Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae
Abstract
1. Introduction
2. Results
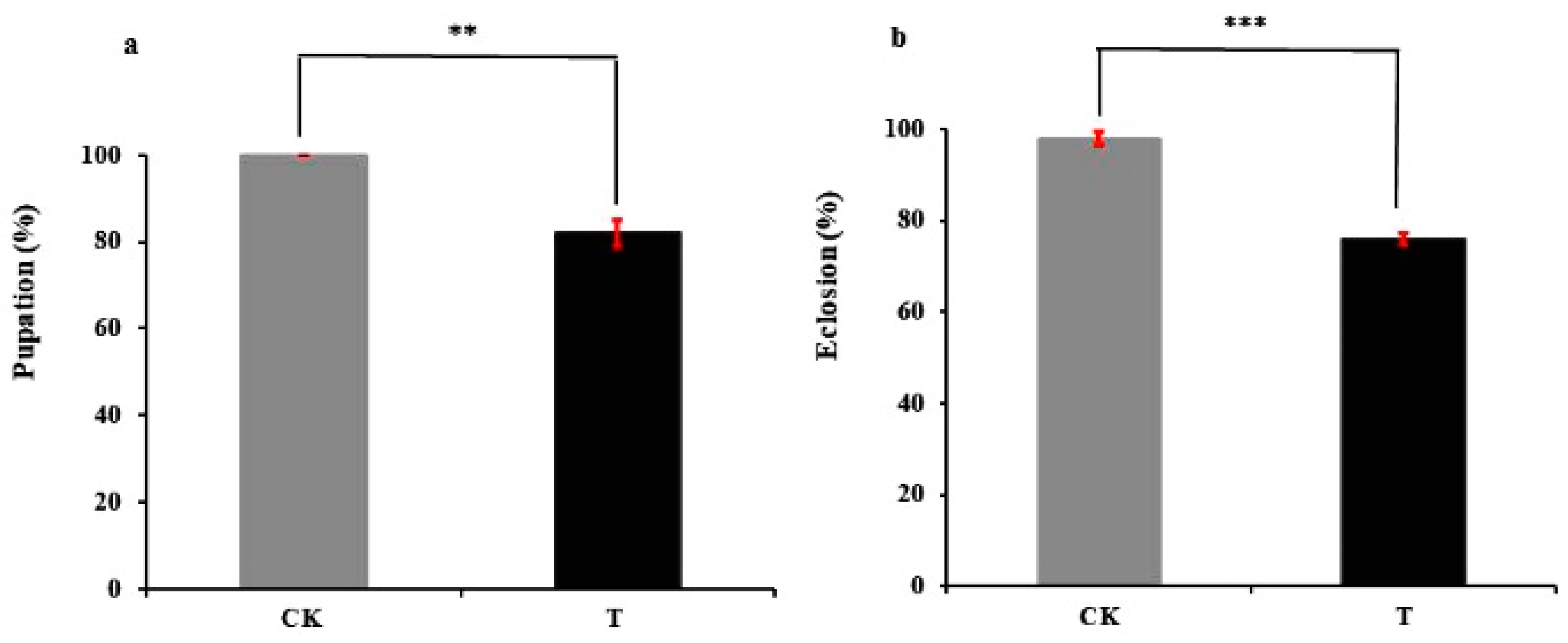
2.1. Azadirachtin Activities against C. sinica
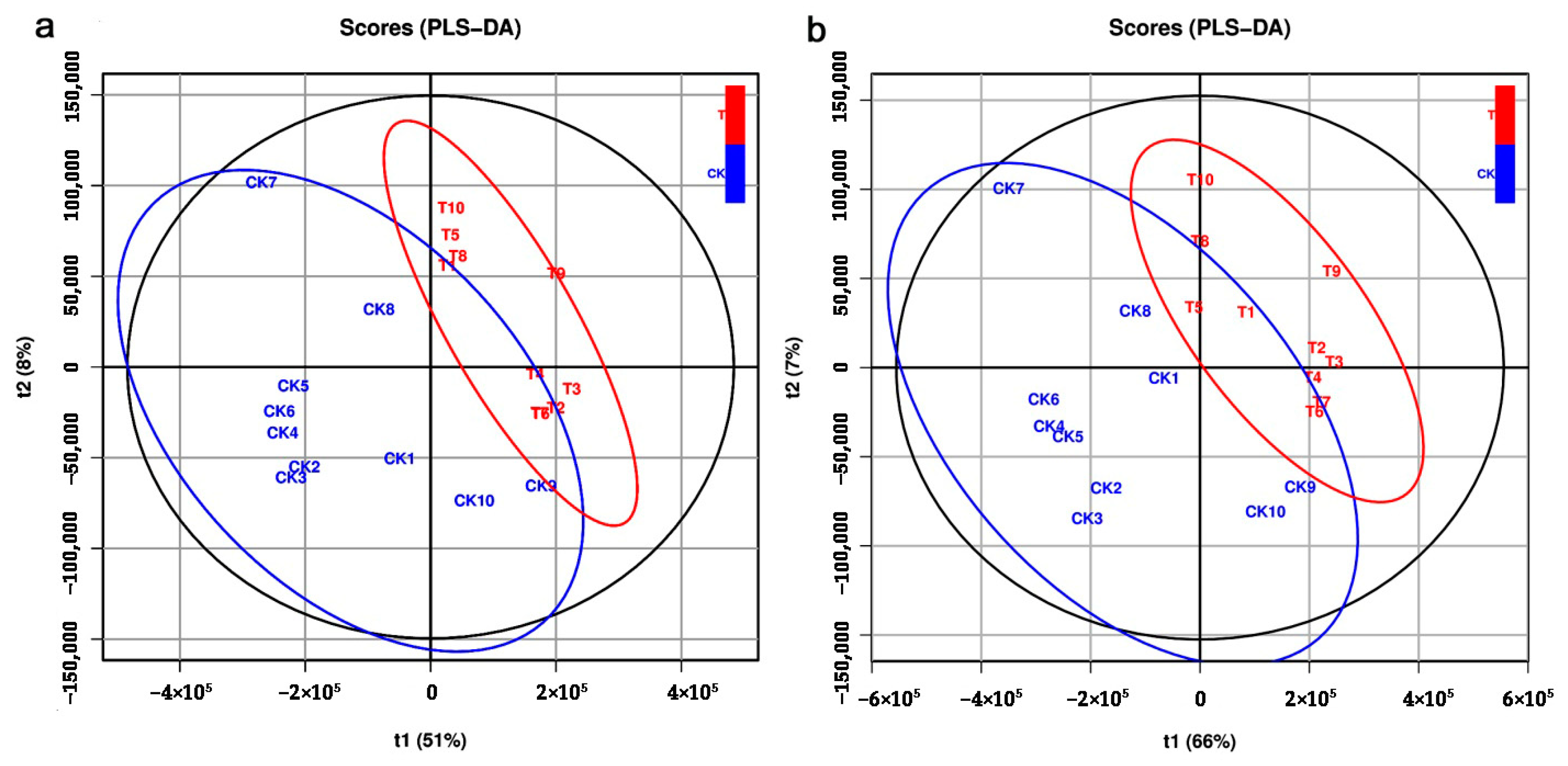
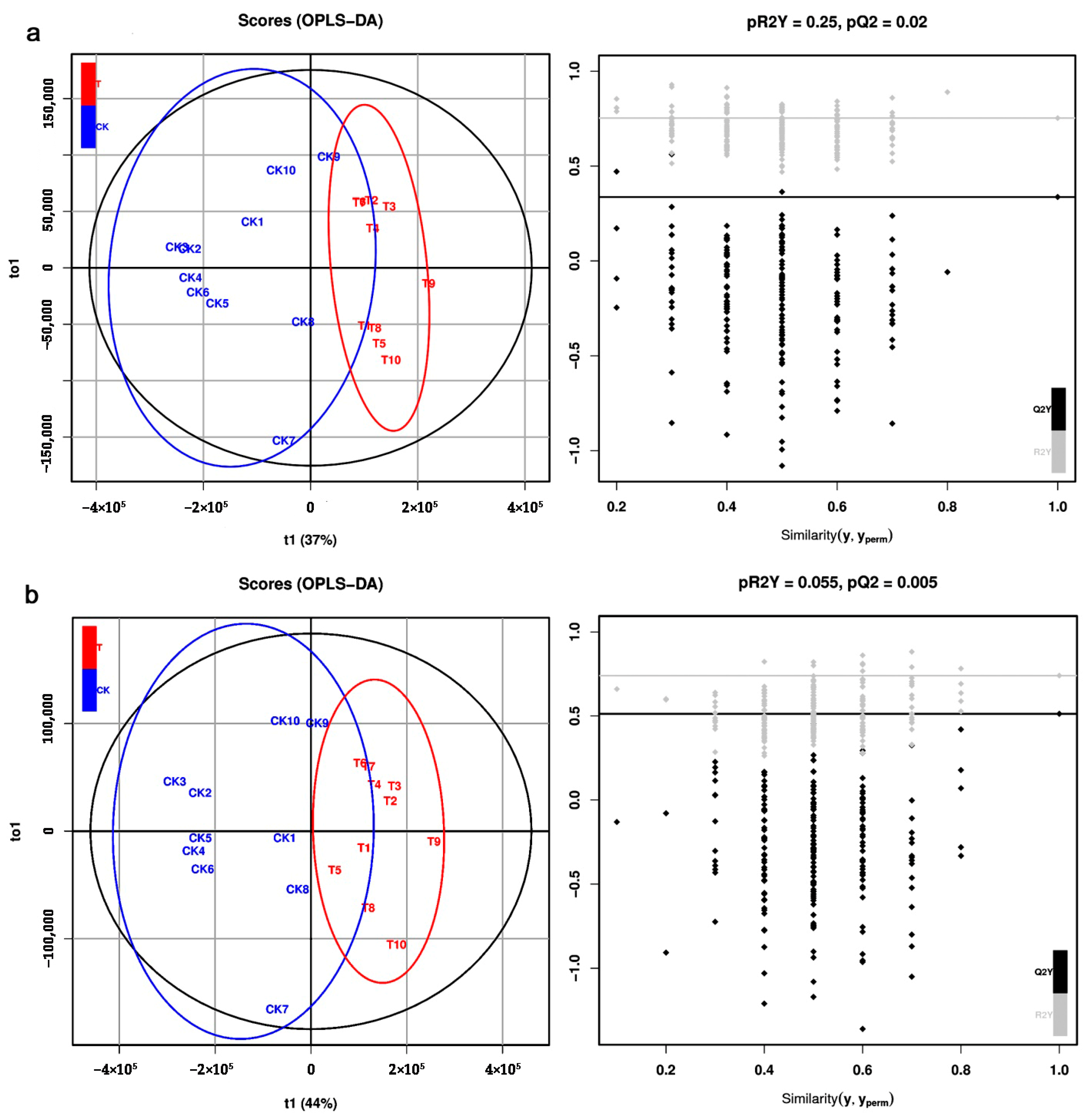
2.2. Metabolic Profiles Analyzed by LC–MS
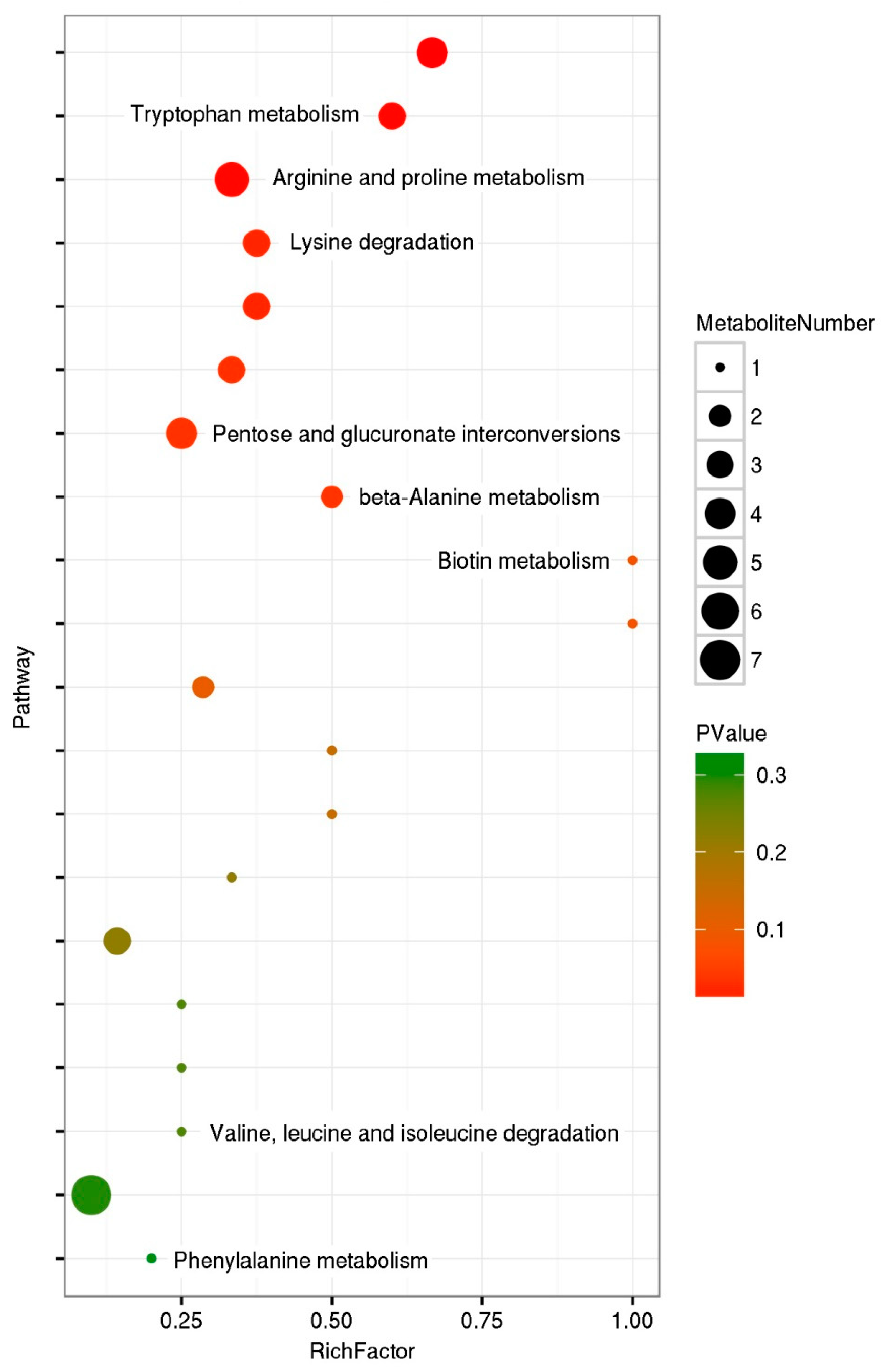
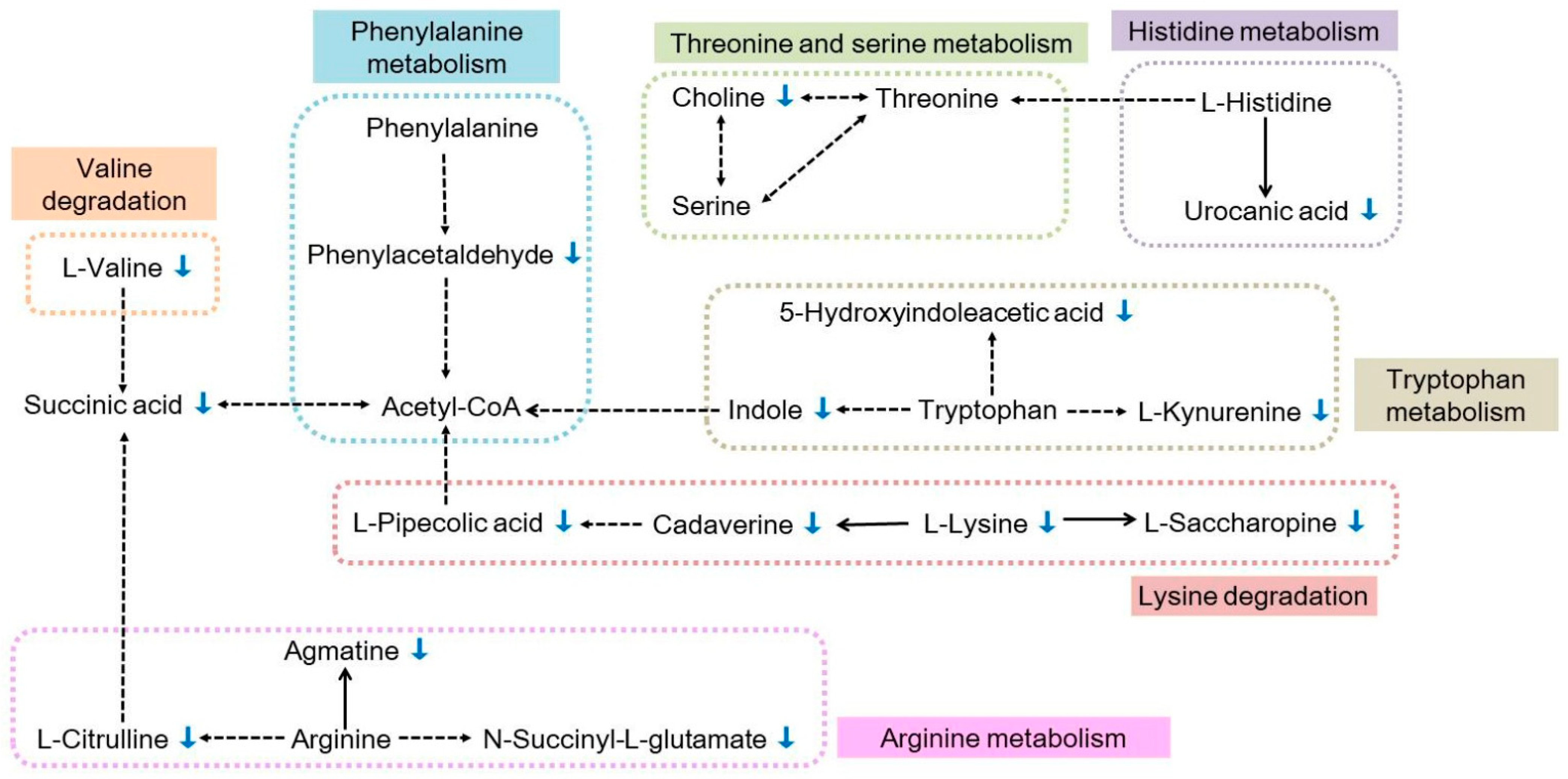
2.3. Changes in Metabolites and Metabolic Pathways of Differentially Abundant Metabolites
3. Discussion
3.1. Metabolite Changes Adversely Affected C. sinica Development
3.2. Key Metabolic Pathways Affected by Feeding on Azadirachtin-Treated P. xylostella Larvae
3.3. Precautions When Azadirachtin and C. sinica Are Used in IPM
4. Materials and Methods
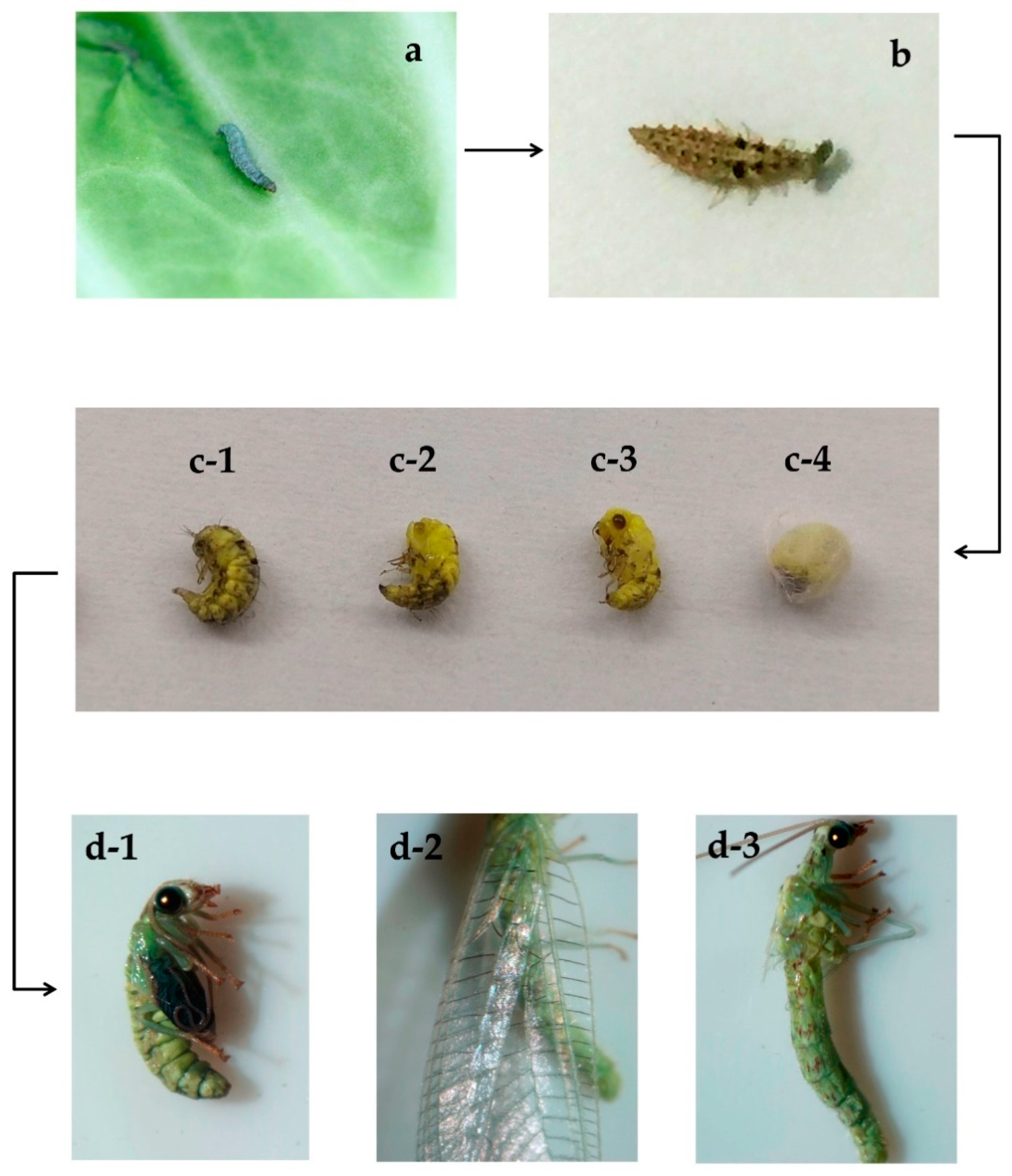
4.1. Chrysopa sinica and Plutella xylostella
4.2. Chemical Reagents and Instruments
4.3. Experimental Procedures and Samples Collection
4.4. Metabolites Extraction and Detection
4.5. Data Preprocessing and Multivariate Statistical Analysis
4.6. KEGG Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Bueno, A.D.F.; Carvalho, G.A.; Santos, A.C.D.; Sosa-Gómez, D.R.; Silva, D.M.D. Pesticide selectivity to natural enemies: Challenges and constraints for research and field recommendation. Ciência Rural 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Martínez-Romero, A.; Ortega-Sánchez, J.L.; Hernández-González, S.I.; Olivas-Calderón, E.H.; Alba-Romero, J.J. Application of neem tree in agriculture, industry, medicine, and environment: A review. Afr. J. Tradit. Complementary Altern. Med. 2016, 13, 191–198. [Google Scholar] [CrossRef][Green Version]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Zanuncio, J.C.; Mourão, S.A.; Martínez, L.C.; Wilcken, C.F.; Ramalho, F.S.; Plata-Rueda, A.; Soares, M.A.; Serrão, J.E. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Reps. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.S.; Hasan, F. Effects of neem-based insecticides on Plutella xylostella (Linn.). Crop Prot. 2012, 34, 18–24. [Google Scholar] [CrossRef]
- Mordue, A.J.; Blackwell, A. Azadirachtin: An update. J. Insect Physiol. 1993, 39, 903–924. [Google Scholar] [CrossRef]
- Schmutterer, H. Potential of azadirachtin-containing pesticides for integrated pest control in developing and industrialized countries. J. Insect Physiol. 1988, 34, 713–719. [Google Scholar] [CrossRef]
- Vasanthakumar, D.; Babu, A.; Shanmugapriyan, R.; Subramaniam, S.R. Impact of Azter (azadirachtin 0.15% EC), a neem-based pesticide, against tea red spider mite, Oligonychus coffeae Neitner (Acarina: Tetranychidae), and its natural enemies. Int. J. Acarol. 2013, 39, 140–145. [Google Scholar] [CrossRef]
