Cryoprotective Metabolites Are Sourced from Both External Diet and Internal Macromolecular Reserves during Metabolic Reprogramming for Freeze Tolerance in Drosophilid Fly, Chymomyza costata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proline and Other Amino Acids
2.2. Trehalose and Other Sugars and Polyols
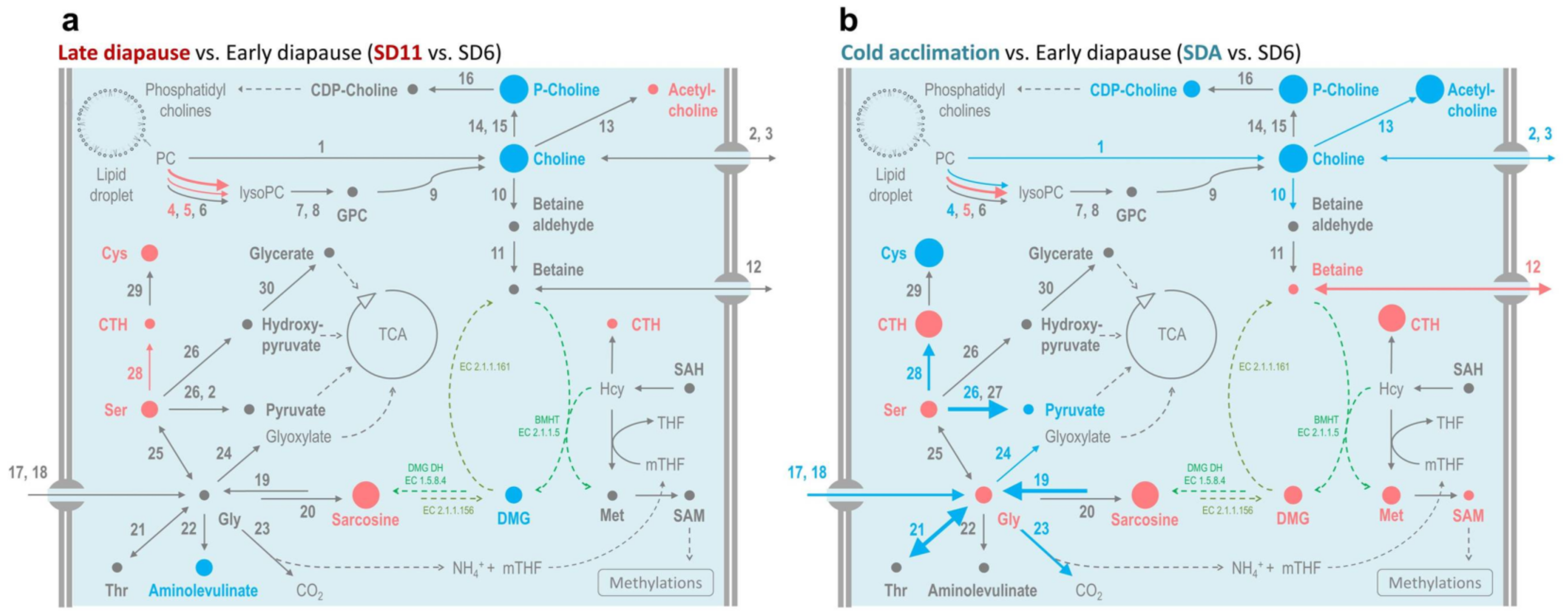
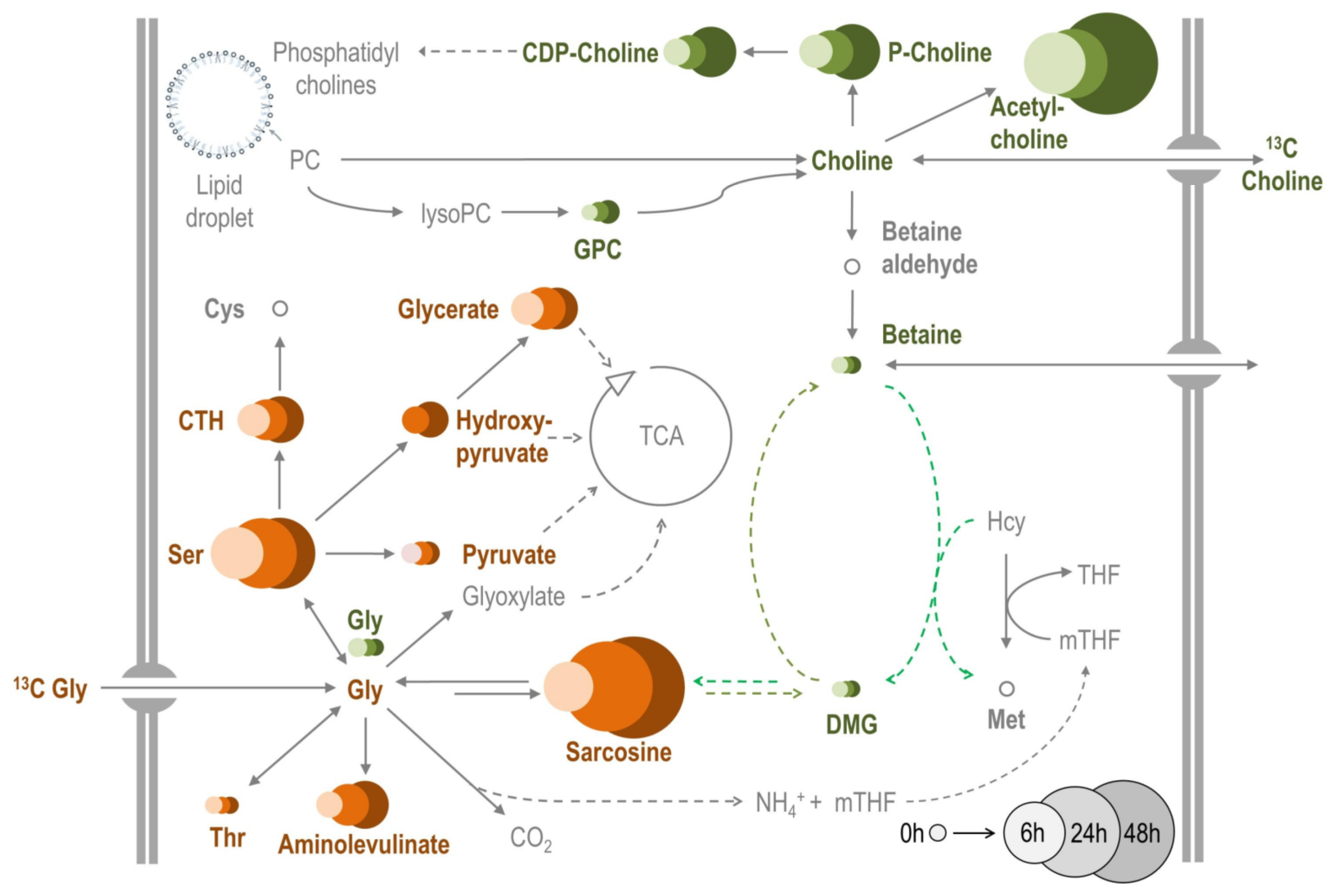
2.3. Betaine, Other Methylamines and Related Compounds
3. Materials and Methods
3.1. Insects
3.2. Extraction of Metabolites and LC-HRMS Platform
3.3. Target Gene Sequences and Gene Expression Analysis
3.4. Supplementary Assays to Clarify the Metabolism of CPs
3.4.1. A Brilliant Blue Larval Food Intake Assay
3.4.2. Total Proteins
3.4.3. PAGE
3.4.4. Total Collagen
3.4.5. Collagenase
3.4.6. Glycogen
3.4.7. Glycogen Phosphorylase
3.4.8. Amylases and Maltases
3.4.9. Fluxomics of 13C-Labelled Metabolic Precursors of Betaine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somero, G. Protons, osmolytes, and fitness of internal milieu for protein function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986, 251, R197–R213. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H.; Siebenaller, J.F. Co-evolution of proteins and solutions: Protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochachka, P.; Somero, G. Biochemical Adaptation. Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002; p. 478. [Google Scholar]
- Lee, R.E.J. A primer on insect cold-tolerance. In Low Temperature Biology of Insects; Denlinger, D.L., Lee, R.E.J., Eds.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Toxopeus, J.; Sinclair, B.J. Mechanisms underlying insect freeze tolerance. Biol. Rev. 2018, 93, 1891–1914. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Storey, K.B. Organic solutes in freezing tolerance. Comp. Biochem. Physiol. A 1997, 117, 319–326. [Google Scholar] [CrossRef]
- Salt, R. Principles of insect cold-hardiness. Annu. Rev. Entomol. 1961, 6, 55–74. [Google Scholar] [CrossRef]
- Lee, R.E. Principles of insect low temperature tolerance. In Insects at Low Temperature; Springer: Berlin/Heidelberg, Germany, 1991; pp. 17–46. [Google Scholar]
- Zachariassen, K.E. Physiology of cold tolerance in insects. Physiol. Rev. 1985, 65, 799–832. [Google Scholar] [CrossRef]
- Holmstrup, M.; Westh, P. Dehydration of earthworm cocoons exposed to cold: A novel cold hardiness mechanism. J. Comp. Physiol. B 1994, 164, 312–315. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Chino, H. Temperature-dependent interconversion between glycogen and trehalose in diapausing pupae of Philosamia cynthia ricini and pryeri. Insect Biochem. 1981, 11, 43–47. [Google Scholar] [CrossRef]
