Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response
Abstract
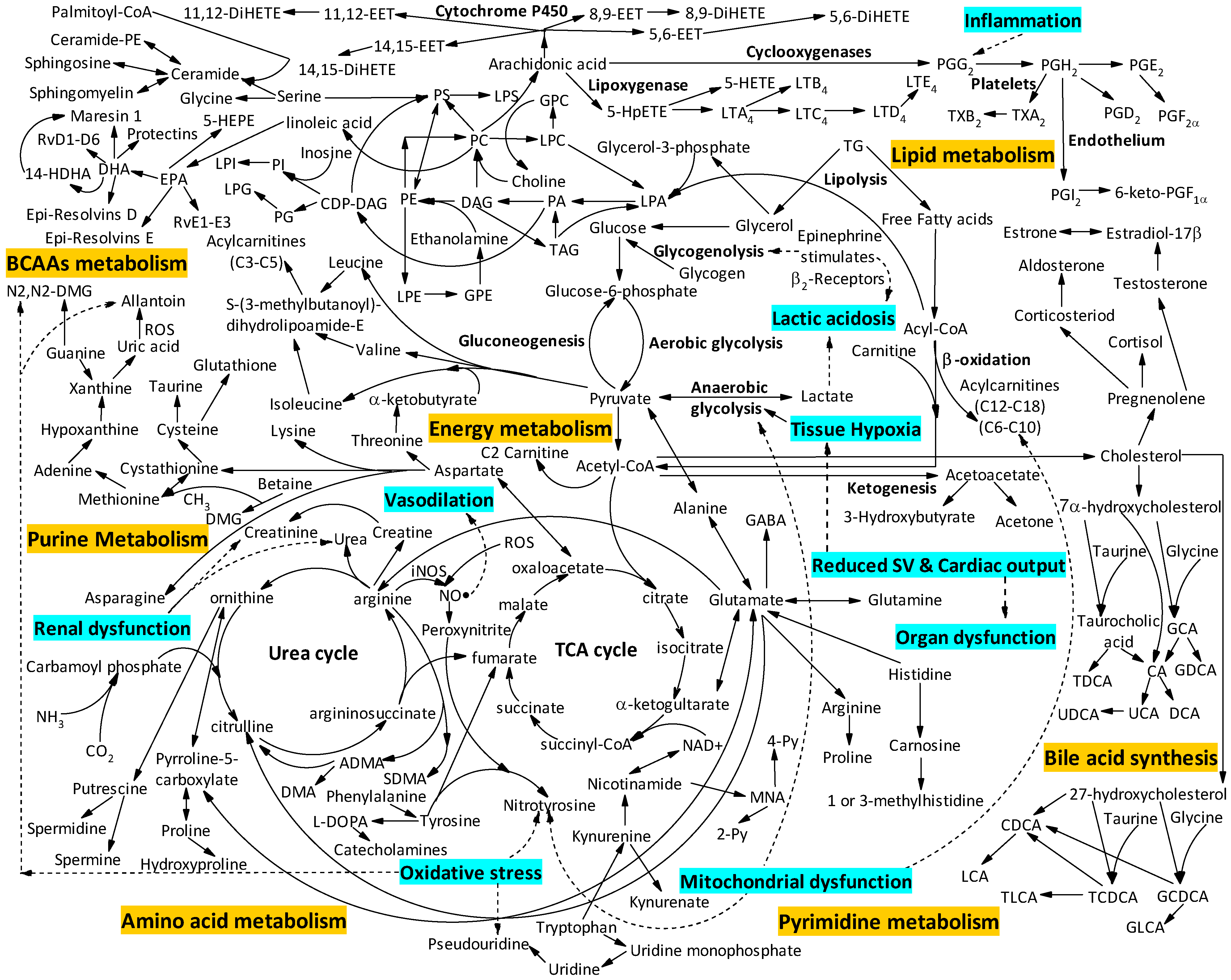
:1. Introduction
2. Metabolomics to Identify Diagnostically Useful Clinical Phenotypes of Patients
2.1. Metabolomic Studies Aiming to Differentiate Sepsis from Other Conditions
2.1.1. Mitochondrial Dysfunction and Energy Metabolism
2.1.2. Amino Acid Metabolism
| Study (Year) | Sample Type | Participants (Septic:Comparator) | Age Group | Comparator | Analytical Technique | Statistical Analysis Methods | Raised in Sepsis | Reduced in Sepsis |
|---|---|---|---|---|---|---|---|---|
| Li et al. [42] (2021) | Serum | 84:59 | Paediatric | Healthy control | HPLC-MS (targeted) | ANOVA, t-test, PCA, OPLS-DA, HCA, LR | D-mannose, d-quinovose, glycocholic acid, L-glutamate | PC (O-17:1/0:0), PI (20:4/18:1), PG (23:0/20:4), PE(P-17:0/0:0) |
| Mierzchala-Pasierb et al. [37] (2021) | Serum and urine | 15:15 | Adults | Healthy control | LC-MS (targeted) | MW-U test, t-test, Cox’s PHR, Spearman’s correlation, ROC analysis | Arginine, glycine, thioproline—in both. Histidine, a-aminoisobutyric acid, sarcosine aminoadipic acid, tyrosine, phenylalanine, leucine, lysine, isoleucine, ornithine, threonine, 4-hydroxyproline, glutamine 3-methyl-histidine, asparagine, aminopimelic acid—in serum. Citrulline—in urine | Histidine, aminoadipic acid, 3-methyl-histidine and aminopimelic acid—in urine. Dipeptide Gly-Pro—in serum |
| Jaurila et al. [27] (2020) | Serum | 44:14 | Adults | Healthy control | 1H-NMR (targeted) | MW-U or t-test, FET, correlations | Glucose, glycine, creatinine, 3-hydroxybutyrate, glycoprotein acetyls mostly AGP | Citrate and histidine |
| Lin et al. [21] (2020) | Serum | 31:23 | Adults | Healthy control | GC–MS (targeted) | PLS-DA, t-test, ROC analysis, correlation analysis | Six FAs (including tetradecanoic acid [12-methyl-, methyl ester, (S)-], hexanoic acid, 2-methyloctadecanoic acid, palmitoleic acid, myristoleic acid, and 3-hydroxyoctanoic acid), amino acids (including leucine, glutamic acid, cysteine, methionine, phenylalanine, putrescine, and aspartic acid), six amino acid derivatives, twenty-seven organic acids (including lactic acid, adipic acid, and 3-hydroxypropionic acid), pyruvic acid and (NADP)-NADPH, two TCA cycle derivatives (including DL-gamma-methyl-ketoglutaramate isomer and dimethyl fumarate), seven TCA cycle metabolites | Two BCFAs (3-methyl-2-oxopentanoic acid, 4-methyl-2-oxopentanoic acid), ten saturated FAs (DPA, hexanoic acid, arachidic acid, palmitic acid, margaric acid, 10,13-dimethyltetradecanoic acid, nonadecanoic acid, pentadecanoic acid, propanedioic acid, methyl, ethyl ester, and stearic acid), nine unsaturated FAs (11,14-EDA, 11,14,17-ETA, adrenic acid, arachidonic acid, conjugated linoleic acid, bishomo-gamma-linolenic acid, linoleic acid, DHA, EPA), tryptophan, glutamine, serine, d-Proline, (N-methoxycarbonyl-, octyl ester), asparagine, 2 amino acid derivatives, citraconic acid, citramalic acid, DL-gamma-methylketoglutaramate isomer 1 and four TCA cycle metabolites |
| Sharma et al. [43] (2019) | Plasma | 27:23 | Adults | Healthy control | LC–MS/MS (targeted), ELISA tests | ANOVA, KWT | - | Total cholesterol, HDCH, LDCH, non-HDCH, Apo-A1, Apo-B100 andparaoxonase 1. |
| Sharma et al. [44] (2017) | Plasma | 33:23 | Adults | Healthy control | LC–MS/MS (targeted) and enzymatic colorimetry | Tukey’s HSD test, ANOVA | - | Total cholesterol, HDCH, LDCH, HDFC |