- Vinuela, E.; Adán, A.; Smagghe, G.; Gonzalez, M.; Medina, M.P.; Budia, F.; Vogt, H.; Estal, P.D. Laboratory effects of ingestion of azadirachtin by two pests (Ceratitis capitata and Spodoptera exigua) and three natural enemies (Chrysoperla carnea, Opius concolor and Podisus maculiventris). Biocontrol Sci. Technol. 2000, 10, 165–177. [Google Scholar] [CrossRef]
- Viñuela, E.; Medina, M.P.; Schneider, M.; González, M.; Budia, F.; Adán, A.; Del Estal, P. Comparison of side-effects of spinosad, tebufenozide and azadirachtin on the predators Chrysoperla carnea and Podisus maculiventris and the parasitoids Opius concolor and Hyposoter didymator under laboratory conditions. Bulletin IOBC/WPRS 2001, 24, 25–34. [Google Scholar]
- Lima, D.B.; Melo, J.W.S.; Guedes, N.M.P.; Gontijo, L.M.; Guedes, R.N.C.; Gondim Jr, M.G.C. Bioinsecticide-predator interactions: Azadirachtin behavioral and reproductive impairment of the coconut mite predator Neoseiulus baraki. PLoS ONE 2015, 10, e118343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Xue, J.; Yang, X.; Gong, S. Response of a predatory insect, Chrysopa sinica, toward the volatiles of persimmon trees infested with the herbivore, Japanese wax scale. Int. J. Ecol. 2012, 2012, 653869. [Google Scholar] [CrossRef]
- Gao, F.; Chen, F.; Ge, F. Elevated CO2 lessens predation of Chrysopa sinica on Aphis gossypii. Entomol. Exp. Appl. 2010, 135, 135–140. [Google Scholar] [CrossRef]
- Shu, M.; Liu, W.; Feng, L.; Mou, L.; Ma, N.; Wang, P. Study on the functional response of Chrysopa sinica Tjeder to predation of the eggs of Leptinotarsa decemlineata Say. Xinjiang Agri. Sci. 2011, 48, 328–333. [Google Scholar]
- Liu, Z.; Wyckhuys, K.A.; Wu, K. Migratory adaptations in Chrysoperla sinica (Neuroptera: Chrysopidae). Environ. Entomol. 2011, 40, 449–454. [Google Scholar] [CrossRef]
- Medina, P.; Budia, F.; Tirry, L.; Smagghe, G.; Vinuela, E. Compatibility of spinosad, tebufenozide and azadirachtin with eggs and pupae of the predator Chrysoperla carnea (Stephens) under laboratory conditions. Biocontrol Sci. Technol. 2001, 11, 597–610. [Google Scholar] [CrossRef]
- Ahmad, M.; Ossiewatsch, H.R.; Basedow, T. Effects of neem-treated aphids as food/hosts on their predators and parasitoids. J. Appl. Entomol. 2003, 127, 458–464. [Google Scholar] [CrossRef]
- Medina, P.; Budia, F.; Del Estal, P.; Vinuela, E. Influence of azadirachtin, a botanical insecticide, on Chrysoperla carnea (Stephens) reproduction: Toxicity and ultrastructural approach. J. Econ. Entomol. 2004, 97, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Scudeler, E.L.; Dos Santos, D.C. Effects of neem oil (Azadirachta indica A. Juss) on midgut cells of predatory larvae Ceraeochrysa claveri (Navás, 1911)(Neuroptera: Chrysopidae). Micron 2013, 44, 125–132. [Google Scholar] [CrossRef]
