Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Liriope spicata Fruit
Abstract
:1. Introduction
2. Results
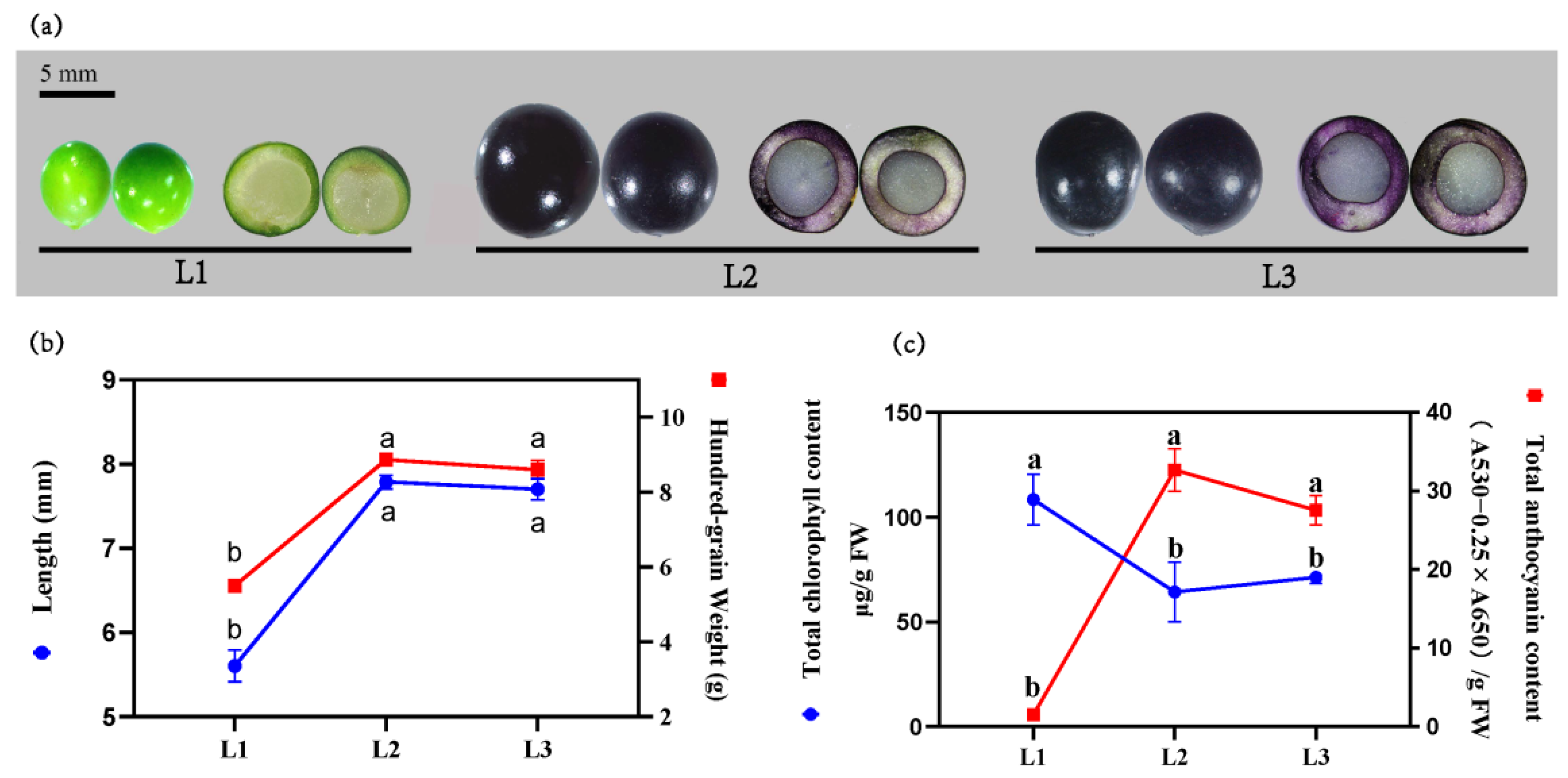
2.1. Relationship between the Color Development and the Variation of Chlorophyll and Anthocyanin
2.2. The Metabolites Profiles of L. spicata Fruits
2.3. Differentially Accumulated Metabolites (DAMs) Analysis on the Fruit
2.4. Differential Expression of Flavonoid Structural Genes and Regulatory Genes (DEGs) Analysis on the Fruit
2.5. Co-Expression Analysis of Genes Related to the Flavonoid Synthesis Pathway
2.6. Relationship between the Genes Expression and Anthocyanins
2.7. Verification by RT–qPCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination on the Pigment Content
4.3. Preparation for Metabonomic Samples
4.4. UPLC Conditions
4.5. Total RNA Extraction and Quality Analysis
4.6. Illumina RNA–Seq Library Construction, Expression Level Estimation and Phylogenetic Analysis
4.7. QRT–PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Anthocyanin composition and changes during kernel development in purple-pericarp supersweet sweetcorn. Food Chem. 2020, 315, 126284. [Google Scholar] [CrossRef]
- Lou, Q.; Liu, Y.; Qi, Y.; Jiao, S.; Tian, F.; Jiang, L.; Wang, Y. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef] [Green Version]
- Tatsuzawa, F.; Tanikawa, N.; Nakayama, M. Red-purple flower color and delphinidin-type pigments in the flowers of Pueraria lobata (Leguminosae). Phytochemistry 2017, 137, 52–56. [Google Scholar] [CrossRef]
- Yuhui, Z.H.A.I.; Jiaqi, L.; Xiang, L.I.; Xiaoning, L.U.O.; Long, L.I.; Qianqian, S.H.I. Effects of cell sap pH on the flower color formation in Primula vulgaris. Acta Hortic. Sin. 2020, 47, 477–491. [Google Scholar]
- Deguchi, A.; Tatsuzawa, F.; Miyoshi, K. A blackish-flowered cultivar of Catharanthus roseus accumulates high concentrations of a novel anthocyanin with a unique feature of aggregation in weak acid solutions. Dye. Pigment. 2020, 173, 108001. [Google Scholar] [CrossRef]
- Deguchi, A.; Tatsuzawa, F.; Hosokawa, M.; Doi, M.; Ohno, S. Quantitative evaluation of the contribution of four major anthocyanins to black flower coloring of dahlia petals. Hortic. J. 2016, 85, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.R.; Davison, P.A.; Bolognesi-Winfield, A.C.; James, C.M.; Srinivasan, N.; Blundell, T.L.; Esch, J.J.; Marks, M.D.; Gray, J.C. The Transparent Testa Glabra1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 1999, 11, 1337–1349. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, Å.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Zhang, R.; Zhang, H.; Zhou, Z.; Liu, C.; Wu, M.; Wang, H.; Dong, H.; Liu, J.; Yao, J.-L.; et al. Identification of gene co-expression networks and key genes regulating flavonoid accumulation in apple (Malus × domestica) fruit skin. Plant Sci. 2020, 304, 110747. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Liu, Y.; Lin-Wang, K.; de Ng, C.; Warran, B.; Wang, L.; Yu, B.; Yang, H.; Wang, J.; Espley, R.V.; Zhang, J.J.P.O. Comparative transcriptome analysis of white and purple potato to identify genes Involved in anthocyanin biosynthesis. PLoS ONE 2015, 10, e0129148. [Google Scholar]