| Mecatti et al. [45] (2018) | Plasma and erythrocytes | 20:20 (Sepsis and septic shock: healthy control) | Adults | Healthy control | LC-MS and GC-MS (targeted) | PCA, OPLS-DA | PCs (C15:0/18:2, C16:0/18:1) only in plasma. Total MUFAs, oleic acid (C18:1 n-9), PS (C18:0/22:1), PC (C16:0/20:4) only in erythrocytes. CL (1′ [18:0/18:2]/3′ [20:0/20:0]) and PC (C16:0/18:2 and C16:0/20:5) in both. | LPC (18:2/0:0), SM (d18:1/16:0), DHA (C22:6 n-3), PC (16:0/20:3) only in plasma. Total n-3 PUFAs, DPA (C22:5 n-3), PC (C15:0/18:2, C16:0/20:1) only in erythrocytes. Nine SMs (d18:1/20:1, d18:1/22:1 (d18:2/22:0), (d18:1/22:0)/(d16:1/24:0), (d18:0/22:0)/(d16:0/24:0), (d18:2/23:0), (d18:1/23:0), (d18:2/24:0)/(d18:1/24:1), (d18:1/24:0)/(d18:0/24:1), d18:1/17:0), 8 LPCs (15:0/0:0, 16:0/0:0, 18:3/0:0, 18:1/0:0, 18:0/0:0, 20:5/0:0, 20:4/0:0, 20:3/0:0), and PC (16:0/20:1) in both. |
| Szelig et al. [36] (2016) | Serum and urine | 20:25 (Septic shock or severe sepsis:healthy control) | Adults | Healthy control | HPLC (targeted) | MW-U or t-test, Kruskal–Wallis and Spearman’s rho test | Serum meta-tyrosine on days 2 and 3. Urinary ortho-tyrosine on days 1 to 5, and urinary para-tyrosine on days 4 and 5. | Serum para-tyrosine on days 1 and 2. Urinary para-tyrosine on day 1. |
| Liang et al. [19] (2015) | Urine | 1282:1346 (Septic shock: healthy control) | Adults | Healthy control | UPLC-MS (untargeted) | OPLS-DA, ROC analysis | Hippuric acid, 3-methyluridine, acetylcysteine | Kynurenic acid, glycine |
| Su et al. [38] (2015) | Serum | 35:18 | Adults | Healthy control | LC-MS/MS (targeted) | ANOVA, Χ2 and t-test, ROC curves, Pearson correlation. | Arginine, aspartic acid, homocitrulline, ethanolamine, glutamine, glutamic acid, phenylalanine, taurine, SAAs—on ICU admission | Cystathionine, EAAs, anserine BCAAs, BCAA/AAA ratio, asparagine, carnosine, citrulline, histidine, Isoleucine, isoleucine, valine, lysine, ornithine, phosphoethanolamine, proline, sarcosine, threonine, tryptophan, tyrosine—on ICU admission |
| Fanos et al. [22] (2014) | Urine | 9:16 | Neonates | Healthy control | 1H-NMR and GC-MS (untargeted) | OPLS-DA | Lactate, glucose and maltose | 2,3,4-trihydroxybutyric acid, rabitol 3,4-dihydroxybutanoic acid, ribonic acid, 3,4,5-trihydroxypentanoic acid, 2-ketogluconic acid, pseudouridine. |
| Mickiewicz et al. [23] (2013) | Serum | 21:13—Infants 20:18—Toddlers 14:9—School Age | Infants, Toddlers, School Age | Healthy control | 1H-NMR (targeted and untargeted) | PCA, PLS-DA, OPLS-DA, ROC analysis | 2-Hydroxybutyrate, 2-hydroxyisovalerate, lactate in all three. Creatinine and 2-oxoisocaproate in infants and school age. Phenylalanine in school age and toddlers. 3-hydroxybutyrate, acetone, betaine, glucose and isobutyrate in toddlers. Arginine, carnitine, creatine, creatine phosphate, histidine, myo-inositol, O-acetylcarnitine in school age. | 2-Aminobutyrate in infants and toddlers; acetate, adipate, threonine in infants. Glutamine in toddlers and citrate in school age. |
| Stringer et al. [46] (2011) | Plasma | 13:6 | Adults | Healthy control | 1H-NMR (targeted) | Spearman’s correlation, t-test | Adenosine, Total Glutathione, PS | Sphingomyelin |
| Gaddnas et al. [47] (2009) | Serum | 44:15 (Severe sepsis:healthy controls) | Adults | Healthy control | Radioimmunological assays | Χ2-test or FET, MW-U test, ROC analysis | Procollagen type III aminoterminal propeptides and crosslinked telopeptides of type I collagen. | - |
| Drobnik et al. [48] (2003) | Plasma | 102:56 | Adults | Healthy control | LC-MS/MS (targeted) | MW-U test, ROC analysis | Ceramides (C16:0, C18:0, C20:0, C22:1, C24:1 and total form), LPC (16:0, 18:0, 18:1, 18:2 and total form), LPC-PC ratio (16:0, 18:0, 18:1,18:2 and total form) | Ceramides (C23:0, C24:0) |
| Reisinger et al. [39] (2021) | Serum | 52:25 | Adults | ICU controls (without sepsis or bacteremia) | 1H-NMR (untargeted) and Bruker IVDr lipoprotein analysis | LR, LMM, FET, Χ2 or MW-U test, PCA, PLS and OPLS-DA, correlations | VLPN, TG, V4 and V5TG, V2-V4FC, V4PL, L1TG, VLAB and ABA1 | Valine, leucine, isoleucine, HDFC, H1-H3FC, HDCH, H1-H3CH, HDPL, H2-H4PL, TPA1, HDA1 and H1-H4A1 |
