Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat
Abstract
:1. Introduction
2. Results
2.1. Quality Parameters of Breast and Thigh Muscles from Two Chicken Lines
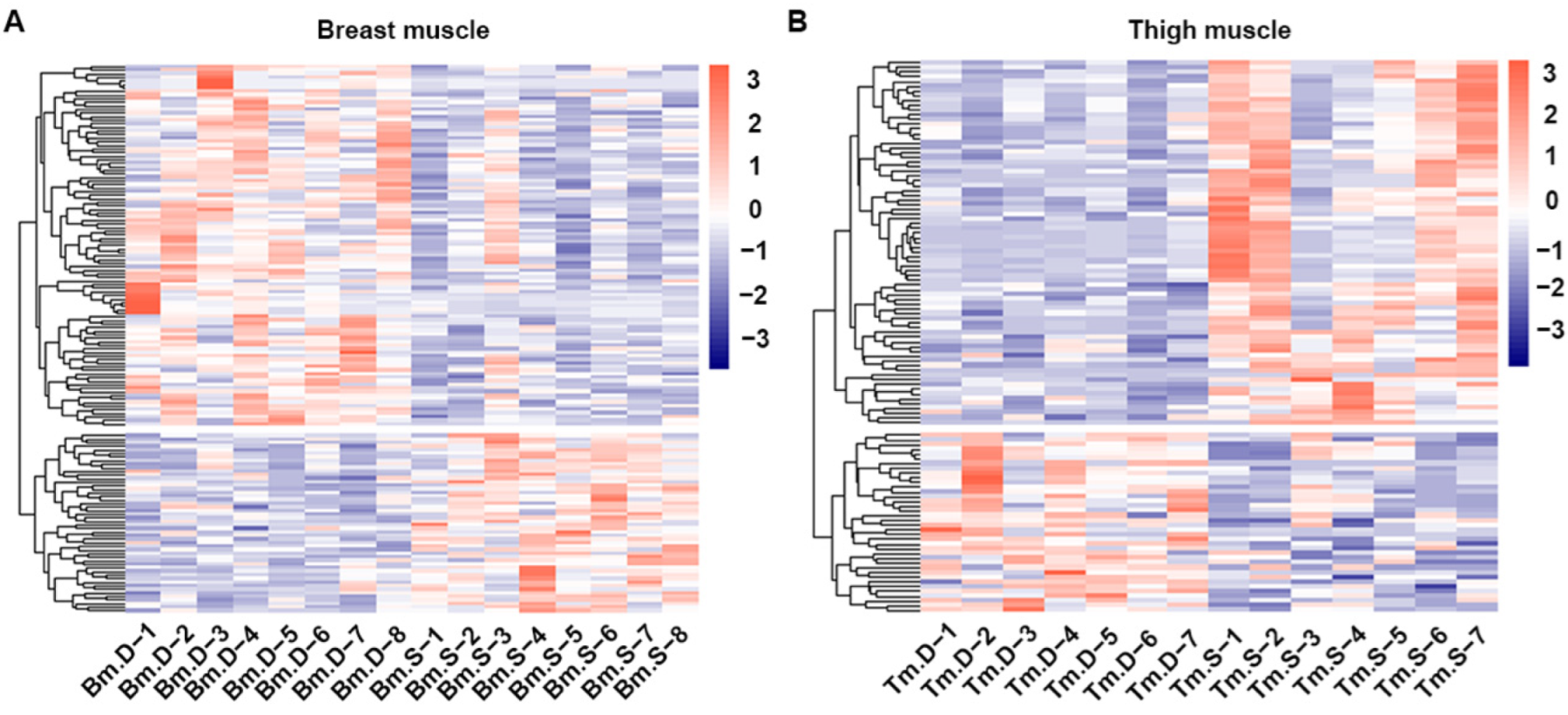
2.2. Quality Control of Metabolomics from Muscle Samples
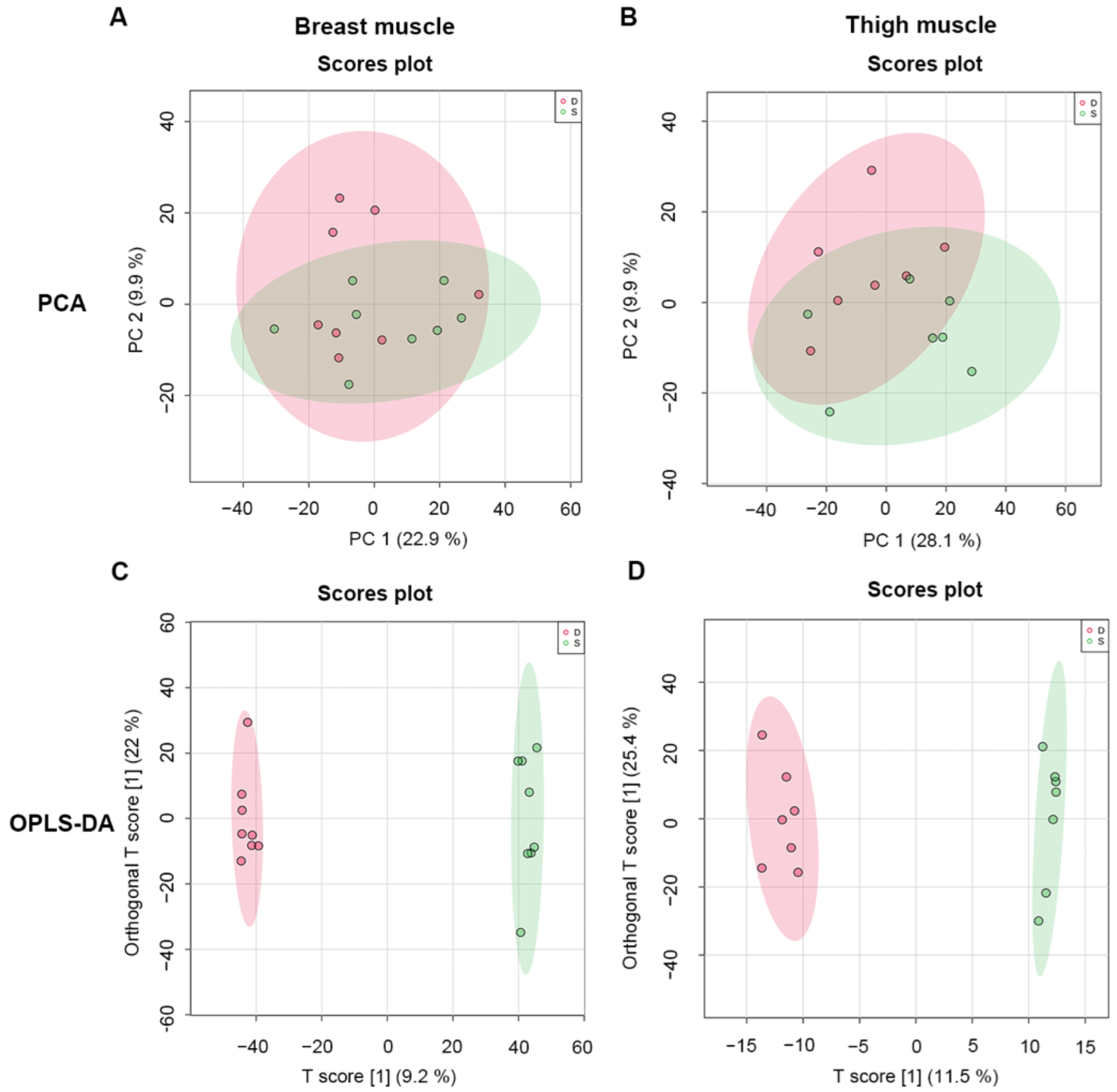
2.3. Multivariate Statistical Analysis of Metabolomics
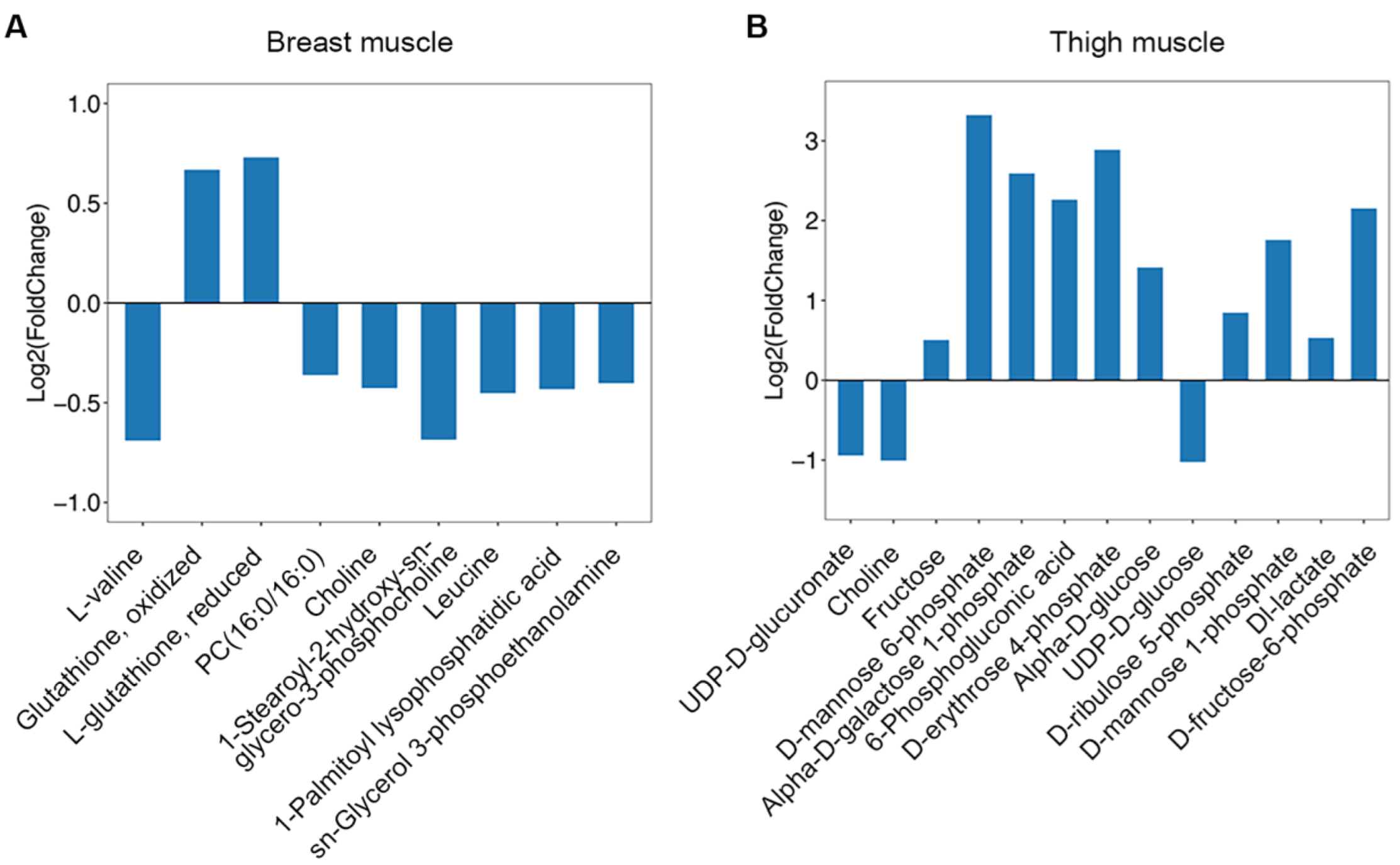
2.4. Screening and Correlation Analysis of Differentiated Metabolites
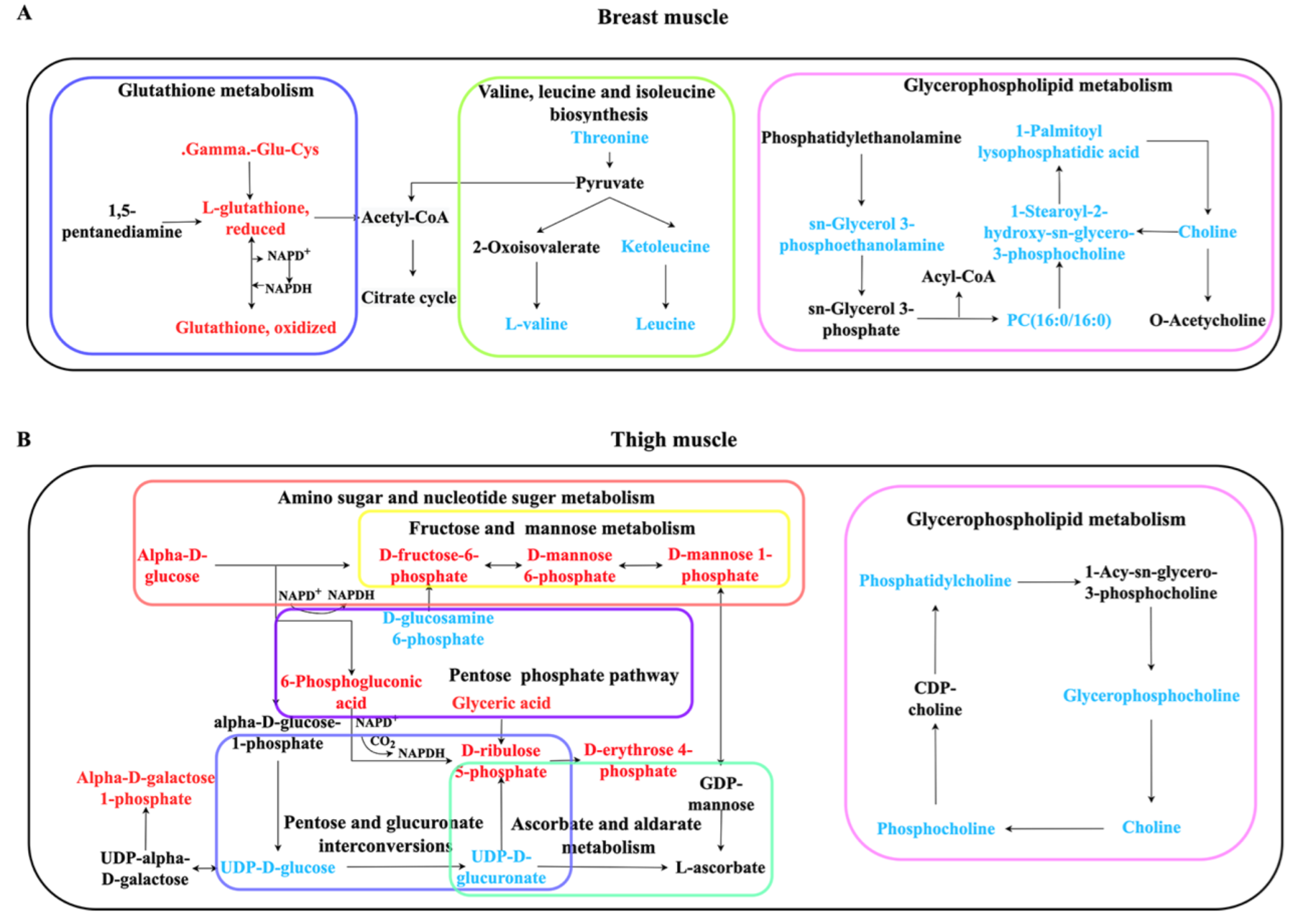
2.5. Pathway Enrichment Analysis
3. Discussion
4. Experimental Design
4.1. Animals
4.2. Assay of Meat Quality
4.3. Sample Preparation for Metabolomics
4.4. Measurement of Metabolites
4.5. Analysis of Metabolomic Data
4.6. RNA Extraction and Sequencing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorshorst, B.; Molin, A.-M.; Rubin, C.-J.; Johansson, A.M.; Strömstedt, L.; Pham, M.-H.; Chen, C.-F.; Hallböök, F.; Ashwell, C.; Andersson, L. A Complex Genomic Rearrangement Involving the Endothelin 3 Locus Causes Dermal Hyperpigmentation in the Chicken. PLoS Genet. 2011, 7, e1002412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imsland, F.; Feng, C.; Boije, H.; Bed’hom, B.; Fillon, V.; Dorshorst, B.; Rubin, C.-J.; Liu, R.; Gao, Y.; Gu, X.; et al. The Rose-Comb Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility. PLoS Genet. 2012, 8, e1002775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, J.; Shen, M.; Xie, X.-L.; Liu, G.-J.; Xu, Y.-X.; Lv, F.-H.; Yang, H.; Yang, Y.-L.; Liu, C.-B.; et al. Whole-Genome Resequencing of Wild and Domestic Sheep Identifies Genes Associated with Morphological and Agronomic Traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A Deletion in the Bovine Myostatin Gene Causes the Double-Muscled Phenotype in Cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef]
- Gim, G.-M.; Kwon, D.-H.; Eom, K.-H.; Moon, J.; Park, J.-H.; Lee, W.-W.; Jung, D.-J.; Kim, D.-H.; Yi, J.-K.; Ha, J.-J.; et al. Production of MSTN-Mutated Cattle without Exogenous Gene Integration Using CRISPR-Cas9. Biotechnol. J. 2021, e2100198. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, Y.M.; Suh, Y.; Shin, S.; Lee, J.; Hwang, S.; Lee, K. Research Note: Association of Temporal Expression of Myostatin with Hypertrophic Muscle Growth in Different Japanese Quail Lines. Poult. Sci. 2020, 99, 2926–2930. [Google Scholar] [CrossRef]