- Storey, J.M.; Storey, K.B. Winter survival of the gall fly larva, Eurosta solidaginis: Profiles of fuel reserves and cryoprotectants in a natural population. J. Insect Physiol. 1986, 32, 549–556. [Google Scholar] [CrossRef]
- Rickards, J.; Kelleher, M.J.; Storey, K.B. Strategies of freeze avoidance in larvae of the goldenrod gall moth, Epiblema scudderiana: Winter profiles of a natural population. J. Insect Physiol. 1987, 33, 443–450. [Google Scholar] [CrossRef]
- Koštál, V.; Tollarova, M.; Šula, J. Adjustments of the enzymatic complement for polyol biosynthesis and accumulation in diapausing cold-acclimated adults of Pyrrhocoris apterus. J. Insect Physiol. 2004, 50, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Storey, K.B. Triggering of cryoprotectant synthesis by the initiation of ice nucleation in the freeze tolerant frog, Rana sylvatica. J. Comp. Physiol. B 1985, 156, 191–195. [Google Scholar] [CrossRef]
- Calderon, S.; Holmstrup, M.; Westh, P.; Overgaard, J. Dual roles of glucose in the freeze-tolerant earthworm Dendrobaena octaedra: Cryoprotection and fuel for metabolism. J. Exp. Biol. 2009, 212, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Koštál, V.; Zahradníčková, H.; Šimek, P. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl. Acad. Sci. USA 2011, 108, 13041–13046. [Google Scholar] [CrossRef] [Green Version]
- Rozsypal, J.; Moos, M.; Šimek, P.; Koštál, V. Thermal analysis of ice and glass transitions in insects that do and do not survive freezing. J. Exp. Biol. 2018, 221, 170464. [Google Scholar] [CrossRef] [Green Version]
- Des Marteaux, L.E.; Hůla, P.; Koštál, V. Transcriptional analysis of insect extreme freeze tolerance. Proc. R. Soc. B 2019, 286, 20192019. [Google Scholar] [CrossRef] [Green Version]
- Kučera, L.; Moos, M.; Štetina, T.; Korbelová, J.; Vodrážka, P.; Marteaux, L.D.; Grgac, R.; Hula, P.; Rozsypal, J.; Faltus, M.; et al. A mixture of innate cryoprotectants is key for freeze tolerance and cryopreservation of a drosophilid fly larva. bioRxiv 2022. [Google Scholar]
- Denlinger, D.L. Relationship between cold hardiness and diapause. In Insects at Low Temperature; Lee, R.E., Denlinger, D.L., Eds.; Chapmann and Hall: New York, NY, USA, 1991; pp. 174–198. [Google Scholar]
- MacRae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef]
- Ragland, G.J.; Denlinger, D.L.; Hahn, D.A. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 2010, 107, 14909–14914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koštál, V.; Štětina, T.; Poupardin, R.; Korbelová, J.; Bruce, A.W. Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling. Proc. Natl. Acad. Sci. USA 2017, 114, 8532–8537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Biochemistry of cryoprotectants. In Insects at Low Temperature; Springer: Berlin/Heidelberg, Germany, 1991; pp. 64–93. [Google Scholar]