| Grauslys et al. [24] (2020) | Serum | 55:58 | Paediatrics | SIRS | 1H-NMR (targeted) | PCA, PLS-DA, t-test | 3-hydroxtbutyrate, lactate, urea, valine, phenylalanine | 2-hydroxyisobutyrate, acetate, acetone, leucine, pyruvate. |
| Antcliffe et al. [40] (2017) | Serum | 15:26 (Pneumonia:brain injury) | Adults | SIRS (brain injury) | 1H-NMR (targeted and untargeted) | PCA, OPLS-DA, CV-ANOVA, ROC analysis | Lipids, formate, phenylalanine, N and O-glycoproteins, unidentified metabolite (at 3.570–3.575 ppm). Lipoproteins (V5FC, L1-L4TG, L1PL, HDTG, H1and H2TG, L1 and L6AB). | Phospholipids (choline), glutamine and alanine. Lipoproteins (H3 and H4FC, L5 and L6FC, HDA1 and H4A1, TPA1 and TPA2, HDA2 and H4A2, HDCH, H3 and H4CH, L6CH, H4PL, L6PL). |
| Neugebauer et al. [20] (2016) | Serum | 322:84 (Total) 123:42 (Test) 59:24 (Confirmation) | Adults | SIRS | LC-MS/MS (targeted) | MW-U test or t-test, HCA, LR, ROC analysis, KMSA | Serine, spermine, spermidine, aspartate, phenylalanine, total dimethylarginine, kynurenine, acetylornithine, acylcarnitine C6(C4:1-DC), PCaa (C32:0), SM (C16:1) | SM (C22:3, C20:2, C24:0, C26:1), SM-OH (C22:1, C24:1), LPCa (C24:0, C14:0), PCaa (C32:0, C32:2, C36:6, C40:4, C42:6), PCae (C44:4), acylcarnitine C16:2(OH) |
| Kauppi et al. [26] (2016) | Whole blood | 65:49 (Bacteremic sepsis:SIRS) | Adults | SIRS | GC-TOF-MS (Untargeted) | H-MCR, OPLS-DA | Myristic acid, pyruvic acid | Isoleucine, norleucine, citric acid and a phosphocholine-like derivative |
| Mickiewicz et al. [32] (2015) | Serum and plasma | 37:20 (Septic shock: SIRS) | Adults | SIRS | 1H-NMR (targeted), cytokine and chemokine assay kits | PCA, OPLS-DA, ROC analysis | Proline, 3-hydroxybutyrate, isobutyrate, phenylalanine, myoinositol, 2-hyroxybutyrate, O-acetylcarnitine, urea, IP-10, HGF, IL-2Ra, IL-1Ra, IL-18 | Valine, arginine, threonine, glutamate, glucose, methanol, propylene glycol, TNF-β, IL-1α, MCP-3 |
| Su et al. [38] (2015) | Serum | 35:14 | Adults | SIRS | LC-MS/MS (targeted) | ANOVA, Χ2 test, t-test, Pearson correlation, ROC analysis | Homocitrulline, cystathionine, ethanolamine—at ICU admission | Anserine, phosphoethanolamine, lysine, δ-hydroxylysine, phosphoserine—at ICU admission |
| Mickiewicz et al. [25] (2014) | Serum | 39:20 (Septic shock: SIRS) | Adults | SIRS | 1H-NMR (targeted) | PCA, OPLS-DA, ROC analysis | Sucrose, lactate, myoinositol, proline, O-acetylcarnitine, isobutyrate, succinate, urea, creatinine, creatine, 2-hydroxyisovalerate, trimethylamine-N-oxide, 3-hydroxybutyrate, phenylalanine | Isoleucine, leucine, valine, lysine, glycine, serine, glutamine, alanine, threonine, glucose, mannose, glutamate, arginine, 2-aminobutyrate, methanol, 2-oxobutyrate, creatine phosphate |
| Su et al. [34] (2014) | Serum | 35:15 | Adults | SIRS | LC-MS/MS (targeted) | PCA, PLS and OPL-DA, MW-U test, ROC analysis | S-(3-methylbutanoyl)-dihydrolipoamide-E and N-nonanoyl glycine | Lactitol dehydrate and S-phenyl-D-cysteine |
| Mickiewicz et al. [23] (2013) | Serum | 21:13—Infants 20:16—Toddlers 14:9—School Age | Infants, Toddlers, School Age | SIRS | 1H-NMR (untargeted and targeted) | PCA, PLS-DA, OPLS-DA, ROC analysis | 2-Hydroxybutyrate and glycerol in infants and school age. Glucose in infants and toddlers. Arginine in toddlers and school age. Citrate only in toddlers. Lactate, alanine, asparagine, creatine, creatine phosphate, creatinine 2-oxoisocaproate, ethanol, methanol, phenylalanine, taurine in school age. | Taurine in infants and toddlers. Adipate, alanine, glutamate, glycine, hopoxanthine, isoleucine, lactate, methionine, ornithine, serine, pyruvate, suberate, threonine in infants. |