- Wang, D.; Wang, N.; Li, N.; Li, H. Identification of Differentially Expressed Proteins in Adipose Tissue of Divergently Selected Broilers. Poult. Sci. 2009, 88, 2285–2292. [Google Scholar] [CrossRef]
- Ding, S.-R.; Li, G.-S.; Chen, S.-R.; Zhu, F.; Hao, J.-P.; Yang, F.-X.; Hou, Z.-C. Comparison of Carcass and Meat Quality Traits between Lean and Fat Pekin Ducks. Anim. Biosci. 2021, 34, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Soglia, F.; Baldi, G.; Laghi, L.; Mudalal, S.; Cavani, C.; Petracci, M. Effect of White Striping on Turkey Breast Meat Quality. Animal 2018, 12, 2198–2204. [Google Scholar] [CrossRef]
- Le Bihan-Duval, E.; Millet, N.; Remignon, H. Broiler Meat Quality: Effect of Selection for Increased Carcass Quality and Estimates of Genetic Parameters. Poult. Sci. 1999, 78, 822–826. [Google Scholar] [CrossRef]
- Fujii, J.; Otsu, K.; Zorzato, F.; de Leon, S.; Khanna, V.K.; Weiler, J.E.; O’Brien, P.J.; MacLennan, D.H. Identification of a Mutation in Porcine Ryanodine Receptor Associated with Malignant Hyperthermia. Science 1991, 253, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Byrem, T.M.; Zarosley, J.; Booren, A.M.; Strasburg, G.M. Skeletal Muscle Calcium Channel Ryanodine Binding Activity in Genetically Unimproved and Commercial Turkey Populations. Poult. Sci. 1999, 78, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.B.; Barnard, R.J.; Edgerton, V.R.; Gillespie, C.A.; Stempel, K.E. Metabolic Profiles of Three Fiber Types of Skeletal Muscle in Guinea Pigs and Rabbits. Biochemistry 1972, 11, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E.; Sosnicki, A. Relationship between Muscle Growth and Poultry Meat Quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef]
- Huo, W.; Weng, K.; Li, Y.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of Muscle Fiber Characteristics and Glycolytic Potential between Slow- and Fast-Growing Broilers. Poult. Sci. 2022, 101, 101649. [Google Scholar] [CrossRef]
- Kim, G.-D.; Jeong, J.-Y.; Jung, E.-Y.; Yang, H.-S.; Lim, H.-T.; Joo, S.-T. The Influence of Fiber Size Distribution of Type IIB on Carcass Traits and Meat Quality in Pigs. Meat Sci. 2013, 94, 267–273. [Google Scholar] [CrossRef]