- Šlachta, M.; Vambera, J.; Zahradníčková, H.; Košťál, V. Entering diapause is a prerequisite for successful cold-acclimation in adult Graphosoma lineatum (Heteroptera: Pentatomidae). J. Insect Physiol. 2002, 48, 1031–1039. [Google Scholar] [CrossRef]
- Hayakawa, Y. Activation mechanism of insect fat body phosphorylase by cold phosphorylase kinase, phosphatase and ATP level. Insect Biochem. 1985, 15, 123–128. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Biochemical strategies of overwintering in the gall gly larva, Eurosta solidaginis: Effect of low temperature acclimation on the activities of enzymes of intermediary metabolism. J. Comp. Physiol. 1981, 144, 191–199. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Molecular biology of freezing tolerance. Compr. Physiol. 2013, 3, 1283–1308. [Google Scholar]
- Pfister, T.D.; Storey, K.B. Insect freeze tolerance: Roles of protein phosphatases and protein kinase A. Insect Biochem. Mol. Biol. 2006, 36, 18–24. [Google Scholar] [CrossRef]
- Wanner, L.A.; Junttila, O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 1999, 120, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Minami, A.; Arakawa, K.; Fujikawa, S.; Takezawa, D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J. Plant Physiol. 2005, 162, 169–180. [Google Scholar] [CrossRef]
- Morgan, T.; Chippendale, G. Free amino acids of the haemolymph of the southwestern corn borer and the European corn borer in relation to their diapause. J. Insect Physiol. 1983, 29, 735–740. [Google Scholar] [CrossRef]
- Fields, P.G.; Fleurat-Lessard, F.; Lavenseau, L.; Febvay, G.; Peypelut, L.; Bonnot, G. The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 1998, 44, 955–965. [Google Scholar] [CrossRef]
- Shimada, K.; Riihimaa, A. Cold acclimation, inoculative freezing and slow cooling: Essential factors contributing to the freezing-tolerance in diapausing larvae of Chymomyza costata (Diptera: Drosophilidae). Cryo Lett. 1988, 9, 5–10. [Google Scholar]
- Koštál, V.; Renault, D.; Rozsypal, J. Seasonal changes of free amino acids and thermal hysteresis in overwintering heteropteran insect, Pyrrhocoris apterus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 245–251. [Google Scholar] [CrossRef]
- Koštál, V.; Korbelová, J.; Poupardin, R.; Moos, M.; Šimek, P. Arginine and proline applied as food additives stimulate high freeze tolerance in larvae of Drosophila melanogaster. J. Exp. Biol. 2016, 219, 2358–2367. [Google Scholar] [CrossRef] [Green Version]