| Schmerler et al. [30] (2012) | Plasma | 69:74 (Total) 30:33 (Training) 39:41 (Test) | Adults | SIRS | LC-MS/MS (targeted) | MW-U or t-test, ROC analysis | Acylcarnitines (C3, C5, C6 (C4:1-DC), C8, C10:1). PCaa (C32:0, C34:1, C36:1), PCae (C34:1) | - |
| Su et al. [38] (2015) | Serum | 12:23 | Adults | Severe sepsis | LC-MS/MS (targeted) | ANOVA, Χ2 test, t-test, Pearson correlation, ROC analysis | Taurine (on days 1, 3, 5, 7, 10, and 14), cystine (on days 3, 7, 10, and 14), SAAs (on days 5, 10, and 14), whilst arginine, asparagine, aspartic acid, glutamic acid, leucine, serine, tryptophan, BCAAs, BCAA/AAA ratio—at certain time points | 3-methyl-L-histidine, α-aminoadipic acid, α-amino-n-butyric acid, argininosuccinic acid, β-amino-isobutyric acid, carnosine, cystathionine, glutamine, phenylalanine, proline—at certain timepoints |
| Su et al. [34] (2014) | Serum | 10:25 | Adults | Severe sepsis | LC-MS/MS (targeted) | MW-U, PCA, PLS and OPL-DA, ROC analysis | Ne, Ne-dimethyllysine, glyceryl-phosphoryl-ethanolamine, 2-phenylacetamide, D-cysteine | - |
| Beloborodova et al. [28] (2019) | Serum | 35:48 (Late: early-stage sepsis) | Adults | Early-stage sepsis | GC-MS | MW-U, Spearman’s correlation | Succinic acid, fumaric acid, p-HPhLA | - |
2.1.3. Urea Cycle
2.1.4. Lipoproteins and Lipids
2.2. Metabolomic Studies Aiming to Identify Site of Infection and Causative Organism
3. Metabolomics to Identify Prognostically Useful Clinical Phenotypes of Sepsis
3.1. Metabolomic Studies of Sepsis Survival
3.1.1. Energy Metabolism
| Study (Year) | Sample Type | Participants (Survivors:Non-Survivors) | Age Group | Analytical Technique | Statistical Analysis Methods | Raised in Non-Survivors | Reduced in Non-Survivors |
|---|---|---|---|---|---|---|---|
| Jones et al. [88] (2022) c | Serum | 113:39 | Adults | UHPLC-MS (targeted) | PCA, PLSDA, FET MW-U test, MELR, Cox PHR, KMSA | 14,15-dihydroxyeicosatrienoic acid (DHET) | - |
| Mierzchala-Pasierb et al. [37] (2021) | Serum and urine | 11:4 | Adults | UPLC-MS (targeted) | MW-U test, Cox PHR analysis | Serum 4-hydroxyproline and Glutamine | - |
| Li et al. [42] (2021) c | Serum | 74:10 | Paediatric | HPLC-MS (targeted) | ANOVA, t-test, PCA, OPLS-DA, HCA, LR | Adenine, indolelactic acid, LPS (18:1/0:0), Ile-Tyr, kynurenine, glutamate, acetylcarnitine, tyrosine, tryptophan, palmitoylcarnitine, methionine, proline, acetylneuraminate and N2,N2-dimethylguanosine | PC (14:0/0:0,17:0/0:0, O-18:1/0:0), PI (18:0/22:5,18:0/22:6) |
| Reisinger et al. [39] (2021) c | Serum | 34:19 30:16 on days 3 and 7 | Adults | 1H-NMR (untargeted) and Bruker IVDr lipoprotein analysis | LR, LMM, FET, Χ2 or MW-U test, PCA, PLS and OPLS-DA correlations | - | BCAAs (valine, leucine, isoleucine) |
| Jaurila et al. [27] (2020) | Serum | 33:11 | Adults | 1H-NMR (targeted) | MW-U or t-test, FET, correlations | Lactate and citrate | - |
| Khaliq et al. [89] (2020) c | Plasma | 12:8 | Adults | LC-MS/MS (targeted) and industrial clinical chemistry system | PCA, mixed effects type-III ANOVA, Tukey HSD test, Random forests, linear SVMs | Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and troponin T, putrescine, acylcarnitines (mostly short-chain acylcarnitines), amino acids (aspartate, tyrosine, phenylalanine, histidine) on days 0–3 or at any specific timepoint | HDCH, LDCH, 4 LPCs, 28 PCs, 11 SM-OH (C14:1, C16:1, C22:2, C23:0), SM (C16:0, C18:0, C18:1, C20:2, C22:3, C24:0, C26:0) on days 0–3 or at any specific timepoint |
| Wang et al. [16] (2020) c | Plasma | 134:54 | Adults | LC-MS (targeted) | MW-U test, PLS-DA, ROC analysis | Isoleucine, alanine, acetylcarnitine, lactic acid pyruvic acid | LPG (22:0), and LPC (24:0) |