- Scudeler, E.L.; Padovani, C.R.; Dos Santos, D.C. Effects of neem oil (Azadirachta indica A. Juss) on the replacement of the midgut epithelium in the lacewing Ceraeochrysa claveri during larval-pupal metamorphosis. Acta Histochem. 2014, 116, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Mordue, A.J. Present concepts of the mode of action of azadirachtin from neem. In Neem: Today and in the New Millennium; Springer: Berlin/Heidelberg, Germany, 2004; pp. 229–242. [Google Scholar]
- Nisbet, A.J. Azadirachtin from the neem tree Azadirachta indica: Its action against insects. An. Soc. Entomológica Brasil 2000, 29, 615–632. [Google Scholar]
- Zhou, Y.; Zhang, P.W.; Chen, X.T.; Liu, B.J.; Cheng, D.M.; Zhang, Z.X. Integrated LC–MS and GC–MS-based untargeted metabolomics studies of the effect of azadirachtin on Bactrocera dorsalis larvae. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Sengottayan, S. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar]
- Lai, D.; Jin, X.; Wang, H.; Yuan, M.; Xu, H. Gene expression profile change and growth inhibition in Drosophila larvae treated with azadirachtin. J. Biotechnol. 2014, 185, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lai, D.; Yuan, M.; Xu, H. Growth inhibition and differences in protein profiles in azadirachtin-treated Drosophila melanogaster larvae. Electrophoresis 2014, 35, 1122–1129. [Google Scholar] [CrossRef]
- Rharrabe, K.; Amri, H.; Bouayad, N.; Sayah, F. Effects of azadirachtin on post-embryonic development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2008, 44, 290–294. [Google Scholar] [CrossRef]
- Khosravi, R.; Sendi, J.J. Effect of neem pesticide (Achook) on midgut enzymatic activities and selected biochemical compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 2013, 53, 238–247. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, M.; Shi, P. Sublethal effects of azadirachtin on lipid metabolism and sex pheromone biosynthesis of the Asian corn borer Ostrinia furnacalis. Phytoparasitica 2012, 40, 361–368. [Google Scholar] [CrossRef]
- Amirmohammadi, F.; Sendi, J.J.; Zibaee, A. Biomonitoring of the genotoxic and oxidative the effect of neemon mortality and physiological indices of Hyphantria cunea (Drury)(Lepidoptera). Munis. Entomol. Zool. 2012, 7, 489–495. [Google Scholar]