- Koštál, V.; Šimek, P.; Zahradníčková, H.; Cimlová, J.; Štětina, T. Conversion of the chill susceptible fruit fly larva (Drosophila melanogaster) to a freeze tolerant organism. Proc. Natl. Acad. Sci. USA 2012, 109, 3270–3274. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, L.; Chen, H.; Koštál, V.; Simek, P.; Moos, M.; Denlinger, D.L. Shifts in metabolomic profiles of the parasitoid Nasonia vitripennis associated with elevated cold tolerance induced by the parasitoid’s diapause, host diapause and host diet augmented with proline. Insect Biochem. Mol. Biol. 2015, 63, 34–46. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, X.; Yan, J.; Yan, L.-Y.; Jin, X.-H.; Zhu, X.-H.; He, Z.-Z.; Liu, J.; Li, R.; Qiao, J. L-proline: A highly effective cryoprotectant for mouse oocyte vitrification. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Dou, M.; Li, Y.; Sun, Z.; Li, L.; Rao, W. L-proline feeding for augmented freeze tolerance of Camponotus japonicus Mayr. Sci. Bull. 2019, 64, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Phang, J.M.; Liu, W.; Hancock, C.N.; Fischer, J.W. Proline metabolism and cancer: Emerging links to glutamine and collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71. [Google Scholar] [CrossRef] [Green Version]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. USA 1998, 95, 7799–7804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Trüper, H.G.; Galinski, E.A. Biosynthesis and fate of compatible solutes in extremely halophilic phototrophic eubacteria. FEMS Microbiol. Rev. 1990, 6, 247–254. [Google Scholar] [CrossRef]
- Sakamoto, A.; Valverde, R.; Chen, T.H.; Murata, N. Transformation of Arabidopsis with the codA gene for choline oxidase enhances freezing tolerance of plants. Plant J. 2000, 22, 449–453. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef]
- Sizeland, P.C.; Chambers, S.T.; Lever, M.; Bason, L.M.; Robson, R.A. Organic osmolytes in human and other mammalian kidneys. Kidney Int. 1993, 43, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Pajares, M.A.; Pérez-Sala, D. Betaine homocysteine S-methyltransferase: Just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. CMLS 2006, 63, 2792–2803. [Google Scholar] [CrossRef] [Green Version]
- Nyyssölä, A.; Kerovuo, J.; Kaukinen, P.; von Weymarn, N.; Reinikainen, T. Extreme halophiles synthesize betaine from glycine by methylation. J. Biol. Chem. 2000, 275, 22196–22201. [Google Scholar] [CrossRef] [Green Version]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Kivirikko, K.I.; Kishida, Y.; Sakakibara, S.; Prockop, D.J. Hydroxylation of (X-Pro-Gly) n by protocollagen proline hydroxylase Effect of chain length, helical conformation and amino acid sequence in the substrate. Biochim. Biophys. Acta (BBA) Protein Struct. 1972, 271, 347–356. [Google Scholar] [CrossRef]