| Evans et al. [90] (2019) f | Serum | 7:4 | Adults | LC-MS (untargeted) | MW-U test, Student’s t-test, generalised estimation equations | N-Methyl-phenylalanine, glucosamine, isoleucyl-proline/leucyl proline, histamine, adipoyl-L-carnitine, methoxytryptophol, fibrinopeptide A, N,N-dimethylguanosine, N-(3-acetamidopropyl)pyrrolidin-2-one, allysine | N-Acetyl-L-phenylalanine, phenylalanyl-tyrosine |
| Chung et al. [91] (2019) c | Plasma | Derivation—69:21 Validation—96:24 | Adults | UHPLC-MS (targeted) | Cox PHR, t-test, LR, ROC curves, KMSA | Acetylcarnitine (in both cohorts) | - |
| Huang et al. [92] (2019) e | Plasma | 63:30 | Adults | UPLC-UV (targeted) | Χ2, MW-U, KWT and t-test, KMSA, Cox PHR, ROC | Leucine and phenylalanine | - |
| Liu et al. [75] (2019) b | Serum | 40:29 at 0 h 32:19 at 24 h | Adults | 1H-NMR (untargeted and targeted) | PCA, OPLS-DA, t-test, ROC analysis | Lactate, pyruvate, alanine, glutamate, glutamine, lysine, 1-methylhistidine, tyrosine, phenylalanine, citrate at 0 h and 24 h. Methionine, fumarate, acetate, urea and 3-hydroxybutyrate at 0 h. Creatinine, 3-hydroxyisovalerate and lipids at 24 h | N-acetyl glycoproteins—0 h and 24 h |
| Cambiaghi et al. [93] (2018) c | Plasma | 9:8 | Adults | LC-MS/MS (targeted) | Elastic net LR, LDA, PLS-DA | Day 7 to day 1 ratios of PCaa (C34:3, C36:3, C36:6, C42:1, C42:5), PCaeC30:1, SDMA, total dimethylarginine, proline, tyrosine | Day 7 to Day 1 ratios of LPC aC24:0, methionine, PCaa (C40:6, C42:6, C42:2), PCae (C30:2 and C42:5) |
| Winkler et al. [49] (2018) c | Plasma | 89:31 | Adults | LC-MS/MS (targeted) | Χ2, MW-U test, KWT, KMSA | SDMA on days 1, 3 and 7, ADMA on days 1 and 3 | - |
| Cirstea et al. [58] (2017) c,e | 186:14—Day 28 172:28—Day 90 | Adults | Photometric analysis | KMSA, ROC analysis | - | HDCH | |
| Dalli et al. [94] (2017) b | Plasma | 13:9 | Adults | LC-MS/MS (targeted) | Wilcoxon paired signed rank test, FET, PLS-DA | PGF2α (on days 0, 3 and 7), RvD5 (on days 3 and 7), RvE1, 17-HDHA, 18-HEPE, 15-HETE (on days 1 and 3), LTB4, RvE2,4S,14S-diHDHA and 5S,15S-diHETE (on day 7), 17R-PD1, 7-HDHA and 15-HEPE (on day 1), 17-epi-RvD1, 17-epi-PD1, 5-HETE and 5S,12S-diHETE (on day 3). | - |
| Wang et al. [95] (2017) c | Plasma | CAPSOD—90:31 HAI–VAP—20:16 | Adults | LC-MS/MS (targeted) | MW-U test, HCA, SVMs, ROC curves | Methylthioadenosine (MTA) in both cohorts | - |
| Sharma et al. [44] (2017) b | Plasma | 20:13—Day 1 14:9—Day7 | Adults | LC-MS/MS (targeted) and enzymatic colorimetry | ANOVA, Tukey HSD test | No significant difference in lipoproteins. | |
| Liu et al. [73] (2016) b | Serum | 21:29 | Adults | UPLC-MS (untargeted) | ANOVA, Tukey HSD test | Citrate, succinate, malate, α-ketoglutartae, amino acids (proline, BCAAAs, glutamine, glutamate, phenylalanine, betaine, creatine, creatinine, tyrosine), lactate, bile acids (GUDCA, GUDCS, GCDCA, GCA, UDCA), acyl carnitines (C6, C10, C12), indoxylactate, indoxysulfate, LPC 14:0 | Ornithine, citrulline, argininosuccinate, acetylcarnitine, FFA (16:0,18:0), LPE (18:0,18:2,20:3,20:4), acylcarnitines (C16, C18) |
| Ferrario et al. [96] (2016) c,e | Plasma | 9:11 (90-day mortality) 11:9 (28-day mortality) | Adults | LC-MS/MS (targeted) | Unpaired Wilcoxon and paired Wilcoxon signed rank test, Multivariate Elastic Net regression analysis | Acetylcarnitine (on day 1) and kynurenine (on day 7)—based on 28-Day mortality. | PCs and LPCs species (on days 1 and 7)—based on 28-day and 90-day mortality. Six saturated long-chain LPC (aC16:0, aC18:0) and polyunsaturated very long-chain PC (aaC32:3, aaC34:4, aaC36:4, aeC40:1) at day 7—on both 28 and 90-day mortality. |
| Garcia-Simon et al. [80] (2015) d | Urine | 48:12 | Adults | 1H-NMR (targeted and untargeted) | ANOVA, PCA, PLS-DA, LR, ROC analysis | Ethanol, glucose, hippurate and an unknown metabolite (located at 1.40–1.45 ppm)—at 0 h and 24 h | Phenylalanine &arginine at 0 h and 24 h. Glutamine and methionine at 0 h |