- Dawkar, V.V.; Barage, S.H.; Barbole, R.S.; Fatangare, A.; Grimalt, S.; Haldar, S.; Heckel, D.G.; Gupta, V.S.; Thulasiram, H.V.; Svatoš, A. Azadirachtin-A from Azadirachta indica impacts multiple biological targets in cotton bollworm Helicoverpa armigera. ACS Omega 2019, 4, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Snart, C.J.; Hardy, I.C.; Barrett, D.A. Entometabolomics: Applications of modern analytical techniques to insect studies. Entomol. Exp. Appl. 2015, 155, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.; Faccio, A.T.; Canuto, G.A.B.; Da Cruz, P.L.R.; Ribeiro, H.C.; Tavares, M.F.M.; Sussulini, A. Metabolomics: Definitions and significance in systems biology. In Metabolomics: From Fundamentals to Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–17. [Google Scholar]
- Idle, J.R.; Gonzalez, F.J. Metabolomics. Cell Metab. 2007, 6, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Chen, Q.; Hou, Y.; Xia, Q.; Zhao, P. Metabolomics analysis of the larval head of the silkworm, Bombyx mori. Int. J. Mol. Sci. 2016, 17, 1460. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Jabaji, S. Metabolomics–A robust bioanalytical approach for the discovery of the modes-of-action of pesticides: A review. Pestic. Biochem. Phys. 2011, 100, 105–117. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Xu, W. Global metabolomic analyses of the hemolymph and brain during the initiation, maintenance, and termination of pupal diapause in the cotton bollworm, Helicoverpa armigera. PLoS ONE 2014, 9, e99948. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.J.; Wang, L.J.; Zhang, Y.A.; Wang, Q.H.; Wang, Y.Z.; Zhao, T.H.; Cai, W.Z. Radiation-Induced Metabolomic Changes in Sterile Male Μοnochamus alternatus (Coleoptera: Cerambycidae). J. Insect Sci. 2014, 14, 166. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F.; El Shesheny, I.; Alfaress, S.; Jones, S.E.; Rogers, M.E. Metabolomic analyses of the haemolymph of the Asian citrus psyllid Diaphorina citri, the vector of huanglongbing. Physiol. Entomol. 2017, 42, 134–145. [Google Scholar] [CrossRef]
- Liigand, P.; Heering, A.; Kaupmees, K.; Leito, I.; Girod, M.; Antoine, R.; Kruve, A. The evolution of electrospray generated droplets is not affected by ionization mode. J. Am. Soc. Mass Spectr. 2017, 28, 2124–2131. [Google Scholar] [CrossRef]
- Mattila, J.; Hietakangas, V. Regulation of carbohydrate energy metabolism in Drosophila melanogaster. Genetics 2017, 207, 1231–1253. [Google Scholar]
- Thompson, S.N.; Borchardt, D.B.; Wang, L. Dietary nutrient levels regulate protein and carbohydrate intake, gluconeogenic/glycolytic flux and blood trehalose level in the insect Manduca sexta L. J. Comp. Physiol. B 2003, 173, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Sörensen, B. The Alpha-Linolenic Acid Requirements of Developing Heliothines. Ph.D. Thesis, Friedrich-Schiller-Universität, Jena, Germany, 17 June 2013. Available online: https://www.db-thueringen.de/receive/dbt_mods_00022437 (accessed on 15 June 2021).