- Von-Hafe, M.; Borges-Canha, M.; Vale, C.; Leite, A.R.; Sérgio Neves, J.; Carvalho, D.; Leite-Moreira, A. Nonalcoholic Fatty Liver Disease and Endocrine Axes—A Scoping Review. Metabolites 2022, 12, 298. [Google Scholar] [CrossRef]
- Vang, S.; Cochran, P.; Sebastian Domingo, J.; Krick, S.; Barnes, J.W. The Glycobiology of Pulmonary Arterial Hypertension. Metabolites 2022, 12, 316. [Google Scholar] [CrossRef]
- Otter, D.; Cao, M.; Lin, H.-M.; Fraser, K.; Edmunds, S.; Lane, G.; Rowan, D. Identification of Urinary Biomarkers of Colon Inflammation in IL10-/- Mice Using Short-Column LCMS Metabolomics. J. Biomed. Biotechnol. 2011, 2011, 974701. [Google Scholar] [CrossRef]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X.; et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol. Cell 2020, 80, 525–540.e9. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, Y.; Sevak, J.K.; Kumar, S.; Kumar, N.; Gopinath, S.D. Metabolomic Analysis of Primary Human Skeletal Muscle Cells during Myogenic Progression. Sci. Rep. 2020, 10, 11824. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, W.; Yang, H.; Yan, Z.; Ge, C.; Liao, G.; Su, H. 1H NMR-Based Water-Soluble Lower Molecule Characterization and Fatty Acid Composition of Chinese Native Chickens and Commercial Broiler. Food Res. Int. 2021, 140, 110008. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1H NMR-Based Metabolic Characterization of Chinese Wuding Chicken Meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Feng, C.; Shao, M.; Shi, K.; Zhao, Q.; Duan, Y.; Shen, Y.; Tian, J.; He, K.; Li, D.; Yu, M.; et al. Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens, 10 March 2022, PREPRINT (Version 1). Available online: https://www.researchsquare.com/article/rs-1400092/v1 (accessed on 10 March 2022). [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Singh, M.; Lim, A.J.; Muir, W.I.; Groves, P.J. Comparison of Performance and Carcass Composition of a Novel Slow-Growing Crossbred Broiler with Fast-Growing Broiler for Chicken Meat in Australia. Poult. Sci. 2021, 100, 100966. [Google Scholar] [CrossRef]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber Characteristics and Meat Quality of Different Muscular Tissues from Slow- and Fast-Growing Broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Production System on Broiler Carcass and Meat Quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, Color, and Texture of Thai Indigenous and Broiler Chicken Muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Farmer, L.J.; Perry, G.C.; Lewis, P.D.; Nute, G.R.; Piggott, J.R.; Patterson, R.L.S. Responses of Two Genotypes of Chicken to the Diets and Stocking Densities of Conventional UK and Label Rouge Production Systems—II. Sensory Attributes. Meat Sci. 1997, 47, 77–93. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat Quality of Slow- and Fast-Growing Chicken Genotypes Fed Low-Nutrient or Standard Diets and Raised Indoors or with Outdoor Access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef]
- Berri, C.; Debut, M.; Santé-Lhoutellier, V.; Arnould, C.; Boutten, B.; Sellier, N.; Baéza, E.; Jehl, N.; Jégo, Y.; Duclos, M.J.; et al. Variations in Chicken Breast Meat Quality: Implications of Struggle and Muscle Glycogen Content at Death. Br. Poult. Sci. 2005, 46, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chartrin, P.; Méteau, K.; Juin, H.; Bernadet, M.D.; Guy, G.; Larzul, C.; Rémignon, H.; Mourot, J.; Duclos, M.J.; Baéza, E. Effects of Intramuscular Fat Levels on Sensory Characteristics of Duck Breast Meat. Poult. Sci. 2006, 85, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Baeza, E.; Salichon, M.R.; Marche, G.; Juin, H. Effect of Sex on Growth, Technological and Organoleptic Characteristics of the Muscovy Duck Breast Muscle. Br. Poult. Sci. 1998, 39, 398–403. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Alkhedaide, A.Q.; Ramadan, A.A.I.; Hafez, A.-E.S.E.; Hussein, M.A. Potential Impact of Stocking Density on Growth, Carcass Traits, Indicators of Biochemical and Oxidative Stress and Meat Quality of Different Broiler Breeds. Poult. Sci. 2021, 100, 101442. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, X.; Huang, J.; Huan, C.; Li, C. H2O2-Induced Oxidative Stress Impairs Meat Quality by Inducing Apoptosis and Autophagy via ROS/NF-ΚB Signaling Pathway in Broiler Thigh Muscle. Poult. Sci. 2022, 101, 101759. [Google Scholar] [CrossRef]
