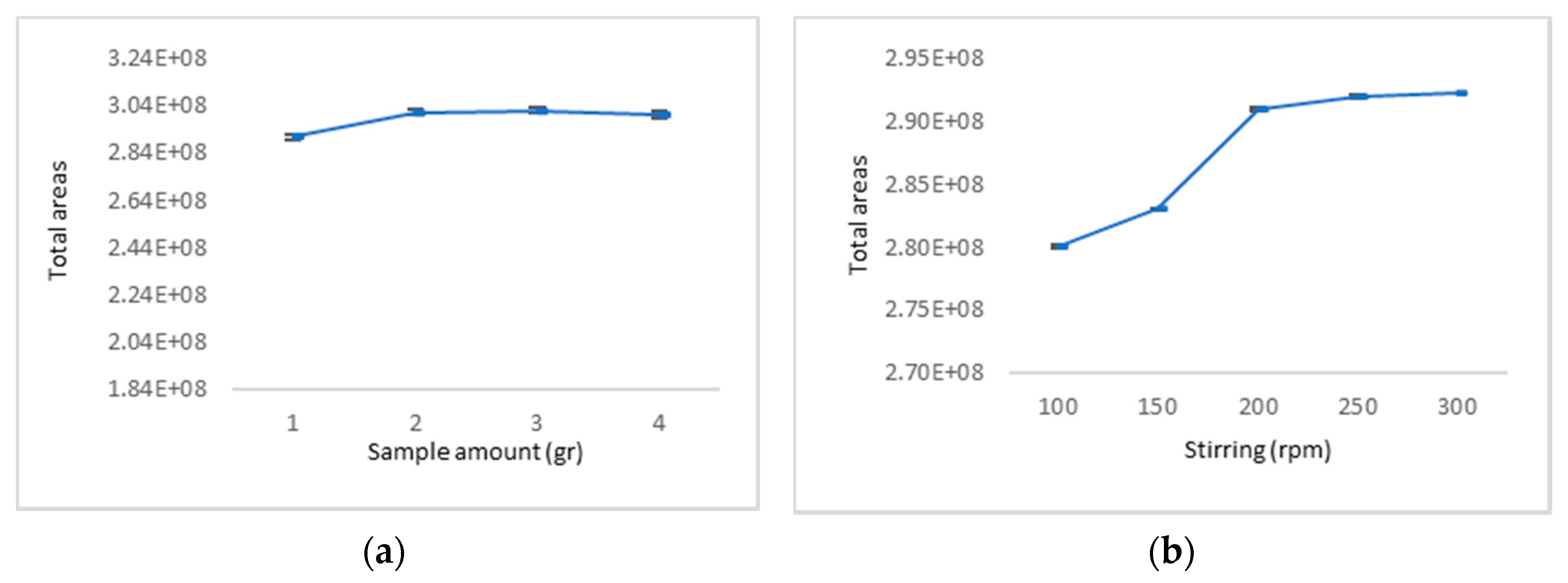
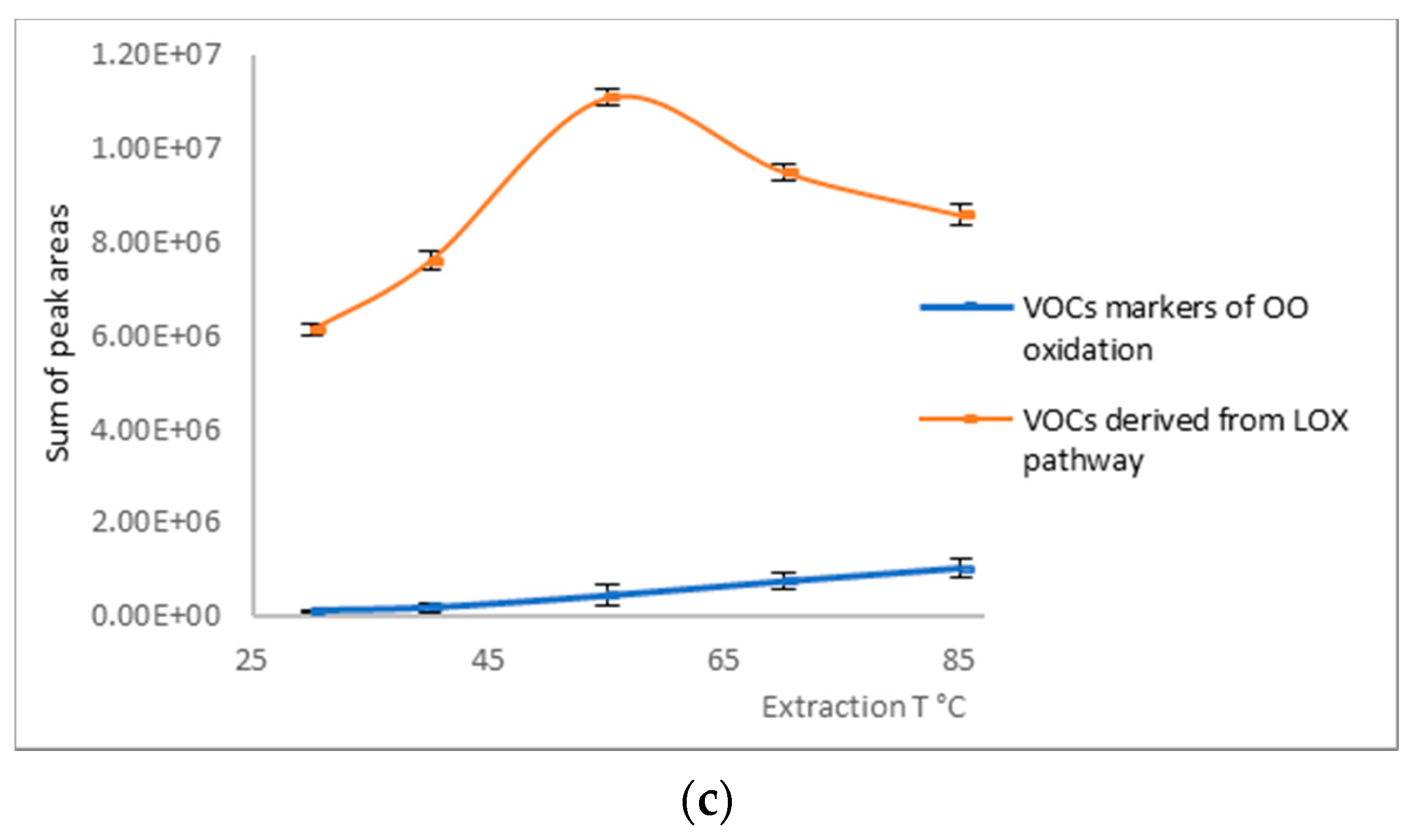
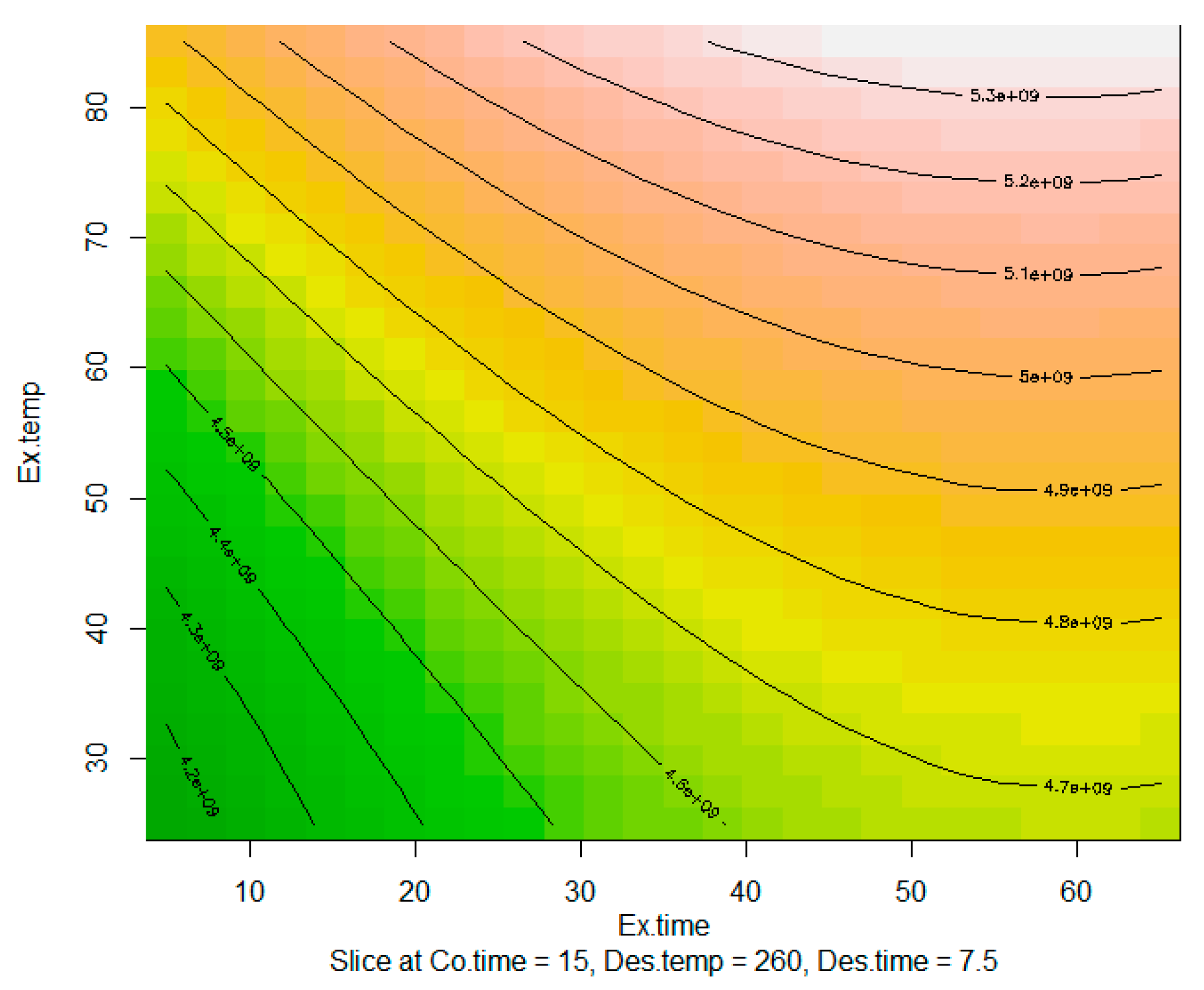

| Multiple R-Squared | 0.9028 | ||||
| Adjusted R-Squared | 0.7262 | ||||
| p-Value | 4.02 × 10−3 | ||||
| Df | Sum Sq | Mean Sq | F Value | Pr (>F) | |
| FO (x1, x2, x3, x4, x5) | 5 | 1.2055 × 1018 | 2.4111 × 1017 | 16.3748 | 9.02 × 10−5 |
| TWI (x1, x2, x3, x4, x5) | 10 | 1.9382 × 1017 | 1.9382 × 1016 | 1.3164 | 0.3283 |
| PQ (x1, x2, x3, x4, x5) | 5 | 1.0584 × 1017 | 2.1168 × 1016 | 1.4376 | 0.2855 |
| Residuals | 11 | 1.6197 × 1017 | 1.4724 × 1016 | ||
| Lack of Fit | 6 | 4.5182 × 1016 | 7.5303 × 1015 | 0.3224 | 0.8996 |
| Pure Error | 5 | 1.1679 × 1017 | 2.3357 × 1016 |
| Compound Name | Rt | RIexp | RI Lit | Chania Mean (n = 17) | ±SD | Heraklion Mean (n = 26) | ±SD | Lasithi Mean (n = 10) | ±SD | Rethymnon Mean (n = 10) | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||||
| Isobutanol | 2 | 626 | 622 | 0.06 | 0.05 | 0.06 | 0.05 | 0.1 | 0.04 | 0.07 | 0.06 |
| 1-Penten-3-ol, C5 | 2.54 | 683 | 686 | 1.96 | 0.38 | 1.69 | 0.49 | 1.45 | 0.49 | 1.05 | 0.37 |
| 3-Methylbutan-1-ol | 3.33 | 738 | 744 | 0.12 | 0.05 | 0.14 | 0.05 | 0.15 | 0.05 | 0.13 | 0.07 |
| 1-Pentanol | 3.89 | 771 | 766 | 0.09 | 0.08 | 0.08 | 0.06 | 0.04 | 0.05 | 0.17 | 0.05 |
| (Z)-2-Penten-1-ol, C5 | 3.92 | 774 | 767 | 1.39 | 0.25 | 1.05 | 0.36 | 0.86 | 0.35 | 0.85 | 0.19 |
| (Z)-Hex-3-en-1-ol, C6-LnA | 5.53 | 857 | 858 | 0.12 | 0.04 | 0.12 | 0.05 | 0.09 | 0.05 | 0.19 | 0.06 |
| (E)-Hex-2-en-1-ol, C6-LnA | 5.82 | 871 | 866 | 1.99 | 1.75 | 2.23 | 2.49 | 0.72 | 2.36 | 7.96 | 3.45 |