- Chang, C.L. Effect of amino acids on larvae and adults of Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2004, 97, 529–535. [Google Scholar] [CrossRef]
- Florkin, M. The free amino acids of insect haemolymph. In Biochemistry of Insects; Levenbook, L., Ed.; Pergamon Press: Oxford, UK, 1959; pp. 63–77. [Google Scholar]
- Terra, W.C.; de Bianchi, A.G.; Gambarini, A.G.; Lara, F.J.S. Haemolymph amino acids and related compounds during cocoon production by larvae of the fly Rhynchosciara americana. J. Insect Physiol. 1973, 19, 2097–2106. [Google Scholar] [CrossRef]
- Gammie, S.C.; Truman, J.W. Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth Manduca sexta. J. Neurosci. 1997, 17, 4389–4397. [Google Scholar] [CrossRef]
- Scott, R.L.; Diao, F.; Silva, V.; Park, S.; Luan, H.; Ewer, J.; White, B.H. Non-canonical eclosion hormone-expressing cells regulate Drosophila ecdysis. iScience 2020, 23, 101108. [Google Scholar] [CrossRef] [PubMed]
- Cerstiaens, A.; Huybrechts, J.; Kotanen, S.; Lebeau, I.; Meylaers, K.; De Loof, A.; Schoofs, L. Neurotoxic and neurobehavioral effects of kynurenines in adult insects. Biochem. Bioph. Res. Co. 2003, 312, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Navrotskaya, V.; Wnorowski, A.; Turski, W.; Oxenkrug, G. Effect of kynurenic acid on pupae viability of Drosophila melanogaster cinnabar and cardinal eye color mutants with altered tryptophan-kynurenine metabolism. Neurotox. Res. 2018, 34, 324–331. [Google Scholar] [CrossRef]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160333. [Google Scholar] [CrossRef]
- Newsholme, P.; Stenson, L.; Sulvucci, M.; Sumayao, R.; Krause, M. 1.02—Amino acid metabolism. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 3–14. [Google Scholar]
- Chaudhary, S.; Kanwar, R.K.; Sehgal, A.; Cahill, D.M.; Barrow, C.J.; Sehgal, R.; Kanwar, J.R. Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [PubMed]
- Westerhuis, J.A.; Hoefsloot, H.C.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.; van Duijnhoven, J.P.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic. Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef]

| Mode | KEGG Class | Pathway | Regulation | p-Value † | VIP ‡ | Name | Formula | M/Z § |
|---|---|---|---|---|---|---|---|---|
| ESI+ | Amino acid metabolism | Glycine, serine, and threonine metabolism | down | 6.86 × 10−3 | 11.98 | Choline | C5H13NO | 104.1068832 |
| Arginine and proline metabolism | up | 9.49 × 10−3 | 1.75 | Putrescine | C4H12N2 | 89.10725487 | ||
| Lysine degradation | down | 4.08 × 10−2 | 1.33 | Cadaverine | C5H14N2 | 103.122903 | ||
| Tryptophan metabolism | down | 3.44 × 10−2 | 1.11 | Indole | C8H7N | 118.0650873 | ||
