A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family
Abstract
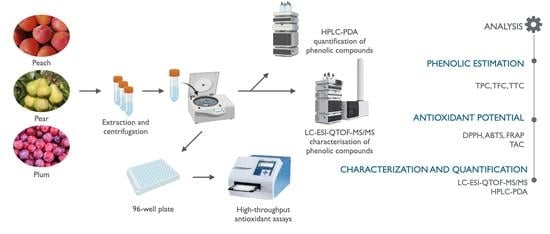
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Evaluation
2.1.1. Phenolic Estimation (TPC, TFC, and TTC)
2.1.2. Antioxidant Potential (DPPH, ABTS, FRAP, and TAC)
2.2. Characterization and Quantification Using LC-ESI-QTOF-MS/MS and HPLC-PDA
2.2.1. Qualitative Characterization Using LC-ESI-QTOF-MS/MS
2.2.2. Phenolic Acids and Derivatives
Hydroxybenzoic Acids
Hydroxycinnamic Acids
2.2.3. Flavonoids
Dihydroflavonols and Dihydrochalcones
Flavanols
Flavonols
Isoflavonoids
2.2.4. Other Polyphenols
2.3. Quantitative Characterization of 3Ps Extracts Using HPLC-PDA
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Extraction of Phenolic Compounds
3.4. Phenolic Compounds Estimation and Antioxidant Assays
3.4.1. Determination of Total Phenolic Content (TPC)
3.4.2. Determination of Total Flavonoids Content (TFC)
3.4.3. Determination of Total Tannins Content (TTC)
3.4.4. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Antioxidant Assay
3.4.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4.6. 2′-Azino-Bis-(3-Ethylbenzo-Thiazoline-6-Sulfonic Acid) (ABTS+) Radical Scavenging Assay
3.4.7. Total Antioxidant Capacity (TAC)
3.5. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds
3.6. HPLC-PDA Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Foreign Agriculture Service. Stone Fruit Annual. 2018. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Stone%20Fruit%20Annual_Madrid_EU-28_8-24-2018.pdf (accessed on 13 January 2022).
- Michailidis, M.; Karagiannis, E.; Nasiopoulou, E.; Skodra, C.; Molassiotis, A.; Tanou, G. Peach, Apple, and Pear Fruit Quality: To Peel or Not to Peel? Horticulturae 2021, 7, 85. [Google Scholar] [CrossRef]
- Annual Apple and Pear Australia Limited (APAL) Report. Special Purpose Financial Report. 2018–2019. Available online: https://apal.org.au/wp-content/uploads/2019/08/5.0-APAL-Annual-Report-FINAL-04.10.18.pdf (accessed on 20 March 2020).
- Butac, M.; Bozhkova, V.; Zhivondov, A.; Milošević, N.; Bellini, E.; Nencetti, V.; Blazek, J.; Balsemin, E.; Lafarque, B.; Kaufmane, E.; et al. Overview of plum breeding in Europe. Acta Hortic. 2013, 2013, 91–98. [Google Scholar] [CrossRef]
- EuroFresh Distrbution Report. EuroFresh Distrbution Magazine. 2020. Available online: https://www.eurofresh-distribution.com/magazine-ed/166-2020-marapr (accessed on 4 April 2020).