- Miller, M.M.; Popova, L.B.; Meleshkevitch, E.A.; Tran, P.V.; Boudko, D.Y. The invertebrate B0 system transporter, D. melanogaster NAT1, has unique D-amino acid affinity and mediates gut and brain functions. Insect Biochem. Mol. Biol. 2008, 38, 923–931. [Google Scholar] [CrossRef] [Green Version]
- LaFever, K.S.; Wang, X.; Page-McCaw, P.; Bhave, G.; Page-McCaw, A. Both Drosophila matrix metalloproteinases have released and membrane-tethered forms but have different substrates. Sci. Rep. 2017, 7, 44560. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Kostal, V.; Shimada, K.; Hayakawa, Y. Induction and development of winter larval diapause in a drosophilid fly, Chymomyza costata. J. Insect Physiol. 2000, 46, 417–428. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—The insect ‘blood’sugar. Adv. Insect Physiol. 2003, 31 (Suppl. SC), 205–285. [Google Scholar]
- Fraenkel, G.; Friedman, S.; Hinton, T.; Laszlo, S.; Noland, J.L. The effect of substituting carnitine for choline in the nutrition of several organisms. Arch. Biochem. Biophys. 1955, 54, 432–439. [Google Scholar] [CrossRef]
- Geer, B.; Vovis, G.; Yund, M. Choline activity during the development of Drosophila melanogaster. Physiol. Zool. 1968, 41, 280–292. [Google Scholar] [CrossRef]
- Gallazzini, M.; Burg, M.B. What’s new about osmotic regulation of glycerophosphocholine. Physiology 2009, 24, 245–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koštál, V.R.; Berková, P.; Šimek, P. Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp. Biochem. Physiol. B 2003, 135, 407–419. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burg, M.B.; Kwon, E.D.; Peters, E.M. Glycerophosphocholine and betaine counteract the effect of urea on pyruvate kinase. Kidney Int. Suppl. 1996, 57, 100–104. [Google Scholar]
- Garcia-Perez, A.; Burg, M.B. Renal medullary organic osmolytes. Physiol. Rev. 1991, 71, 1081–1115. [Google Scholar] [CrossRef]
- Kostal, V.; Noguchi, H.; Shimada, K.; Hayakawa, Y. Developmental changes in dopamine levels in larvae of the fly Chymomyza costata: Comparison between wild-type and mutant-nondiapause strains. J. Insect Physiol. 1998, 44, 605–614. [Google Scholar] [CrossRef]
- Lakovaara, S. Malt as a culture medium for Drosophila species. Drosoph. Inf. Serv. 1969, 44, 128. [Google Scholar]