| Lysine biosynthesis | down | 2.11 × 10−6 | 2.07 | L-Saccharopine | C11H20N2O6 | 277.1390289 | ||
| Phenylalanine metabolism | down | 2.18 × 10−2 | 5.22 | Phenylacetaldehyde | C8H8O | 121.0647704 | ||
| Arginine biosynthesis | down | 5.84 × 10−3 | 2.74 | L-Citrulline | C6H13N3O3 | 176.1028923 | ||
| Arginine and proline metabolism | up | 8.30 × 10−4 | 11.94 | Spermidine | C7H19N3 | 146.1651255 | ||
| Tryptophan metabolism | down | 1.83 × 10−2 | 5.89 | L-Kynurenine | C10H12N2O3 | 209.0919249 | ||
| Arginine and proline metabolism | down | 9.08 × 10−3 | 4.49 | Agmatine | C5H14N4 | 131.1290899 | ||
| Arginine and proline metabolism | up | 3.60 × 10−3 | 2.51 | 1-Pyrroline-4-hydroxy-2-carboxylate | C5H7NO3 | 130.0498415 | ||
| Histidine metabolism | down | 2.19 × 10−2 | 2.51 | Urocanic acid | C6H6N2O2 | 139.0502099 | ||
| Lysine degradation | up | 1.17 × 10−2 | 2.85 | Deoxycarnitine | C7H15NO2 | 146.1175269 | ||
| Lysine degradation | down | 1.27 × 10−3 | 2.68 | L-Pipecolic acid | C6H11NO2 | 130.0862821 | ||
| Arginine and proline metabolism | down | 2.11 × 10−2 | 1.28 | N-Succinyl-L-glutamate | C9H13NO7 | 248.2077594 | ||
| Cysteine and methionine metabolism | up | 2.01 × 10−3 | 3.80 | Sulfite | SO32- | 83.084995 | ||
| Carbohydrate metabolism | Glyoxylate and dicarboxylate metabolism | down | 2.99 × 10−3 | 4.62 | L-threo-3-Methylaspartate | C5H9NO4 | 148.0603521 | |
| Amino sugar and nucleotide sugar metabolism | down | 1.80 × 10−3 | 1.72 | N-Acetylneuraminate | C11H19NO9 | 310.1127173 | ||
| Amino sugar and nucleotide sugar metabolism | up | 1.47 × 10−2 | 1.72 | CDP-abequose | C15H25N3O14P2 | 534.3283127 | ||
| Pentose and glucuronate interconversions | down | 3.45 × 10−4 | 16.41 | CDP-ribitol | C14H25N3O15P2 | 538.3194126 | ||
| Amino sugar and nucleotide sugar metabolism | down | 2.76 × 10−3 | 1.13 | UDP-L-Ara4N | C14H23N3O15P2 | 536.3039803 | ||
| Amino sugar and nucleotide sugar metabolism | down | 7.69 × 10−4 | 1.18 | CDP-4-dehydro-3,6-dideoxy-D-glucose | C15H23N3O14P2 | 532.3185085 | ||
| Amino sugar and nucleotide sugar metabolism | down | 4.66 × 10−3 | 2.99 | N-Acetylmuramic acid 6-phosphate | C11H20NO11P | 374.2531432 | ||
| Digestive system | Bile secretion | down | 1.26 × 10−2 | 7.39 | L-Carnitine | C7H15NO3 | 162.1123783 | |
| Bile secretion | down | 3.80 × 10−4 | 6.22 | Acyclovir | C8H11N5O3 | 226.216309 | ||
| Lipid metabolism | Glycerophospholipid metabolism | up | 1.57 × 10−2 | 7.49 | sn-Glycero-3-phosphocholine | C8H21NO6P+ | 258.1097776 | |
| Glycerophospholipid metabolism | up | 4.97 × 10−2 | 2.77 | LysoPC(16:1(9Z)) | C24H48NO7P | 494.3234809 | ||
| Glycerophospholipid metabolism | up | 3.07 × 10−2 | 3.58 | Glycerylphosphorylethanolamine | C5H14NO6P | 216.062969 | ||