| Hexan-1-ol, C6-LA | 5.9 | 875 | 862 | 1.77 | 0.9 | 1.62 | 0.88 | 0.99 | 0.9 | 4.31 | 1.12 |
| 1-Octanol | 9.76 | 1076 | 1074 | 0.08 | 0.02 | 0.08 | 0.03 | 0.07 | 0.02 | 0.07 | 0.04 |
| 1-Nonanol | 11.48 | 1176 | 1172 | 0.06 | 0.03 | 0.1 | 0.05 | 0.1 | 0.04 | 0.05 | 0.04 |
| (Z)-11-Hexadecen-1-ol | 12.31 | 1228 | - | 0.05 | 0.01 | 0.05 | 0.02 | 0.05 | 0.02 | 0.03 | 0.02 |
| Aldehydes | |||||||||||
| 3-Methylbutanal | 2.26 | 652 | 650 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 | 0.03 | 0.05 | 0.03 |
| (E)-2-Pentenal, C5 | 3.52 | 749 | 754 | 0.08 | 0.03 | 0.06 | 0.03 | 0.06 | 0.02 | 0.04 | 0.02 |
| (E)-2-Pentenal, C5 | 3.69 | 760 | 754 | 0.3 | 0.08 | 0.25 | 0.09 | 0.22 | 0.09 | 0.17 | 0.07 |
| Pentanal | 2.75 | 703 | 695 | 0.99 | 0.44 | 0.87 | 0.4 | 0.75 | 0.39 | 0.37 | 0.22 |
| 3-Hexenal, C6-LnA | 4.48 | 806 | 795 | 2.85 | 2.78 | 1.39 | 1.85 | 0.83 | 1.66 | 1.84 | 0.87 |
| Hexanal, C6-LA | 4.52 | 808 | 798 | 3.51 | 1.42 | 3.1 | 1.06 | 2.75 | 1.07 | 4.22 | 1.08 |
| 2-Hexenal, C6-LnA | 5.47 | 854 | 854 | 0.19 | 0.05 | 0.16 | 0.05 | 0.15 | 0.05 | 0.22 | 0.07 |
| (2E)-Hexenal, C6-LnA | 5.61 | 862 | 856 | 25.43 | 9.62 | 22.16 | 9.57 | 21.51 | 9.23 | 32.67 | 13.59 |
| Heptanal | 6.6 | 908 | 903 | 0.19 | 0.07 | 0.21 | 0.07 | 0.17 | 0.06 | 0.12 | 0.06 |
| 2,4 Hexadienal | 6.79 | 919 | 916 | 1.95 | 0.71 | 1.43 | 0.61 | 1.22 | 0.57 | 1.66 | 0.34 |
| Benzeneacetaldehyde | 9.39 | 1055 | 1049 | 0.03 | 0.01 | 0.04 | 0.02 | 0.06 | 0.02 | 0.06 | 0.02 |
| Nonanal | 10.38 | 1111 | 1099 | 0.98 | 0.47 | 1.14 | 0.54 | 1.17 | 0.5 | 0.45 | 0.47 |
| Acids | |||||||||||
| Acetic acid | 1.72 | 596 | 595 | 0.56 | 0.56 | 0.52 | 0.56 | 0.34 | 0.49 | 0.57 | 0.89 |