| Su et al. [38] (2015) c | Serum | 20:15 | Adults | LC-MS/MS (targeted) | ANOVA, Χ2 test, t-test, Pearson correlation, ROC analysis | α-aminoadipic acid, ethanolamine, cystathionine, and phenylalanine—at certain time points | Taurine (on days 10 and 14), BCAA/AAA ratio (on day 14), SAA (on days 7, 10, and 14) whilst arginine, glutamic acid, serine, and tryptophan at certain timepoints |
| Lee et al. [97] (2015) c | Serum | 65:52 | Adults | Commercial kits with automated analysers | LMM, MW-U test or Student’s t-test, Cox PHR, ROC analysis | - | Cholesterol, TG, HDL, LDL, and Apo A-I—On days 0, 1, 3 and 7 |
| Mickiewicz et al. [32] (2015) | Serum and plasma | 8:8 | Adults | 1H-NMR (targeted) and cytokine and chemokine kits | PCA, OPLS-DA, ROC analysis | 2-hydroxyisovalerate, fructose, IL-8, IL-9 and growth-regulated oncogene alpha (GRO-α). | Tumour necrosis factor (TNF)-β, beta-nerve growth factor (β-NGF) and dimethylamine |
| Kamisoglu et al. [84] (2015) c | Plasma | 90:31 | Adults | LC-Q-orbitrap-MS and DSQ GC-MS (untargeted) | Welch’s t-test, Kolmogorov–Smirnov test | 2-methylbutyroylcarnitine tiglylcarnitine, acetylcarnitine, hexanoylcarnitine octanoylcarnitine, propionylcarnitine, butyrylcarnitine, decanoylcarnitie, cis-4-decenoyl carnitine at 0 h and 24 h whilst deoxycarnitine only at 24 h. | 1-eicosatrienoyl-GPC (20:3), 1-palmitoleoyl-GPC (16:1), 2-palmitoyl GPC (16:0) at 0 h and 24 h. 1-palmitoyl-GPC (16:0) 1-stearoyl-GPC (18:0), 1-oleoyl-GPC (18:1), 1-linoleoyl-GPC (18:2), 1-arachidonyl-GPC (20:4) at 24 h. |
| Mickiewicz et al. [25] (2014) | Serum | 4:4 | Adults | 1H-NMR (targeted) | PCA, OPLS-DA, ROC analysis | 20 metabolites were significant in differentiating survivors from non-survivors (results not reported) | - |
| Rogers et al. [72] (2014) c | Plasma | RoCI—60:30 CAPSOD—115:34 | Adults | GC-MS and LC-MS (targeted) | LR, Bayesian networks | Kynurenine lactate, p-HPhLA, ornithine, 3-hydroxyisovalerate, 2-hydroxyisovalerate, N-acetylalanine, sucrose, N-acetylserine, xanthine, allantoin, N2,N2-dimethylguanosine, 1-methylimidazoleacetate, glycocholate, GCDCA, TCDCA, taurocholate, cortisol, carnitines (C3, C4, C5, C5-OH, C5:1 and C6), γ-glutamylphenyl-alanine, γ-glutamyl-tyrosine—in both cohorts. | 1-arachidonoyl-GPC (20:4), 1-arachidonoyl-GPE (20:4),), 1-palmitoyl-GPC (16:0), 2-palmitoyl-GPC (16:0), 1-linoleoyl-GPC (18:2), 1-stearoyl-GPC (18:0))—in both cohorts |
| Su et al. [34] (2014) a | Serum | 26:9 | Adults | LC-MS/MS (targeted) | MW-U, ROC analysis, PCA, PLS and OPL-DA | S-succinyl glutathione, GPC, PG (22:2(13Z,16Z)/0:0), S-(3-methylbutanoyl)dihydrolipoamide-E) | - |
| Mickiewicz et al. [23] (2013) | Serum | 10:10—Model 1 13:10—Model 2 | Infants, Toddlers, School Age | 1H-NMR (untargeted and targeted) | PCA, PLS-DA, OPLS-DA, ROC analysis | Eleven metabolites from model 1 and eighteen metabolites from model 2 were significant in separating survivors from non-survivors. (Metabolites not reported) | - |
| Langley et al. [70] (2013) c | Plasma | Derivation set—90:31 CAPSOD—34:18 RoCI—36:25 | Adults | UPLC-MS/MS (Targeted and untargeted) and GC-MS (untargeted) | ANOVA, LR, SVMs | Seventeen amino acid catabolites (lactate, p-HPhLA, 4-hydroxyproline, 3-methoxytyrosine), sixteen carnitine esters (Cis-4-decenoylcarnitine, 2-methylbutyroylcarnitine, butyroylcarnitine, hexanoylcarnitine), citrate, malate, pyruvate, dihydroxyacetone, phosphate, eleven nucleic acid catabolites, and four FFAs | Seven GPC and GPE esters, anabolic steroids, cortisone |