| Membrane transport | ABC transporters | up | 1.58 × 10−3 | 1.84 | Ferrichrome | C27H42FeN9O12 | 741.5250124 | |
| ABC transporters | down | 1.28 × 10−3 | 2.09 | Mannopine | C11H22N2O8 | 311.3132994 | ||
| Metabolism of cofactors and vitamins | Porphyrin and chlorophyll metabolism | up | 4.05 × 10−3 | 1.01 | Biliverdin | C33H34N4O6 | 583.2537613 | |
| Biotin metabolism | down | 4.20 × 10−3 | 1.06 | Biotin | C10H16N2O3S | 245.0953355 | ||
| Thiamine metabolism | up | 2.49 × 10−3 | 2.39 | Thiamin triphosphate | C12H19N4O10P3S | 506.2968678 | ||
| Nicotinate and nicotinamide metabolism | down | 1.75 × 10−2 | 1.09 | 2,3-Dimethylmaleate | C6H8O4 | 145.1335282 | ||
| Metabolism of other amino acids | beta-Alanine metabolism | up | 3.18 × 10−3 | 3.47 | Pantothenic acid | C9H17NO5 | 220.1178677 | |
| ESI− | Amino acid metabolism | Valine, leucine and isoleucine degradation | down | 3.54 × 10−3 | 2.84 | L-VALINE | C5H11NO2 | 116.0717418 |
| Lysine biosynthesis | down | 3.88 × 10−3 | 3.80 | L-LYSINE | C6H14N2O2 | 145.098347 | ||
| Tryptophan metabolism | down | 1.95 × 10−2 | 1.18 | 5-Hydroxyindoleacetic acid | C10H9NO3 | 190.0509535 | ||
| Lysine biosynthesis | down | 7.28 × 10−6 | 1.02 | L-Saccharopine | C11H20N2O6 | 275.1248028 | ||
| Alanine, aspartate and glutamate metabolism | up | 2.46 × 10−2 | 1.09 | Succinic acid semialdehyde | C4H6O3 | 101.0244019 | ||
| Biosynthesis of other secondary metabolites | Caffeine metabolism | down | 5.30 × 10−3 | 1.07 | 7-Methylxanthosine | C11H15N4O6 | 298.2467506 | |
| Carbohydrate metabolism | Citrate cycle (TCA cycle) | down | 9.43 × 10−3 | 1.64 | Succinic acid | C4H6O4 | 117.0193765 | |
| Pentose and glucuronate interconversions | up | 1.46 × 10−3 | 1.60 | Ribitol | C5H12O5 | 151.0611315 | ||
| Glycolysis / Gluconeogenesis | up | 2.63 × 10−5 | 2.23 | D-Glycerate 3-phosphate | C3H7O7P | 184.9855899 | ||
| Fructose and mannose metabolism | up | 4.08 × 10−2 | 3.58 | Mannitol | C6H14O6 | 181.0717565 | ||
| Amino sugar and nucleotide sugar metabolism | down | 2.78 × 10−3 | 1.02 | Uridine diphosphate-N-acetylglucosamine | C17H27N3O17P2 | 606.0741142 | ||
| C5-Branched dibasic acid metabolism | up | 4.61 × 10−2 | 1.54 | Itaconate, Itaconic acid | C5H6O4 | 129.0194034 | ||
| Ascorbate and aldarate metabolism | up | 5.48 × 10−3 | 1.48 | Threonic acid | C4H8O5 | 135.0299386 | ||
| Galactose metabolism | up | 9.68 × 10−3 | 2.24 | Tagatose | C6H12O6 | 179.0560745 | ||
| Pentose and glucuronate interconversions | down | 1.10 × 10−3 | 2.43 | CDP-ribitol | C14H25N3O15P2 | 536.3041792 | ||
| Pentose and glucuronate interconversions | down | 3.65 × 10−4 | 11.32 | CDP-ribitol | C14H25N3O15P2 | 536.3053925 | ||