| 3-Methylbutanoic acid | 5.35 | 852 | 858 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 |
| Hexanoic acid | 8.04 | 981 | 981 | 0.73 | 0.71 | 0.55 | 0.51 | 0.21 | 0.49 | 0.7 | 0.35 |
| Heptanoic acid | 9.72 | 1074 | 1076 | 0.1 | 0.03 | 0.09 | 0.03 | 0.06 | 0.03 | 0.09 | 0.03 |
| 2-Ethylhexanoic acid | 10.52 | 1118 | 1123 | 0.58 | 0.55 | 0.37 | 0.45 | 0 | 0.41 | 0.37 | 0.41 |
| Octanoic acid | 11.36 | 1170 | 1177 | 0.14 | 0.06 | 0.13 | 0.05 | 0.06 | 0.05 | 0.14 | 0.04 |
| Nonanoic acid | 12.92 | 1268 | 1270 | 0.25 | 0.1 | 0.25 | 0.1 | 0.1 | 0.09 | 0.26 | 0.09 |
| Esters | |||||||||||
| Ethyl acetate | 1.89 | 615 | 614 | 0.22 | 0.12 | 0.27 | 0.13 | 0.29 | 0.12 | 0.22 | 0.16 |
| 3-Methylbutyl acetate | 6.04 | 882 | 883 | 0.13 | 0.08 | 0.13 | 0.07 | 0.18 | 0.07 | 0 | 0.11 |
| (Z)-Pent-2-en-1-yl acetate | 6.76 | 918 | 916 | 0.59 | 0.33 | 0.39 | 0.25 | 0.27 | 0.23 | 0.51 | 0.11 |
| (3Z)-Hex-3-en-1-yl acetate, C6-LnA | 8.55 | 1009 | 1005 | 18.49 | 9.03 | 12.15 | 7.19 | 13.46 | 7.22 | 7.97 | 4.09 |
| Hexyl acetate, C6-LA | 8.68 | 1016 | 1011 | 4.5 | 2.44 | 3.07 | 1.85 | 4.21 | 1.89 | 2.81 | 1.51 |
| Ethers | |||||||||||
| 1-Methoxy-2-propanol | 2.36 | 654 | 650 | 0.08 | 0.03 | 0.11 | 0.08 | 0.1 | 0.07 | 0.08 | 0.03 |
| 1-Methoxyhexane | 4.99 | 830 | 831 | 0 | 0 | 0 | 0.02 | 0 | 0.06 | 0 | 0 |
| (3Z)-1-Methoxy-3-hexene | 5.05 | 838 | 832 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0 | 0 |
| Ketones | |||||||||||
| 1-Penten-3-one, C5 | 2.58 | 687 | 679 | 1.96 | 0.92 | 1.65 | 1.17 | 1.46 | 1.07 | 0.7 | 1.15 |
| 3-Pentanone | 2.71 | 701 | 694 | 1.65 | 0.92 | 1.26 | 0.72 | 1.04 | 0.75 | 1.51 | 0.77 |