| Seymour et al. [71] (2013) e | Plasma | 15:15 | Adults | UHPLC-MS/MS and GC-MS (untargeted) | Wilcoxon signed rank and t-test, random forests with supervised classification | Urea, cortisol, cortisone, fumarate, Kynurenate, 2-Py, pyridoxate, cofactors/vitamins, taurocholate, sulfated bile acids, sulfated hormones, N2, N2-dimethylguanosine, N1-methyladenosine, pseudouridine, allantoin, 10-hepatodecenoic acid, N6-carbamoylthreonyladenosine | GPEs and xenobiotics (paraxanthine and caffeine) |
| Gaddnas et al. [47] (2009) d | Serum | 33:11 | Adults | Radioimmunological assays | Χ2 test, FET, MW-U, ROC | Procollagen type III aminoterminal propeptides and crosslinked telopeptides of type I collagen | - |
| Chien et al. [98] (2005) d | Serum | 44:19 | Adults | Enzymatic and turbidimetric methods using kits | MW-U, FET, multivariate LR, KMSA | - | HDCH and Apo-A1 (on days 1 to 4) |
| Vermont et al. [99] (2005) | Serum | 46:10 | Paediatrics | Enzymatic colorimetric assay | Non-parametric test, FET | - | Total cholesterol |
| Drobnik et al. [48] (2003) d | Plasma | 63:39 | Adults | LC-MS/MS (targeted) | MW-U test, ROC curves | Cer-SM ratios—on day 4 and day 11. Cer-SM to LPC-PC ratios—on day 1, 4 and 11 | LPC-PC ratios—on day 4 and day 11 |
| van Leeuwen et al. [53] (2003) | Plasma | 10:7 | Adults | DGU and enzymatic methods | ANOVA, t-test, MLRA | No significant differences in lipoproteins | - |
| Sprung et al. [100] (1991) | Plasma | 10:5 | Adults | Postcolumn IEC with ninhydrin detection | Spearman’s correlations, t-test | AAAs (tyrosine, phenylalanine), SAAs (taurine, methionine, and cysteine), ammonia and GABA | - |
| Roth et al. [81] (1982) | Plasma | 7:7 | Adults | Automatic amino acid analyser (Liquimat III) | Student’s t-test | Muscle valine and leucine and plasma levels of glucose, glucagon, phosphoserine, cysteine, valine, phenylalanine and 3-methylhistidine | Muscle glutamine, proline and lysine |
3.1.2. Amino Acid and Nucleotide Metabolism
3.1.3. Urea Cycle
3.1.4. Lipoprotein and Lipid Metabolism
3.1.5. Steroid Metabolism
3.2. Metabolomic Studies of Organ Dysfunction
4. Use of Metabolomics to Identify Response to Treatment
5. Use of Metabolomics to Understand Changes to the Microbiome in Sepsis
6. Use of Metabolomics to Identify Novel Sub-Phenotypes
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perner, A.; Gordon, A.C.; Angus, D.C.; Lamontagne, F.; Machado, F.; Russell, J.A.; Timsit, J.F.; Marshall, J.C.; Myburgh, J.; Shankar-Hari, M.; et al. The intensive care medicine research agenda on septic shock. Intensive Care Med. 2017, 43, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L. The Clinical Challenge of Sepsis Identification and Monitoring. PLoS Med. 2016, 13, e1002022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.M.; Artigas, A.; Phillips, G.S.; Rhodes, A.; Beale, R.; Osborn, T.; Vincent, J.-L.; Townsend, S.; Lemeshow, S.; Dellinger, R.P. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: A prospective cohort study. Lancet Infect. Dis. 2012, 12, 919–924. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Namendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.N.; Vincent, J.L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Perner, A.; Gordon, A.C.; De Backer, D.; Dimopoulos, G.; Russell, J.A.; Lipman, J.; Jensen, J.U.; Myburgh, J.; Singer, M.; Bellomo, R.; et al. Sepsis: Frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016, 42, 1958–1969. [Google Scholar] [CrossRef]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [Green Version]