- Gisbert, C.; Dumm, J.M.; Prohens, J.; Vilanova, S.; Stommel, J.R. A Spontaneous eggplant (Solanum melongena L.) color mutant conditions anthocyanin-free fruit pigmentation. HortScience 2016, 51, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Fantini, E.; Bassolino, L.; Povero, G.; Spelt, C.; Buti, S.; Giuliano, G.; Quattrocchio, F.; Koes, R.; Perata, P.J.P.O. Tomato R2R3-MYB proteins SlANT1 and SlAN2: Same protein activity, different roles. PLoS ONE 2015, 10, e0136365. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Deng, C.; Wang, J.; Lu, C.; Li, Y.; Kong, D.; Hong, Y.; Huang, H.; Dai, S. CcMYB6-1 and CcbHLH1, two novel transcription factors synergistically involved in regulating anthocyanin biosynthesis in cornflower. Plant Physiol. 2020, 151, 271–283. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ichihi, A.; Tanaka, Y.; Fujishige, S.; Koeda, S.; Shimizu, K. The R2R3-MYB transcription factor MiMYB1 regulates light dependent red coloration of ‘Irwin’ mango fruit skin. Sci. Hortic. 2020, 272, 109567. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Xu, Z.S.; Feng, K.; Que, F.; Wang, F.; Xiong, A.S. A MYB transcription factor, DcMYB6, is involved in regulating anthocyanin biosynthesis in purple carrot taproots. Sci. Rep. 2017, 7, 45324. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Reichelt, M.; Yoshida, K.; Gershenzon, J.; Constabel, C.P. Two R2R3-MYB proteins are broad repressors of flavonoid and phenylpropanoid metabolism in poplar. Plant J. 2018, 96, 949–965. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Zhou, L.; Han, L.; Zou, H.; Miao, K.; Wang, Y. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, D.; Xu, J.; Guo, D.; Li, H.; Wang, Y.; Mei, W.; Peng, S. Identification of the bHLH gene family in Dracaena cambodiana reveals candidate genes involved in flavonoid biosynthesis. Ind. Crops Prod. 2020, 150, 112407. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Yang, Y.-P. Advances in the study of the systematics of Ophiopogoneae in Asparagaceae. Plant Divers. Resour. 2015, 37, 365–375. [Google Scholar]
- Huang, G.; Zeng, Y.; Wei, L.; Yao, Y.; Dai, J.; Liu, G.; Gui, Z. Comparative transcriptome analysis of mulberry reveals anthocyanin biosynthesis mechanisms in black (Morus atropurpurea Roxb.) and white (Morus alba L.) fruit genotypes. BMC Plant Biol. 2020, 20, 279. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, L.; Zhao, C.; Li, K.; Shang, C.; Hou, L.; Wang, M.; Shi, J.; Fan, S.; Wang, X. Comparative transcriptome analysis of anthocyanin synthesis in black and pink peanut. Plant Signal Behav. 2020, 15, 1721044. [Google Scholar] [CrossRef]
- Meng, G.; Clausen, S.K.; Rasmussen, S.K. Transcriptome analysis reveals candidate genes related to anthocyanin biosynthesis in different carrot genotypes and tissues. Plants 2020, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Choung, M.G.; Hwang, Y.S.; Kim, G.P.; Ahn, K.G.; Shim, H.S.; Hong, S.B.; Choi, J.H.; Yu, C.Y.; Chung, I.M.; Kim, S.H.; et al. Antimelanogenic effect and whitening of anthocyanin rich fraction from seeds of Liriope platyphylla. Korean J. Med. Crop Sci. 2013, 21, 361–371. [Google Scholar] [CrossRef]