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, X.; Chu, K. Qualitative and Quantitative Analysis of Phenolic Acids, Flavonoids and Iridoid Glycosides in Yinhua Kanggan Tablet by UPLC-QqQ-MS/MS. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Total Phenolic, Flavonoid Distribution and Antioxidant Capacity in Skin, Pulp and Fruit Extracts of Plum Cultivars. J. Food Biochem. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic Composition and Antioxidant Properties of Different Peach [Prunus persica (L.) Batsch] Cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Saidani, F.; Gimenez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of Phenolic Compounds in Plum Fruits (Prunus salicina L. and Prunus domestica L.) by High-Performance Liquid Chromatography/Tandem Mass Spectrometry and Characterization of Varieties by Quantitative Phenolic Fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef]
- Wang, Z.; Barrow, C.; Dunshea, F.; Suleria, H. A Comparative Investigation on Phenolic Composition, Characterization and Antioxidant Potentials of Five Different Australian Grown Pear Varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Barrow, C.; Dunshea, F.; Suleria, H. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C. Bioactive Compounds from Plant Origin: Extraction, Applications, and Potential Health Benefits; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Kadam, D.; Lele, S.S. Extraction, characterization and bioactive properties of Nigella sativa seedcake. J. Food Sci. Technol. 2017, 54, 3936–3947. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Content of bioactive compounds and antioxidant capacity in skin tissues of pear. J. Funct. Foods 2016, 23, 40–51. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of Phenolic Compounds in Australian Grown Berries by LC-ESI-QTOF-MS/MS and Determination of Their Antioxidant Potential. Antioxidants 2020, 10, 26. [Google Scholar] [CrossRef]
- Leng, Z.; Zhong, B.; Wu, H.; Liu, Z.; Rauf, A.; Bawazeer, S.; Suleria, H.A.R. Identification of Phenolic Compounds in Australian-Grown Bell Peppers by Liquid Chromatography Coupled with Electrospray Ionization-Quadrupole-Time-of-Flight-Mass Spectrometry and Estimation of Their Antioxidant Potential. ACS Omega 2022, 7, 4563–4576. [Google Scholar] [CrossRef]
- Lucci, P.; Martins, C.P.B.; Núñez, O.; Gallart-Ayala, H. Liquid Chromatography–High-Resolution Mass Spectrometry in Environmental and Food Analysis; World Scientific Pub Co Pte Ltd.: Singapore, 2015; pp. 325–345. [Google Scholar]
- Lucci, P.; Saurina, J.; Nuñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldner, K.; Michaelis, S.V.; Stammler, J.; Neumüller, M.; Treutter, D. Different phenotypes of plum cultivars due to specific phenolic profiles. Acta Hortic. 2019, 2019, 137–144. [Google Scholar] [CrossRef]
- Liao, X.; Greenspan, P.; Pegg, R.B. Characterizing the phenolic constituents and antioxidant capacity of Georgia peaches. Food Chem. 2019, 271, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mitic, V.; Ilic, M.; Dimitrijevic, M.; Cvetkovic, J.; Ciric, S.; Jovanovic, V.S. Chemometric characterization of peach, nectarine and plum cultivars according to fruit phenolic content and anti-oxidant activity. Fruits 2015, 71, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic Compounds and Chemical Characteristics of Pears (Pyrus communis L.). Int. J. Food Prop. 2014, 18, 536–546. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and properties of the polyphenolic extracts obtained from industrial plum pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- Erbil, N.; Murathan, Z.T.; Arslan, M.; Ilcim, A.; Sayin, B. Antimicrobial, Antioxidant, and Antimutagenic Activities of Five Turkish Pear Cultivars. Erwerbs-Obstbau 2017, 60, 203–209. [Google Scholar] [CrossRef]