| 2,2-Dimethyl-3-heptanone | 9.64 | 1070 | - | 5.07 | 5.21 | 2.07 | 3.54 | 0.85 | 3.14 | 3.22 | 1.13 |
| Terpenic Compounds | |||||||||||
| .alpha.-Pinene | 7 | 930 | 932 | 0.01 | 0.04 | 0.02 | 0.07 | 0 | 0.06 | 0.18 | 0.03 |
| .beta.-Pinene | 8.05 | 983 | 980 | 0.07 | 0.26 | 0.07 | 0.31 | 0 | 0.27 | 0 | 0.15 |
| 6-Methyl-5-hepten-2-one | 8.2 | 990 | 985 | 0.61 | 0.41 | 0.56 | 0.33 | 0.51 | 0.31 | 0.78 | 0.34 |
| Alpha-Terpinene | 8.79 | 1018 | 1017 | 0 | 0 | 0 | 0.01 | 0 | 0.01 | 0.02 | 0 |
| 3-Carene | 8.79 | 1018 | 1018 | 0 | 0.01 | 0.01 | 0.01 | 0 | 0.01 | 0.02 | 0 |
| (Z)-beta-Ocimene | 9.29 | 1050 | 1048 | 0.05 | 0.02 | 0.07 | 0.03 | 0.07 | 0.03 | 0.09 | 0.02 |
| o-Cymene | 8.95 | 1031 | 1037 | 0.14 | 0.48 | 0.2 | 0.59 | 0 | 0.54 | 1.33 | 0 |
| d-Limonene | 9.02 | 1035 | 1041 | 0.7 | 1.83 | 0.99 | 3.29 | 0.1 | 2.87 | 0.19 | 4.33 |
| (+)-2-Carene | 9.04 | 1035 | 1031 | 0 | 0 | 0.06 | 0.23 | 0 | 0.23 | 0.92 | 0 |
| Neral | 12.61 | 1247 | 1244 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| Citral | 13.07 | 1276 | 1276 | 0 | 0.01 | 0 | 0.02 | 0 | 0.02 | 0 | 0.02 |
| (+)-Cyclosativene | 14.67 | 1384 | 1368 | 0 | 0 | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.01 |
| Copaene | 14.73 | 1388 | 1392 | 0.03 | 0.01 | 0.05 | 0.03 | 0.06 | 0.03 | 0.1 | 0.02 |
| Eremophilene | 16.34 | 1505 | 1503 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| alpha-Farnesene | 16.39 | 1509 | 1509 | 0.05 | 0.03 | 0.06 | 0.03 | 0.05 | 0.03 | 0.05 | 0.07 |
| Kessane | 16.94 | 1551 | 1530 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liguloxide | 17.07 | 1561 | 1533 | 0 | 0 | 0.01 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| Others | |||||||||||