- Shen, J.; Shao, W.; Du, Z.; Lu, H.; Li, J. Integrated metabolomic and transcriptomic analyses reveal differences in the biosynthetic pathway of anthocyanins in Fragaria nilgerrensis and Fragaria pentaphylla. Sci. Hortic. 2020, 271, 109476. [Google Scholar] [CrossRef]
- Xue, Q.; Fan, H.; Yao, F.; Cao, X.; Liu, M.; Sun, J.; Liu, Y. Transcriptomics and targeted metabolomics profilings for elucidation of pigmentation in Lonicera japonica flowers at different developmental stages. Ind. Crops Prod. 2020, 145, 111981. [Google Scholar] [CrossRef]
- Li, B.J.; Zheng, B.Q.; Wang, J.Y.; Tsai, W.C.; Lu, H.C.; Zou, L.H.; Wan, X.; Zhang, D.Y.; Qiao, H.J.; Liu, Z.J.; et al. New insight into the molecular mechanism of colour differentiation among floral segments in orchids. Commun. Biol. 2020, 3, 89. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Mei, X.; Rothenberg, D.O.; Yang, Z.; Zhang, W.; Wan, S.; Yang, H.; Zhang, L. Metabolome and Transcriptome Analysis Reveals Putative Genes Involved in Anthocyanin Accumulation and Coloration in White and Pink Tea (Camellia sinensis) Flower. Molecules 2020, 25, 190. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Tan, L.; Zou, Y.; Tan, X.; Huang, J.; Chen, W.; Tang, Q. The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’ (Camellia sinensis). Molecules 2020, 25, 354. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.J.; Park, J.E.; Lee, K.S.; Seo, W.D.; Song, S.B.; Lee, M.H.; Kim, S.; Kim, J.I.; Oh, E.; Pae, S.B.; et al. Identification of anthocyanin compositions in black seed coated Korean adzuki bean (Vigna angularis) by NMR and UPLC-Q-Orbitrap-MS/MS and screening for their antioxidant properties using different solvent systems. Food Chem. 2021, 346, 128882. [Google Scholar] [CrossRef]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Usman, B.; Zhao, Y.; Li, Q.; Liu, P. Computational and transcriptomic analysis unraveled OsMATE34 as a putative anthocyanin transporter in black rice (Oryza sativa L.) caryopsis. Genes 2021, 12, 583. [Google Scholar] [CrossRef]
- Dong, T.; Han, R.; Yu, J.; Zhu, M.; Zhang, Y.; Gong, Y.; Li, Z. Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis, L.). Food Chem. 2019, 271, 18–28. [Google Scholar] [CrossRef]
- Deguchi, A.; Ohno, S.; Hosokawa, M.; Tatsuzawa, F.; Doi, M. Endogenous post-transcriptional gene silencing of flavone synthase resulting in high accumulation of anthocyanins in black dahlia cultivars. Planta 2013, 237, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.-J.; Li, L.; Wang, S.-B.; Ma, X.-W.; Wu, H.-X.; Xu, H.-X.; Xu, W.-T.; Liang, Q.-Z.; Chen, J.-Z. Review of integrated metabolome and transcriptome analysis used for disclosing physiological mechanism in fruit crops. J. Fruit Sci. 2020, 37, 1413–1424. [Google Scholar]
- Lu, C.; Pu, Y.; Liu, Y.; Li, Y.; Qu, J.; Huang, H.; Dai, S. Comparative transcriptomics and weighted gene co-expression correlation network analysis (WGCNA) reveal potential regulation mechanism of carotenoid accumulation in Chrysanthemum × morifolium. Plant Physiol. Biochem. 2019, 142, 415–428. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Li, W.; Zhang, S.; Xi, W. Identification of key genes and regulators associated with carotenoid metabolism in apricot (Prunus armeniaca) fruit using weighted gene coexpression network analysis. BMC Genom. 2019, 20, 876. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef]