- Fan, R.-F.; Liu, J.-X.; Yan, Y.-X.; Wang, L.; Wang, Z.-Y. Selenium Relieves Oxidative Stress, Inflammation, and Apoptosis within Spleen of Chicken Exposed to Mercuric Chloride. Poult. Sci. 2020, 99, 5430–5439. [Google Scholar] [CrossRef]
- Liu, C.; Chaudhry, M.T.; Zhao, D.; Lin, T.; Tian, Y.; Fu, J. Heat Shock Protein 70 Protects the Quail Cecum against Oxidant Stress, Inflammatory Injury, and Microbiota Imbalance Induced by Cold Stress. Poult. Sci. 2019, 98, 5432–5445. [Google Scholar] [CrossRef]
- Allameh, S.; Toghyani, M. Effect of Dietary Valine Supplementation to Low Protein Diets on Performance, Intestinal Morphology and Immune Responses in Broiler Chickens. Livest. Sci. 2019, 229, 137–144. [Google Scholar] [CrossRef]
- Tomonaga, S.; Kawase, T.; Tsukahara, T.; Ohta, Y.; Shiraishi, J. Metabolism of Imidazole Dipeptides, Taurine, Branched-Chain Amino Acids, and Polyamines of the Breast Muscle Are Affected by Post-Hatch Development in Chickens. Metabolites 2022, 12, 86. [Google Scholar] [CrossRef]
- Senoo, N.; Miyoshi, N.; Kobayashi, E.; Morita, A.; Tanihata, J.; Takeda, S.; Miura, S. Glycerophospholipid Profile Alterations Are Associated with Murine Muscle-Wasting Phenotype. Muscle Nerve 2020, 62, 413–418. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, C.; Chen, Y.; Xing, X.; Zheng, D.; Guo, Z.; Lin, S. Metabolomics Study of Whole-Body Vibration on Lipid Metabolism of Skeletal Muscle in Aging Mice. Int. J. Sports Med. 2021, 42, 464–477. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Mugnai, C.; Pedrazzoli, M. Comparison of Two Chicken Genotypes Organically Reared: Oxidative Stability and Other Qualitative Traits of the Meat. Ital. J. Anim. Sci. 2006, 5, 29–42. [Google Scholar] [CrossRef]
- Chabault, M.; Baéza, E.; Gigaud, V.; Chartrin, P.; Chapuis, H.; Boulay, M.; Arnould, C.; D’Abbadie, F.; Berri, C.; Le Bihan-Duval, E. Analysis of a Slow-Growing Line Reveals Wide Genetic Variability of Carcass and Meat Quality-Related Traits. BMC Genet. 2012, 13, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.; Jiang, X.Y.; Ding, L.R.; Wang, T.; Zhou, Y.M. Effects of Dietary Methionine on Growth Performance, Meat Quality and Oxidative Status of Breast Muscle in Fast- and Slow-Growing Broilers. Poult. Sci. 2017, 96, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Turinsky, J.; Long, C.L. Free Amino Acids in Muscle: Effect of Muscle Fiber Population and Denervation. Am. J. Physiol. 