| 2-Methylpentane | 1.62 | 588 | 573 | 0.04 | 0.03 | 0.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.01 |
| 3-Methylpentane | 3.39 | 741 | 748 | 0.05 | 0.03 | 0.07 | 0.03 | 0.08 | 0.03 | 0.09 | 0.03 |
| Toluene | 3.86 | 770 | 771 | 0.17 | 0.1 | 0.19 | 0.21 | 0.13 | 0.24 | 0.06 | 0.2 |
| 1-Octene | 4.25 | 794 | 792 | 0.17 | 0.1 | 0.15 | 0.08 | 0.14 | 0.07 | 0.21 | 0.05 |
| Octane | 4.42 | 803 | 800 | 1.64 | 0.78 | 2.13 | 1.08 | 2.28 | 1 | 3.86 | 0.79 |
| Styrene | 6.36 | 898 | 899 | 0.04 | 0.04 | 0.04 | 0.06 | 0 | 0.06 | 0.09 | 0.11 |
| o-Xylene | 6.36 | 898 | 899 | 0.04 | 0.05 | 0.06 | 0.07 | 0.06 | 0.07 | 0 | 0.1 |
| (Prop-2-en-1-yl)cyclopentane | 6.31 | 895 | 898 | 0.31 | 0.06 | 0.27 | 0.07 | 0.23 | 0.08 | 0.22 | 0.06 |
| Pentene dimer 1, C5 | 6.4 | 899 | - | 0.22 | 0.05 | 0.19 | 0.06 | 0.16 | 0.06 | 0.15 | 0.05 |
| Pentene dimer 2, C5 | 7.19 | 940 | 947 | 1.65 | 0.41 | 1.41 | 0.45 | 1.21 | 0.47 | 0.98 | 0.33 |
| Pentene dimer 3, C5 | 7.33 | 947 | 949 | 1.9 | 0.46 | 1.54 | 0.53 | 1.35 | 0.53 | 1.22 | 0.34 |
| 4,8-Dimethyl-1,7-nonadiene | 7.38 | 949 | 998 | 0.01 | 0.02 | 0 | 0.01 | 0 | 0.01 | 0 | 0 |
| Propylbenzene | 7.59 | 960 | 962 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 |
| 2,2,6-Trimethyloctane | 7.63 | 961 | 964 | 0 | 0 | 0.01 | 0.03 | 0 | 0.02 | 0 | 0.15 |
| 2,2-Dimethyl-3-heptanone | 7.69 | 965 | 965 | 0.78 | 0.62 | 0.42 | 0.43 | 0.23 | 0.39 | 0.46 | 0.22 |
| 1-Ethyl-2-methylbenzene | 7.73 | 971 | 969 | 0.05 | 0.03 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 |