- Davidović, S.M.; Veljović, M.S.; Pantelić, M.M.; Baošić, R.M.; Natić, M.M.; Dabić, D.Č.; Pecić, S.P.; Vukosavljević, P.V. Physicochemical, Antioxidant and Sensory Properties of Peach Wine Made from Redhaven Cultivar. J. Agric. Food Chem. 2013, 61, 1357–1363. [Google Scholar] [CrossRef]
- Sattar, S.; Ahmad, T.; Nisa, M.-U.; Imran, M.; Holmes, M.; Maycock, J.; Nadeem, M.; Khan, M.K. Microwave processing impact on physicochemical and bioactive attributes of optimized peach functional beverage. J. Food Process. Preserv. 2019, 43, e13952. [Google Scholar] [CrossRef]
- Guan, J.; He, J.; Shen, C.; Li, L.; Wang, Y.; Cheng, Y. Chapter 17-How Cultivars Influence Fruit Composition: Total Phenols, Flavonoids Contents, and Antioxidant Activity in the Pulp of Selected Asian Pears. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 139–145. [Google Scholar]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolic Content and Antioxidant Capacity in Fruits of Plum (Prunus domestica L.) Cultivars “Valjevka” and “Mildora” as Influenced by Air Drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Curi, P.N.; Schiassi, M.C.E.V.; Pio, R.; Peche, P.M.; Albergaria, F.C.; de Souza, V.R. Bioactive compounds and antioxidant activity of fruit of temperate climate produced in subtropical regions. Food Sci. Technol. 2021, 41, 607–614. [Google Scholar] [CrossRef]
- Vo, G.T.; Liu, Z.; Chou, O.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of phenolic compounds in australian grown grapes and their potential antioxidant activities. Food Biosci. 2022, 47, 101644. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Galvis Sánchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2014, 8, 1294–1302. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; da Costa, D.L.M.G.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Dos Santos, L.C. Characterization and Quantification of the Compounds of the Ethanolic Extract from Caesalpinia ferrea Stem Bark and Evaluation of Their Mutagenic Activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.; Jayaprakasha, G.K.; Patil, B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct. 2018, 9, 1235–1244. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Nakabayashi, R.; Yamada, Y.; Suzuki, M.; Sato, M.; Sakata, A.; Akiyama, K.; Sakurai, T.; Matsuda, F.; Aoki, T.; et al. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry 2012, 82, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Delpino-Rius, A.; Eras, J.; Gatius, F.; Balcells, M.; Canela-Garayoa, R. Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Mon-ovarietal Peach Purees and Pear Juices. Molecules 2019, 24, 3289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M.; et al. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298, Erratum in Nat. Methods 2019, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative Analysis of Chemical Constituents of Moringa oleifera Leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef] [Green Version]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Sasot, G.; Martínez-Huélamo, M.; Vallverdu-Queralt, A.; Mercader-Martí, M.; Estruch, R.; Lamuela-Raventós, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution LTQ-Orbitrap-MS approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic con-tents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Jáuregui, O.; Valderas-Martinez, P.; Vallverdú-Queralt, A.; Estruch, R.; Torrado, X.; Lamuela-Raventós, R.M. Sensitive and Rapid UHPLC-MS/MS for the Analysis of Tomato Phenolics in Human Biological Samples. Molecules 2015, 20, 20409–20425. [Google Scholar] [CrossRef] [Green Version]
- Touriño, S.; Fuguet, E.; Jáuregui, O.; Saura-Calixto, F.; Cascante, M.; Torres, J.L. High-resolution liquid chromatography/electrospray ionization time-of-flight mass spectrometry combined with liquid chromatography/electrospray ionization tandem mass spectrometry to identify polyphenols from grape antioxidant dietary fiber. Rapid Commun. Mass Spectrom. 2008, 22, 3489–3500. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Petkovska, A.; Gjamovski, V.; Stanoeva, J.P.; Stefova, M. Characterization of the Polyphenolic Profiles of Peel, Flesh and Leaves of Malus domestica Cultivars Using UHPLC-DAD-HESI-MSn. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef] [Green Version]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef]
- Zhang, M.; Jagdmann, J.G.E.; Van Zandt, M.; Sheeler, R.; Beckett, P.; Schroeter, H. Chemical Synthesis and Characterization of Epicatechin Glucuronides and Sulfates: Bioanalytical Standards for Epicatechin Metabolite Identification. J. Nat. Prod. 2013, 76, 157–169. [Google Scholar] [CrossRef]
- Kelebek, H. LC-DAD–ESI-MS/MS characterization of phenolic constituents in Turkish black tea: Effect of infusion time and tem-perature. Food Chem. 2016, 204, 227–238. [Google Scholar] [CrossRef]
- Reed, K.A. Identification of Phenolic Compounds from Peanut Skin Using Hplc-Msn; Virginia Tech: Blacksburg, VA, USA, 2009. [Google Scholar]
- Lee, J.M.; Im, W.J.; Nam, Y.J.; Oh, K.H.; Lim, J.C.; Whang, W.K.; Sohn, U.D. Acute Toxicity and General Pharmacological Action of QGC EXT. Korean J. Physiol. Pharmacol. 2012, 16, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of Chemical Constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [Green Version]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-qTOF-MS/MS-Based Profiling of Flavan-3-ols and Dimeric Proanthocyanidins in Berries of Two Muscadine Grape Hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Habtemariam, S. α-Glucosidase Inhibitory Activity of Kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef] [Green Version]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Sobeh, M.; Rezq, S.; El-Raey, M.A.; Dmirieh, M.; El-Shazly, A.M.; Mahmoud, M.; Wink, M. Characterization of phenolic compounds from Eugenia supra-axillaris leaf extract using HPLC-PDA-MS/MS and its antioxidant, anti-inflammatory, antipyretic and pain killing activities in vivo. Sci. Rep. 2019, 9, 11122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuan, N.H.; Pandey, R.P.; Thuy, T.T.T.; Park, J.W.; Sohng, J.K. Improvement of Regio-Specific Production of Myricetin-3-O-α-l-Rhamnoside in Engineered Escherichia coli. Appl. Biochem. Biotechnol. 