1990, 258, E485–E491. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Miura, M.; Kodama, J.; Ahmed, S.M.; Itokawa, Y. Role of Iron in Oxidative Stress in Skeletal Muscle Atrophied by Immobilization. Pflug. Arch. 1992, 421, 295–297. [Google Scholar] [CrossRef]
- Bar-Shai, M.; Carmeli, E.; Ljubuncic, P.; Reznick, A.Z. Exercise and Immobilization in Aging Animals: The Involvement of Oxidative Stress and NF-KappaB Activation. Free Radic. Biol. Med. 2008, 44, 202–214. [Google Scholar] [CrossRef]
- Tachibana, N.; Fukao, M.; Irie, T.; Irisawa, Y.; Shirono, H.; Oarada, M.; Nikawa, T.; Fukaya, T. A Diet Including Red Bell Pepper Juice and Soy Protein Suppress Physiological Markers of Muscle Atrophy in Mice. J. Nutr. Sci. Vitaminol. 2020, 66, 449–455. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, X.; Zhang, Y.; Liu, H. UHPLC-QTOF/MS-based Comparative Metabolomics in Pectoralis Major of Fast- and Slow-growing Chickens at Market Ages. Food Sci. Nutr. 2021, 10, 487–498. [Google Scholar] [CrossRef]
- Kiyimba, F.; Hartson, S.D.; Rogers, J.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Changes in Glycolytic and Mitochondrial Protein Profiles Regulates Postmortem Muscle Acidification and Oxygen Consumption in Dark-Cutting Beef. J. Proteom. 2021, 232, 104016. [Google Scholar] [CrossRef]
- Cônsolo, N.R.B.; Rosa, A.F.; Barbosa, L.C.G.S.; Maclean, P.H.; Higuera-Padilla, A.; Colnago, L.A.; Titto, E.A.L. Preliminary Study on the Characterization of Longissimus Lumborum Dark Cutting Meat in Angus × Nellore Crossbreed Cattle Using NMR-Based Metabolomics. Meat Sci. 2021, 172, 108350. [Google Scholar] [CrossRef] [PubMed]
- Bawa, S.; Brooks, D.S.; Neville, K.E.; Tipping, M.; Sagar, M.A.; Kollhoff, J.A.; Chawla, G.; Geisbrecht, B.V.; Tennessen, J.M.; Eliceiri, K.W.; et al. Drosophila TRIM32 Cooperates with Glycolytic Enzymes to Promote Cell Growth. Elife 2020, 9, e52358. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation 2020, 141, 1249–1265. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The Pentose Phosphate Pathway and Cancer. Trends Biochem. Sci. 2014, 39, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.R.; Kauffman, F.C.; Max, S.R. The Pentose Phosphate Pathway in Regenerating Skeletal Muscle. Biochem. J. 1978, 170, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Fernandez, I.E.; Wei, M.; Witting, M.; Aichler, M.; Feuchtinger, A.; Burgstaller, G.; Verleden, S.E.; Schmitt-Kopplin, P.; Eickelberg, O.; et al. Pharmacometabolic Response to Pirfenidone in Pulmonary Fibrosis Detected by MALDI-FTICR-MSI. Eur. Respir. J. 2018, 52, 1702314. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Koorbanally, N.A.; Islam, M.S. Kolaviron Modulates Dysregulated Metabolism in Oxidative Pancreatic Injury and Inhibits Intestinal Glucose Absorption with Concomitant Stimulation of Muscle Glucose Uptake. Arch. Physiol. Biochem. 2020, 1–11. [Google Scholar] [CrossRef]
- Mitsumoto, Y.; Liu, Z.; Klip, A. A Long-Lasting Vitamin C Derivative, Ascorbic Acid 2-Phosphate, Increases Myogenin Gene Expression and Promotes Differentiation in L6 Muscle Cells. Biochem. Biophys. Res. Commun. 1994, 199, 394–402. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, A.; Yu, J.; Wang, L.; Liu, C.; Zhou, X.; Sun, H.; Song, Q.; Wang, X. Insight into the Metabolic Mechanism of Scoparone on Biomarkers for Inhibiting Yanghuang Syndrome. Sci. Rep. 2016, 6, 37519. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Ma, J.; Zhong, Q.; Zhao, M.; Hu, T.; Chen, T.; Qiu, L.; Wen, L. Curcumin Improves Alcoholic Fatty Liver by Inhibiting Fatty Acid Biosynthesis. Toxicol. Appl. Pharmacol. 2017, 328, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, J.; Li, K.; Heng, C.; Jiang, L.; Cai, C.; Zhan, X. Effects of Different Stocking Densities on Tracheal Barrier Function and Its Metabolic Changes in Finishing Broilers. Poult. Sci. 2020, 99, 6307. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Tao, J.Z. Metabolomics and Pathway Analyses to Characterize Metabolic Alterations in Pregnant Dairy Cows on D 17 and D 45 after AI. Sci. Rep. 2018, 8, 5973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Gu, J.; Jiang, X.; Deng, N.; Wu, J.; Zou, L.; Zhu, Y.; Tan, B. Anxiety Disturbs the Blood Plasma Metabolome in Acute Coronary Syndrome Patients. Sci. Rep. 2021, 11, 12897. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.-F.; Xie, H.; Mei, X.-Y.; Gao, J. Buyang Huanwu Tang Alleviates Inflammation and Improves Motor Endplate Functions in DSMA Rat Models by Activating Several Biological Molecules and Associated Signaling Pathways. Am. J. Transl. Res. 2019, 11, 3056–3068. [Google Scholar] [PubMed]
- Estrada-Cortés, E.; Ortiz, W.; Rabaglino, M.B.; Block, J.; Rae, O.; Jannaman, E.A.; Xiao, Y.; Hansen, P.J. Choline Acts during Preimplantation Development of the Bovine Embryo to Program Postnatal Growth and Alter Muscle DNA Methylation. FASEB J. 2021, 35, e21926. [Google Scholar] [CrossRef] [PubMed]

| Tissue | Pathway ID 1 | Pathway Name 2 | N 3 | p Value |
|---|---|---|---|---|
| Breast muscle | ko00290 | Valine, leucine and isoleucine biosynthesis | 3 | 0.00132 |
| ko00564 | Glycerophospholipid metabolism | 5 | 0.00339 | |
| ko00480 | Glutathione metabolism | 4 | 0.00894 | |
| Thigh muscle | ko00520 | Amino sugar and nucleotide sugar metabolism | 11 | 9.33 × 10−9 |
| ko00053 | Ascorbate and aldarate metabolism | 3 | 0.00371 | |
| ko00030 | Pentose phosphate pathway | 4 | 0.00542 | |
| ko00040 | Pentose and glucuronate interconversions | 3 | 0.02082 | |
| ko00051 | Fructose and mannose metabolism | 3 | 0.02082 | |
| ko00564 | Glycerophospholipid metabolism | 4 | 0.02806 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Zhao, Q.; Shao, M.; Duan, Y.; Li, D.; Lu, Y.; Tang, Y.; Feng, C. Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat. Metabolites 2022, 12, 367. https://doi.org/10.3390/metabo12050367
Shi K, Zhao Q, Shao M, Duan Y, Li D, Lu Y, Tang Y, Feng C. Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat. Metabolites. 2022; 12(5):367. https://doi.org/10.3390/metabo12050367
Chicago/Turabian StyleShi, Kai, Qian Zhao, Minghui Shao, Ying Duan, Dongfeng Li, Yangqing Lu, Yanfei Tang, and Chungang Feng. 2022. "Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat" Metabolites 12, no. 5: 367. https://doi.org/10.3390/metabo12050367
APA StyleShi, K., Zhao, Q., Shao, M., Duan, Y., Li, D., Lu, Y., Tang, Y., & Feng, C. (2022). Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat. Metabolites, 12(5), 367. https://doi.org/10.3390/metabo12050367