| 5-Ethyl-2(5H)-furanone | 7.82 | 971 | 968 | 12.91 | 12.72 | 5.39 | 8.7 | 2.22 | 7.76 | 6.5 | 3.27 |
| 2,2,4-Trimethylpentane | 8.2 | 990 | - | 0.15 | 0.5 | 0.05 | 0.34 | 0 | 0.38 | 0 | 0.08 |
| Pentene dimer 4, C5 | 8.27 | 994 | - | 0.88 | 0.23 | 0.77 | 0.23 | 0.66 | 0.24 | 0.58 | 0.18 |
| Pentene dimer 5, C5 | 8.34 | 998 | - | 3.26 | 0.87 | 2.7 | 0.92 | 2.38 | 0.94 | 2.14 | 0.63 |
| 2-Propylfuran | 8.45 | 1004 | - | 0.12 | 0.05 | 0.1 | 0.04 | 0.08 | 0.04 | 0.13 | 0.04 |
| 4-Ethyltoluene | 8.89 | 1029 | - | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 |
| 2,2,4,4,6-Pentamethylheptane | 8.93 | 1030 | - | 0 | 0 | 0 | 0.01 | 0 | 0.04 | 0 | 0.07 |
| 5-Ethyl-2(5H)-furanone | 9.17 | 1044 | - | 0.64 | 0.66 | 0.27 | 0.44 | 0.12 | 0.39 | 0.31 | 0.14 |
| 1-Phenyl-1-propanone | 9.42 | 1059 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | |
| 1,4-Diethylbenzene | 9.53 | 1063 | 1070 | 0 | 0 | 0 | 0.01 | 0 | 0.01 | 0 | 0 |
| 1-Undecene | 10.07 | 1078 | 1082 | 0.04 | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 |
| (E)-4,8-Dimethyl-1,3,7-nonatriene | 10.11 | 1096 | 1098 | 0.06 | 0.02 | 0.09 | 0.04 | 0.09 | 0.04 | 0.05 | 0.03 |
| Methyl benzoate | 10.27 | 1104 | 1106 | 0.04 | 0.01 | 0.05 | 0.02 | 0.05 | 0.02 | 0.03 | 0.01 |
| Naphthalene | 11.88 | 1201 | 1206 | 0.06 | 0.24 | 0 | 0.15 | 0.01 | 0.13 | 0 | 0 |
| (E)-2-Dodecene | 11.96 | 1206 | 1206 | 1.78 | 0.32 | 2.14 | 0.64 | 2.02 | 0.67 | 1.05 | 0.58 |
| 1-Dodecene | 12.96 | 1270 | 1265 | 0.04 | 0.02 | 0.04 | 0.02 | 0.03 | 0.02 | 0.07 | 0.02 |
| Pentadecane | 16.28 | 1501 | 1500 | 0.01 | 0.01 | 0 | 0.01 | 0 | 0.01 | 0.02 | 0.01 |