2013, 171, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.C.; Liu, L.T.; Bian, J.J.; Yan, C.Q.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Wang, W.; Liang, K.; Shi, Z.F.; et al. Identification of multiple constituents in shuganjieyu capsule and rat plasma after oral administration by ul-tra-performance liquid chromatography coupled with electrospray ionization and ion trap mass spectrometry. Acta Chromato-Graph. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liao, J.; Fan, X.; Cheng, Y. An ultra-robust fingerprinting method for quality assessment of traditional Chinese medicine using multiple reaction monitoring mass spectrometry. J. Pharm. Anal. 2021, 11, 88–95. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, J.-A.; Liu, S.-L.; Wang, F.; Wang, H.-Y. Identification and determination of isoflavones in germinated black soybean sprouts by UHPLC−Q-TOF-MS mass spectrometry and HPLC-DAD. Int. J. Food Prop. 2017, 20, 2877–2887. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Punt, A.; Spenkelink, B.; Murk, A.J.; van Leeuwen, F.X.R.; Rietjens, I.M.C.M. Conversion of major soy isoflavone glucosides and aglycones in in vitro intestinal models. Mol. Nutr. Food Res. 2013, 58, 503–515. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Yim, S.-H.; Nam, S.-H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Pérez-Ramírez, I.F.; Amaya-Cruz, D.M.; Gallegos-Corona, M.A.; Ramos-Gomez, M.; Mora, O.; Reynoso-Camacho, R. Polyphenol-rich peach (Prunus persica L.) by-product exerts a greater beneficial effect than dietary fiber-rich by-product on insulin resistance and hepatic steatosis in obese rats. J. Funct. Foods 2018, 45, 58–66. [Google Scholar] [CrossRef]
- Ma, C.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds in Palm Fruits (Jelly and Fishtail Palm) and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011, 126, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Zou, B.; Dong, X.-Q.; Ge, Z.-Z.; Xu, Z.; Du, J.; Li, C.-M. Development of suitable standards for quantitative determination of persimmon phenol contents in Folin-Ciocalteu and vanillin assays. Eur. Food Res. Technol. 2014, 239, 385–391. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Chen, X.-X.; Feng, H.-L.; Ding, Y.-M.; Chai, W.-M.; Xiang, Z.-H.; Shi, Y.; Chen, Q.-X. Structure characterization of proanthocyanidins from Caryota ochlandra Hance and their bioactivities. Food Chem. 2014, 155, 1–8. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
| Assays | Peach | Pear | Plums |
|---|---|---|---|
| TPC (mg GAE/g of sample F.W) | 0.43 ± 0.09 b | 0.29 ± 0.05 c | 0.62 ± 0.01 a |
| TFC (µg QE/g of sample F.W) | 0.29 ± 0.04 a | 0.18 ± 0.09 b | 0.24 ± 0.07 a |
| TTC (mg CE/g of sample F.W) | 0.01 ± 0.08 c | 0.03 ± 0.07 a | 0.02 ± 0.05 b |
| DPPH (mg AAE/g of sample F.W) | 0.20 ± 0.07 b | 0.23 ± 0.09 b | 0.53 ± 0.08 a |
| ABTS (mg AAE/g of sample F.W) | 0.27 ± 0.02 b | 0.12 ± 0.07 c | 0.47 ± 0.01 a |
| FRAP (mg AAE/g of sample F.W) | 0.35 ± 0.04 b | 0.41 ± 0.04 b | 0.56 ± 0.02 a |
| TAC (mg AAE/g of sample F.W) | 0.32 ± 0.09 b | 0.19 ± 0.02 c | 0.41 ± 0.09 a |