- Sun, H.; Guo, K.; Feng, S.; Zou, W.; Li, Y.; Fan, C.; Peng, L. Positive selection drives adaptive diversification of the 4-coumarate: CoA ligase (4CL) gene in angiosperms. Ecol. Evol. 2015, 5, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Endler, A.; Martens, S.; Wellmann, F.; Matern, U. Unusually divergent 4-coumarate: CoA-ligases from Ruta graveolens L. Plant Mol. Biol. 2008, 67, 335–346. [Google Scholar] [CrossRef]
- Liang, L.-J.; Yang, Y.-C.; Wang, E.-H.; Xing, B.-C.; Liang, Z.-S. Research progress on biosynthesis and regulation of plant anthocyanin. Anhui Agric. Sci. 2018, 46, 18–24. [Google Scholar]
- Hong, Y.-H.; Ye, Q.-H.; Li, Z.-K.; Wang, W.; Xie, Q.; Chen, Q.-C.; Chen, J.-Q. Accumulation of anthocyanins in red-flowered strawberry ‘Meihong’ petals and expression analysis of MYB gene. Acta Hortic. Sin. 2021, 48, 1470. [Google Scholar]
- Nitarska, D.; Stefanini, C.; Haselmair-Gosch, C.; Miosic, S.; Walliser, B.; Mikulic-Petkovsek, M.; Regos, I.; Slatnar, A.; Debener, T.; Terefe-Ayana, D.; et al. The rare orange-red colored Euphorbia pulcherrima cultivar 'Harvest Orange' shows a nonsense mutation in a flavonoid 3′-hydroxylase allele expressed in the bracts. BMC Plant Biol. 2018, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Cui, Y.; Ren, J.; Qi, F.; Qu, J.; Huang, H.; Dai, S. Isolation and Functional Analysis of Genes Involved in Polyacylated Anthocyanin Biosynthesis in Blue Senecio cruentus. Front. Plant Sci. 2021, 12, 640746. [Google Scholar] [CrossRef] [PubMed]
- Noda, N. Recent advances in the research and development of blue flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhu, M.; Wang, M.; Xu, Y.; Chen, W.; Yang, G. Transcriptome analysis of calcium-induced accumulation of anthocyanins in grape skin. Sci. Hortic. 2020, 260, 108871. [Google Scholar] [CrossRef]
- Sui, X.; Zhao, M.; Han, X.; Zhao, L.; Xu, Z. RrGT1, a key gene associated with anthocyanin biosynthesis, was isolated from Rosa rugosa and identified via overexpression and VIGS. Plant Physiol. Biochem. 2019, 135, 19–29. [Google Scholar] [CrossRef]
- Qiu, W.; Su, W.; Cai, Z.; Dong, L.; Li, C.; Xin, M.; Fang, W.; Liu, Y.; Wang, X.; Huang, Z.; et al. Combined Analysis of Transcriptome and Metabolome Reveals the Potential Mechanism of Coloration and Fruit Quality in Yellow and Purple Passiflora edulis Sims. J. Agric. Food Chem. 2020, 68, 12096–12106. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kapoor, N.; Aslam, L.; Mahajan, R. Molecular characterization of PgUFGT gene and R2R3-PgMYB transcription factor involved in flavonoid biosynthesis in four tissues of wild pomegranate (Punica granatum L.). J. Genet. 2019, 98, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Kou, M.; Li, C.; Zhang, Y.G. Comparative transcriptome analysis reveals candidate genes involved in anthocyanin biosynthesis in sweetpotato (Ipomoea batatas L.). Plant Physiol. Biochem. 2021, 158, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Ni, J.; Hou, J.; Shu, X.; Zhao, W.; Su, P.; Wang, D.; Shah, F.A.; Huang, S.; et al. The R2R3-MYB transcription factor SsMYB1 positively regulates anthocyanin biosynthesis and determines leaf color in Chinese tallow (Sapium sebiferum Roxb.). Ind. Crops Prod. 2021, 164, 113335. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Yu, C.; Wang, C.; Jin, Y.; Zhang, H. MYB transcription factor PdMYB118 directly interacts with bHLH transcription factor PdTT8 to regulate wound-induced anthocyanin biosynthesis in poplar. BMC Plant Biol. 2020, 20, 173. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.-F.; Huang, H.-L.; Ji, Y.-Z.; Zhao, X.-F.; Qi, J.-L.; Zhang, L.-H.; Zhang, G.-Z. Evaluation of the optimum concentration of chlorophyll extract for determination of chlorophyll content by spectrophotometry. Pratacultural Sci. 2018, 35, 1965–1974. [Google Scholar]
- Hutabarat, R.P.; Xiao, Y.D.; Wu, H.; Wang, J.; Li, D.J.; Huang, W.Y. Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J. Food Qual. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Zhang, X.; Sun, Y.; Wang, H.; Song, J.; Li, X. Transcriptome analyses reveal key genes involved in skin color changes of 'Xinlimei' radish taproot. Plant Physiol. Biochem. 2019, 139, 528–539. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Schultz, A.W.; Wang, J.; Johnson, C.H.; Yannone, S.M.; Patti, G.J.; Siuzdak, G.J.N.P. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat. Protoc. 2013, 8, 451–460. [Google Scholar] [CrossRef]






| Genes | Description | DEGs | ALL | |
|---|---|---|---|---|
| Ⅰ | PAL | phenylalanine ammonia–lyase | 15 | 20 |
| C4H | trans–cinnamate 4–monooxygenase | 9 | 10 | |
| 4CL | 4–coumarate–CoA ligase | 22 | 70 | |
| CHS | chalcone synthase | 20 | 22 | |
| CHI | chalcone isomerase | 8 | 24 | |
| F3H | naringenin 3–dioxygenase | 6 | 6 | |
| F3′5′H | flavonoid 3′,5′–hydroxylase | 2 | 2 | |
| Ⅱ | DFR | dihydroflavonol 4–reductase | 0 | 2 |
| LDOX/ANS | leucoanthocyanidin dioxygenase | 6 | 6 | |
| UFGT | anthocyanidin 3–O–glucosyltransferase | 3 | 5 | |
| OMT | caffeoyl–CoA O–methyltransferase | 7 | 14 | |
| Ⅲ | FLS | flavonol synthase | 3 | 7 |
| FG3 | flavonol–3–O–glucoside | 2 | 2 | |
| Ⅳ | MYB | transcription factor MYB | 52 | 88 |
| bHLH | transcription factor bHLH | 26 | 57 | |
| WD40 | transcription factor WD40 | 0 | 14 |
| Group | Module | Response | DEGs |
|---|---|---|---|
| Ⅰ | darkorange2, bisque4, salmon, darkorange | Positively respond to DAMs | 1609 |
| Ⅱ | Darkgrey, orangered4, brown, lightcyan | Negatively respond to DAMs | 1698 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, S.; Zheng, G.; Zhu, S.; Qian, J.; Liang, L. Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Liriope spicata Fruit. Metabolites 2022, 12, 144. https://doi.org/10.3390/metabo12020144
Gan S, Zheng G, Zhu S, Qian J, Liang L. Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Liriope spicata Fruit. Metabolites. 2022; 12(2):144. https://doi.org/10.3390/metabo12020144
Chicago/Turabian StyleGan, Sichen, Gang Zheng, Shoukuo Zhu, Jieyu Qian, and Lijun Liang. 2022. "Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Liriope spicata Fruit" Metabolites 12, no. 2: 144. https://doi.org/10.3390/metabo12020144
APA StyleGan, S., Zheng, G., Zhu, S., Qian, J., & Liang, L. (2022). Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Liriope spicata Fruit. Metabolites, 12(2), 144. https://doi.org/10.3390/metabo12020144