| 2,4-Di-tert-butylphenol | 16.44 | 1513 | 1517 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 108.1 | 81.42 | 92.67 | 85.77 | |||||||
| C5 | 13.6 | 11.3 | 12.35 | 12.06 | |||||||
| C6 | 54.37 | 42.93 | 47.86 | 42.74 | |||||||
| C5 + C6 | 67.97 | 54.22 | 60.21 | 54.8 | |||||||
| Abbr. | Cube Level 1 | Cube Level 2 | Star Level 1 | Star Level 2 | Units |
|---|---|---|---|---|---|
| Ex.temp | 40 | 70 | 25 | 85 | °C |
| Co.time | 10 | 20 | 5 | 25 | mins |
| Ex.time | 20 | 50 | 5 | 65 | mins |
| Des.temp | 250 | 270 | 240 | 280 | °C |
| Des.time | 5 | 10 | 2.5 | 12.5 | mins |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lioupi, A.; Sampsonidis, I.; Virgiliou, C.; Papoti, V.T.; Zinoviadou, K.G.; Spyros, A.; Theodoridis, G. Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils. Metabolites 2022, 12, 114. https://doi.org/10.3390/metabo12020114
Lioupi A, Sampsonidis I, Virgiliou C, Papoti VT, Zinoviadou KG, Spyros A, Theodoridis G. Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils. Metabolites. 2022; 12(2):114. https://doi.org/10.3390/metabo12020114
Chicago/Turabian StyleLioupi, Artemis, Ioannis Sampsonidis, Christina Virgiliou, Vassiliki T. Papoti, Kyriaki G. Zinoviadou, Apostolos Spyros, and Georgios Theodoridis. 2022. "Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils" Metabolites 12, no. 2: 114. https://doi.org/10.3390/metabo12020114
APA StyleLioupi, A., Sampsonidis, I., Virgiliou, C., Papoti, V. T., Zinoviadou, K. G., Spyros, A., & Theodoridis, G. (2022). Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils. Metabolites, 12(2), 114. https://doi.org/10.3390/metabo12020114