| No | Proposed Compound | Molecular Formula | Retention Time (min) | Mode of Ionization | Molecular Weight | Theoretical (m/z) | Observed (m/z) | MS/MSProduct Ion | Error (ppm) | Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids Hydroxybenzoic acids | ||||||||||
| 1 | Gallic acid 4-O-glucoside | C13H16O10 | 5.039 | [M–H]− | 332.0743 | 331.0670 | 331.0672 | 169, 125 | 0.60 | * Pear, Plum |
| 2 | Vanillic acid 4-sulphate | C8H8O7S | 5.122 | [M–H]− | 247.9991 | 246.9918 | 246.9915 | 167 | −1.21 | Pear |
| 3 | Gallic acid | C7H6O5 | 6.878 | [M–H]− | 170.0215 | 169.0142 | 169.0145 | 125 | 1.77 | Pear |
| 4 | 5-O-Galloylquinic acid | C14H16O10 | 7.143 | [M–H]− | 344.0740 | 343.0670 | 343.0688 | 191 | −0.95 | Pear |
| 5 | 2-Hydroxybenzoic acid | C7H6O3 | 7.607 | [M–H]− | 138.0317 | 137.0244 | 137.0244 | 93 | 0.00 | * Pear, Plum |
| 6 | 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 11.218 | [M–H]− | 300.0845 | 299.0772 | 299.0759 | 255, 137 | −4.35 | Pear |
| 7 | Gallic acid 3-O-gallate | C14H10O9 | 17.066 | [M–H]− | 322.0325 | 321.0252 | 321.0239 | 169 | −4.05 | Pear |
| 8 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 32.179 | [M–H]− | 154.0266 | 153.0193 | 153.0190 | 109 | −1.96 | * Plum, Pear |
| Hydroxycinnamic acids | ||||||||||
| 9 | Isoferulic acid 3-sulfate | C10H10O7S | 5.341 | [M–H]− | 274.015 | 273.0074 | 273.0067 | 193, 178 | −2.56 | Plum |
| 10 | Cinnamic acid | C9H8O2 | 9.366 | [M–H]− | 148.052 | 147.0451 | 147.0444 | 103 | −4.76 | * Plum, Pear |
| 11 | Feruloyl tartaric acid | C14H14O9 | 10.506 | [M–H]− | 326.064 | 325.0565 | 325.0567 | 193, 149 | 0.62 | Pear |
| 12 | 3-Caffeoylquinic acid | C16H18O9 | 13.144 | [M–H]− | 354.095 | 353.0878 | 353.0874 | 253, 190, 144 | −1.13 | Plum |
| 13 | Caffeic acid | C9H8O4 | 13.144 | ** [M–H]− | 180.042 | 179.0350 | 179.0341 | 143,133 | −5.03 | * Plum, Pear |
| 14 | Caffeoyl glucose | C15H18O9 | 14.833 | [M–H]− | 0342.095 | 341.0878 | 341.0887 | 179, 161 | 2.64 | * Plum, Pear |
| 15 | 3-p-Coumaroylquinic acid | C16H18O8 | 18.180 | [M–H]− | 338.100 | 337.0929 | 337.0934 | 265, 173, 162 | 1.48 | Plum |
| 16 | Rosmarinic acid | C18H16O8 | 18.180 | [M–H]− | 360.0845 | 359.0772 | 359.0752 | 179 | −5.57 | * Plum, Pear |
| 17 | m-Coumaric acid | C9H8O3 | 18.180 | [M–H]− | 164.0473 | 163.0400 | 163.0402 | 119 | 1.23 | Plum |
| 18 | Ferulic acid | C10H10O4 | 18.508 | [M–H]− | 194.0579 | 193.0506 | 193.0492 | 178, 149, 134 | −7.25 | * Pear, Plum |
| 19 | Ferulic acid 4-O-glucoside | C16H20O9 | 18.508 | [M–H]− | 356.1107 | 355.1034 | 355.1010 | 193, 178, 149, 134 | −6.76 | * Pear, Plum |
| 20 | 3-Feruloylquinic acid | C17H20O9 | 20.847 | [M–H]− | 368.1107 | 367.1034 | 367.1025 | 298, 288, 192, 191 | −2.45 | * Plum, Pear |
| 21 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 20.897 | [M–H]− | 326.1002 | 325.0929 | 325.0922 | 163 | −2.15 | * Plum, Pear |
| 22 | Ferulic acid 4-O-glucuronide | C16H18O10 | 22.305 | [M–H]− | 370.0900 | 369.0827 | 369.0826 | 193 | −0.27 | * Plum, Pear |
| 23 | Hydroxycaffeic acid | C9H8O5 | 37.033 | [M–H]− | 196.0372 | 195.0299 | 195.0298 | 151 | −0.51 | Plum |
| 24 | Cinnamoyl glucose | C15H18O7 | 60.985 | [M–H]− | 310.1053 | 309.0980 | 309.0965 | 147, 131, 103 | −4.85 | Pear |
| Hydroxyphenylpropanoic acids | ||||||||||
| 25 | Dihydroferulic acid 4-O-glucuronide | C16H20O10 | 5.689 | [M–H]− | 372.1056 | 371.0983 | 371.0986 | 195 | 1.35 | Plum |