- Poupardin, R.; Schöttner, K.; Korbelová, J.; Provazník, J.; Doležel, D.; Pavlinic, D.; Beneš, V.; Koštál, V. Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genom. 2015, 16, 720. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kaun, K.R.; Riedl, C.A.; Chakaborty-Chatterjee, M.; Belay, A.T.; Douglas, S.J.; Gibbs, A.G.; Sokolowski, M.B. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J. Exp. Biol. 2007, 210, 3547–3558. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.e.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Cross, H.; Carpenter, Z.; Smith, G. Effects of intramuscular collagen and elastin on bovine muscle tenderness. J. Food Sci. 1973, 38, 998–1003. [Google Scholar] [CrossRef]
- Bueding, E.; Orrell, S.A. A mild procedure for the isolation of polydisperse glycogen from animal tissues. J. Biol. Chem. 1964, 239, 4018–4020. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Koštál, V.; Tamura, M.; Tollarová, M.; Zahradníčková, H. Enzymatic capacity for accumulation of polyol cryoprotectants changes during diapause development in the adult red firebug, Pyrrhocoris apterus. Physiol. Entomol. 2004, 29, 344–355. [Google Scholar] [CrossRef]






| Phenotype | Acclimation Variant/ Phenotype | Freeze Tolerance at −30 °C * [% Larvae Surviving to Adulthood] | Survival in LN2 * [% Larvae Surviving to Adulthood] |
|---|---|---|---|
| SD3 | Diapause entry | 0 | 0 |
| SD6 | Early diapause maintenance | 65.0 | 7.7 |
| SD11 | Late diapause maintenance | 41.5 | 10.1 |
| SDA | Diapause, cold-acclimated | 75.9 | 42.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moos, M.; Korbelová, J.; Štětina, T.; Opekar, S.; Šimek, P.; Grgac, R.; Koštál, V. Cryoprotective Metabolites Are Sourced from Both External Diet and Internal Macromolecular Reserves during Metabolic Reprogramming for Freeze Tolerance in Drosophilid Fly, Chymomyza costata. Metabolites 2022, 12, 163. https://doi.org/10.3390/metabo12020163
Moos M, Korbelová J, Štětina T, Opekar S, Šimek P, Grgac R, Koštál V. Cryoprotective Metabolites Are Sourced from Both External Diet and Internal Macromolecular Reserves during Metabolic Reprogramming for Freeze Tolerance in Drosophilid Fly, Chymomyza costata. Metabolites. 2022; 12(2):163. https://doi.org/10.3390/metabo12020163
Chicago/Turabian StyleMoos, Martin, Jaroslava Korbelová, Tomáš Štětina, Stanislav Opekar, Petr Šimek, Robert Grgac, and Vladimír Koštál. 2022. "Cryoprotective Metabolites Are Sourced from Both External Diet and Internal Macromolecular Reserves during Metabolic Reprogramming for Freeze Tolerance in Drosophilid Fly, Chymomyza costata" Metabolites 12, no. 2: 163. https://doi.org/10.3390/metabo12020163
APA StyleMoos, M., Korbelová, J., Štětina, T., Opekar, S., Šimek, P., Grgac, R., & Koštál, V. (2022). Cryoprotective Metabolites Are Sourced from Both External Diet and Internal Macromolecular Reserves during Metabolic Reprogramming for Freeze Tolerance in Drosophilid Fly, Chymomyza costata. Metabolites, 12(2), 163. https://doi.org/10.3390/metabo12020163