| Amino sugar and nucleotide sugar metabolism | up | 8.30 × 10−4 | 1.31 | CMP-pseudaminic acid | C22H34N5O15P | 638.5000382 | ||
| Energy metabolism | Oxidative phosphorylation | down | 9.09 × 10−3 | 1.12 | Pyrophosphate | P2O74− | 176.935889 | |
| Lipid metabolism | Fatty acid biosynthesis | up | 2.72 × 10−3 | 11.07 | Caprylic acid | C8H16O2 | 143.1077396 | |
| Fatty acid biosynthesis | up | 7.01 × 10−6 | 15.37 | Myristic acid | C14H28O2 | 227.2016801 | ||
| Fatty acid biosynthesis | down | 3.75 × 10−3 | 10.03 | Palmitoleic acid | C16H30O2 | 253.2172625 | ||
| Primary bile acid biosynthesis | up | 2.73 × 10−3 | 5.33 | Taurine | C2H7NO3S | 124.0073745 | ||
| Fatty acid biosynthesis | up | 7.76 × 10−3 | 5.94 | Palmitic acid | C16H32O2 | 255.2329072 | ||
| alpha-Linolenic acid metabolism | down | 1.33 × 10−3 | 5.58 | Linolenic Acid | C18H30O2 | 277.2171486 | ||
| Linoleic acid metabolism | down | 8.66 × 10−3 | 2.89 | 9-OxoODE | C18H30O3 | 293.2123043 | ||
| Biosynthesis of unsaturated fatty acids | down | 6.24 × 10−3 | 1.21 | Arachidic acid | C20H40O2 | 311.2954346 | ||
| Membrane transport | ABC transporters | up | 6.64 × 10−3 | 4.01 | Ferrichrome | C27H42FeN9O12 | 739.5113096 | |
| Metabolism of cofactors and vitamins | Folate biosynthesis | down | 3.34 × 10−3 | 4.26 | 2-Amino-4-hydroxy-6-(D-erythro-1,2,3-trihydroxypropyl)-7,8-dihydropteridine | C9H13N5O4 | 254.2205895 | |
| Ubiquinone and other terpenoid-quinone biosynthesis | down | 7.16 × 10−4 | 1.66 | (1R,6R)-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | C11H12O6 | 239.2015915 | ||
| Folate biosynthesis | down | 3.34 × 10−2 | 1.37 | Neopterin | C9H11N5O4 | 252.2049325 | ||
| Metabolism of other amino acids | beta-Alanine metabolism | up | 1.37 × 10−2 | 1.77 | Pantothenic Acid (B5) | C9H17NO5 | 218.1033645 | |
| Sensory system | Inflammatory mediator regulation of TRP channels | down | 2.82 × 10−3 | 1.58 | Icilin | C16H13N3O4 | 310.2830475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhou, Y.; Qin, D.; Chen, J.; Zhang, Z. Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae. Metabolites 2022, 12, 158. https://doi.org/10.3390/metabo12020158
Zhang P, Zhou Y, Qin D, Chen J, Zhang Z. Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae. Metabolites. 2022; 12(2):158. https://doi.org/10.3390/metabo12020158
Chicago/Turabian StyleZhang, Peiwen, You Zhou, Deqiang Qin, Jianjun Chen, and Zhixiang Zhang. 2022. "Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae" Metabolites 12, no. 2: 158. https://doi.org/10.3390/metabo12020158
APA StyleZhang, P., Zhou, Y., Qin, D., Chen, J., & Zhang, Z. (2022). Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae. Metabolites, 12(2), 158. https://doi.org/10.3390/metabo12020158