| 26 | Dihydrocaffeic acid 3-O-glucuronide | C15H18O10 | 8.468 | [M–H]− | 358.0900 | 357.0827 | 357.0811 | 181 | −1.96 | Pear |
| 27 | 3-Hydroxy-3-(3-hydroxyphenyl) propionic acid | C9H10O4 | 14.730 | [M–H]− | 182.0579 | 181.0506 | 181.0504 | 163, 135, 119 | −1.1 | Pear |
| Flavonoids | ||||||||||
| Dihydroflavonols | ||||||||||
| 28 | Dihydroquercetin | C15H12O7 | 11.732 | [M–H]− | 304.0583 | 303.0510 | 303.0501 | 285, 275, 151 | −2.97 | Pear |
| Dihydrochalcones | ||||||||||
| 29 | 3-Hydroxyphloretin 2′-O-glucoside | C21H24O11 | 13.819 | [M–H]− | 452.1319 | 451.1246 | 451.1258 | 289, 273 | −2.22 | Pear |
| 30 | Phloridzin | C21H24O10 | 51.681 | [M–H]− | 436.1369 | 435.1326 | 435.1307 | 273 | −4.37 | Peach |
| Flavanols | ||||||||||
| 31 | Procyanidin dimer B1 | C30H26O12 | 15.562 | [M–H]− | 578.1424 | 577.1351 | 577.1348 | 451 | −0.17 | * Plum, Pear, Peach |
| 32 | Procyanidin trimer C1 | C45H38O18 | 19.240 | [M–H]− | 866.2058 | 865.1985 | 865.1961 | 739, 713, 695 | 0.69 | * Plum, Peach |
| 33 | Cinnamtannin A2 | C60H50O24 | 19.422 | [M–H]− | 1154.2690 | 1153.2619 | 1153.2609 | 739 | −0.87 | Plum |
| 34 | (+)-Catechin | C15H14O6 | 19.684 | [M–H]− | 290.0790 | 289.0717 | 289.0706 | 245, 205, 179 | −3.81 | * Pear, Plum |
| 35 | (+)-gallocatechin | C15H14O7 | 19.733 | [M–H]− | 306.0739 | 305.0667 | 305.0648 | 261, 219 | 1.9 | Pear |
| 36 | 3′-O-Methylcatechin | C16H16O6 | 24.124 | [M–H]− | 304.0947 | 303.0874 | 303.0878 | 271, 163 | 1.32 | Pear |
| 37 | (+)-Catechin 3-O-gallate | C22H18O10 | 36.333 | [M–H]− | 442.0900 | 441.0827 | 441.0805 | 289, 169, 125 | −0.45 | Pear |
| Flavanones | ||||||||||
| 38 | Hesperetin 3′-O-glucuronide | C22H22O12 | 47.738 | [M–H]− | 478.1111 | 477.1068 | 477.1054 | 301, 175, 113, 85 | −2.93 | * Peach, Pear |
| Flavones | ||||||||||
| 39 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 43.862 | [M–H]− | 594.1585 | 593.1542 | 593.1539 | 503, 473 | −0.51 | Peach |
| 40 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 46.658 | [M–H]− | 448.1006 | 447.0933 | 447.0935 | 301 | 0.45 | * Plum, Pear, Peach |
| 41 | Apigenin 6-C-glucoside | C21H20O10 | 55.256 | [M–H]− | 432.1056 | 431.0983 | 431.0984 | 413, 341, 311 | 0.23 | Plum |
| Flavonols | ||||||||||
| 42 | Isorhamnetin | C16H12O7 | 20.284 | [M–H]− | 316.0583 | 315.0510 | 315.0504 | 300, 271 | −1.90 | Plum |
| 43 | Quercetin 3-O-glucosyl-xyloside | C26H28O16 | 34.730 | [M–H]− | 596.1377 | 595.1304 | 595.1290 | 265, 138, 116 | −2.35 | Plum |
| 44 | Myricetin 3-O-rhamnoside | C21H20O12 | 39.945 | [M–H]− | 464.0955 | 463.0882 | 463.0847 | 317 | −7.56 | * Pear, Plum, Peach |
| 45 | Kaempferol 3-O-(2″-rhamnosyl-galactoside) 7-O-rhamnoside | C33H40O19 | 42.036 | [M–H]− | 740.2164 | 739.2091 | 739.2106 | 593, 447, 285 | 2.03 | Plum |
| 46 | Quercetin 3-O-arabinoside | C20H18O11 | 45.598 | [M–H]− | 434.0849 | 433.0776 | 433.0780 | 301 | 0.92 | * Plum, Pear |
| Isoflavonoids | ||||||||||
| 47 | 6″-O-Acetyldaidzin | C23H22O10 | 4.413 | [M–H]− | 458.1213 | 457.1140 | 457.1125 | 221 | −3.28 | Plum |
| 48 | Violanone | C17H16O6 | 20.267 | [M–H]− | 316.0947 | 315.0874 | 315.0868 | 300, 285, 135 | −1.9 | Plum |
| 49 | Glycitin | C22H22O10 | 30.071 | [M–H]− | 446.1213 | 447.1286 | 445.1150 | 285 | 2.25 | Pear |
| Other polyphenols | ||||||||||
| Hydroxybenzaldehydes | ||||||||||
| 50 | 4-Hydroxybenzaldehyde | C7H6O2 | 44.769 | ** [M–H]− | 122.0368 | 121.0295 | 121.0298 | 77 | 2.48 | * Plum, Pear |
| Hydroxycoumarins | ||||||||||
| 51 | Coumarin | C9H6O2 | 20.913 | ** [M+H]+ | 146.0368 | 147.0441 | 147.0448 | 103, 91 | 1.38 | Plum |
| No. | Compounds Name | RT | Standard Curve | * Peach | * Pear | * Plum |
|---|---|---|---|---|---|---|
| Phenolic Acids | ||||||
| 1 | Gallic acid | 6.836 | 2531.9x + 12238 | 0.02 ± 0.47 b | 4.70 ± 0.98 a | - |
| 2 | Protocatechuic acid | 12.569 | 1824x − 16182 | - | 2.10 ± 1.52 a | 1.70 ± 2.13 ab |
| 3 | Caftaric acid | 13.774 | 3500.2x − 43822 | 0.01 ± 0.91 b | 0.30 ± 3.20 b | 4.50 ± 3.74 a |
| 4 | p-hydroxybenzoic acid | 19.704 | 1387.5x + 5575.1 | 3.87 ± 0.94 a | 0.90 ± 0.37 b | 4.30 ± 1.78 a |
| 5 | Sinapic acid | 38.745 | 46102x + 724718 | 3.87 ± 1.78 a | 0.93 ± 1.59 b | - |
| 6 | Ferulic acid | 39.823 | 65160x + 2000000 | 0.21 ± 3.67 b | 0.73 ± 1.20 b | 3.44 ± 4.78 a |
| 7 | Chlorogenic acid | 20.579 | 3043.6x + 4706.3 | 7.26 ± 3.41 ab | 5.26 ± 2.47 b | 11.86 ± 3.24 a |
| 8 | Caffeic acid | 25.001 | 5622.4x + 23944 | - | 0.09 ± 2.74 b | 3.24 ± 0.08 a |
| 9 | Syringic acid | 26.326 | 2900.6x + 65091 | 2.23 ± 4.15 a | - | 0.03 ± 2.13 b |
| 10 | Coumaric acid | 34.455 | 6418.4x + 60121 | - | - | 0.02 ± 1.96 a |
| Total phenolic acids | 17.47±15.33 b | 15.01±14.07 b | 29.09±19.84 b | |||
| Flavonoids | ||||||
| 1 | Polydatin | 34.966 | 45035x + 80265 | 2.45 ± 9.14 ab | 3.17 ± 0.15 a | 0.89 ± 1.74 b |
| 2 | Epicatechin gallate | 38.015 | 22958x − 26657 | 0.75 ± 1.32 b | 2.13 ± 0.41 b | 3.12 ± 1.96 a |
| 3 | Catechin | 20.240 | 779.41x + 2373.3 | 7.31 ± 4.02 a | 1.92 ± 3.67b | 0.30 ± 3.67 b |
| 4 | Epicatechin | 26.739 | 680.52x + 14866 | 0.07 ± 3.17 b | 1.73 ± 1.29 a | 0.49 ± 2.13 b |
| 5 | q-3-O-galactoside | 40.659 | 23472x + 185001 | 3.21± 1.23 a | 0.98 ± 5.21 b | 0.06 ± 0.04 b |
| 6 | Q-3-O-rhamnoside | 45.172 | 16282x + 40330 | 2.12 ± 3.17 a | 2.32 ± 1.47 a | - |
| 7 | kaempferol-3-O-glucoside | 47.111 | 22405x − 33766 | 3.85± 0.04 a | 0.03 ± 2.15 b | 1.28 ± 6.47 b |
| 8 | Resveratrol | 58.685 | 7338.8x + 50349 | 0.01 ± 3.14 b | - | 0.09 ± 1.27 a |
| 9 | Quercetin | 70.098 | 2585.7x − 29267 | 0.08 ± 7.15 b | - | 4.03 ± 3.19 a |
| 10 | Kaempferol | 80.347 | 4425.8x − 110841 | 3.03 ± 2.15 a | 1.54 ± 3.57 b | 2.96 ± 4.12 a |
| Total flavonoids | 22.88 ± 34.53 a | 13.82 ± 17.92 b | 13.22 ± 24.59 b | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, A.; Liu, Z.; Wu, H.; Zhong, B.; Ciborowski, M.; Suleria, H.A.R. A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family. Metabolites 2022, 12, 271. https://doi.org/10.3390/metabo12030271
Hameed A, Liu Z, Wu H, Zhong B, Ciborowski M, Suleria HAR. A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family. Metabolites. 2022; 12(3):271. https://doi.org/10.3390/metabo12030271
Chicago/Turabian StyleHameed, Ahsan, Ziyao Liu, Hanjing Wu, Biming Zhong, Michal Ciborowski, and Hafiz Ansar Rasul Suleria. 2022. "A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family" Metabolites 12, no. 3: 271. https://doi.org/10.3390/metabo12030271
APA StyleHameed, A., Liu, Z., Wu, H., Zhong, B., Ciborowski, M., & Suleria, H. A. R. (2022). A Comparative and Comprehensive Characterization of Polyphenols of Selected Fruits from the Rosaceae Family. Metabolites, 12(3), 271. https://doi.org/10.3390/metabo12030271