- Assfalg, M.; Bertini, I.; Colangiuli, D.; Luchinat, C.; Schäfer, H.; Schütz, B.; Spraul, M. Evidence of different metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nepi, S.; Saccenti, E.; Schäfer, H.; Schütz, B.; Spraul, M.; Tenori, L. Individual Human Phenotypes in Metabolic Space and Time. J. Proteome Res. 2009, 8, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabonomics: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Schlotterbeck, G.; Ross, A.; Dieterle, F.; Senn, H. Metabolic profiling technologies for biomarker discovery in biomedicine and drug development. Pharmacogenomics 2006, 7, 1055–1075. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Teng, S.; Li, K. Prediction of sepsis mortality using metabolite biomarkers in the blood: A meta-analysis of death-related pathways and prospective validation. BMC Med. 2020, 18, 83. [Google Scholar] [CrossRef] [Green Version]
- Trongtrakul, K.; Thonusin, C.; Pothirat, C.; Chattipakorn, S.C.; Chattipakorn, N. Past Experiences for Future Applications of Metabolomics in Critically Ill Patients with Sepsis and Septic Shocks. Metabolites 2021, 12, 1. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Liu, H.; Zhang, T.; Jiang, Y.; Xing, H.; Zhang, A.-h. Potential urine biomarkers from a high throughput metabolomics study of severe sepsis in a large Asian cohort. RSC Adv. 2015, 5, 102204–102209. [Google Scholar] [CrossRef]
- Neugebauer, S.; Giamarellos-Bourboulis, E.J.; Pelekanou, A.; Marioli, A.; Baziaka, F.; Tsangaris, I.; Bauer, M.; Kiehntopf, M. Metabolite Profiles in Sepsis: Developing Prognostic Tools Based on the Type of Infection. Crit. Care Med. 2016, 44, 1649–1662. [Google Scholar] [CrossRef]
- Lin, S.H.; Fan, J.; Zhu, J.; Zhao, Y.S.; Wang, C.J.; Zhang, M.; Xu, F. Exploring plasma metabolomic changes in sepsis: A clinical matching study based on gas chromatography-mass spectrometry. Ann. Transl. Med. 2020, 8, 1568. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Caboni, P.; Corsello, G.; Stronati, M.; Gazzolo, D.; Noto, A.; Lussu, M.; Dessì, A.; Giuffrè, M.; Lacerenza, S.; et al. Urinary 1H-NMR and GC-MS metabolomics predicts early and late onset neonatal sepsis. Early Hum. Dev. 2014, 90, S78–S83. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Vogel, H.J.; Wong, H.R.; Winston, B.W. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am. J. Respir Crit. Care Med. 2013, 187, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauslys, A.; Phelan, M.M.; Broughton, C.; Baines, P.B.; Jennings, R.; Siner, S.; Paulus, S.C.; Carrol, E.D. Title NMR-based metabolic profiling provides diagnostic and prognostic information in critically ill children with suspected infection. Sci. Rep. 2020, 10, 20198. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Duggan, G.E.; Winston, B.W.; Doig, C.; Kubes, P.; Vogel, H.J.; Alberta Sepsis, N. Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit. Care Med. 2014, 42, 1140–1149. [Google Scholar] [CrossRef]
- Kauppi, A.M.; Edin, A.; Ziegler, I.; Molling, P.; Sjostedt, A.; Gylfe, A.; Stralin, K.; Johansson, A. Metabolites in Blood for Prediction of Bacteremic Sepsis in the Emergency Room. PLoS ONE 2016, 11, e0147670. [Google Scholar] [CrossRef]
- Jaurila, H.; Koivukangas, V.; Koskela, M.; Gaddnas, F.; Myllymaa, S.; Kullaa, A.; Salo, T.; Ala-Kokko, T.I. 1H NMR Based Metabolomics in Human Sepsis and Healthy Serum. Metabolites 2020, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Beloborodova, N.; Pautova, A.; Sergeev, A.; Fedotcheva, N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites 2019, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Otsubo, C.; Bharathi, S.; Uppala, R.; Ilkayeva, O.R.; Wang, D.; McHugh, K.; Zou, Y.; Wang, J.; Alcorn, J.F.; Zuo, Y.Y.; et al. Long-chain Acylcarnitines Reduce Lung Function by Inhibiting Pulmonary Surfactant. J. Biol. Chem. 2015, 290, 23897–23904. [Google Scholar] [CrossRef] [Green Version]
- Schmerler, D.; Neugebauer, S.; Ludewig, K.; Bremer-Streck, S.; Brunkhorst, F.M.; Kiehntopf, M. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J. Lipid Res. 2012, 53, 1369–1375. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, B.; Tam, P.; Jenne, C.N.; Leger, C.; Wong, J.; Winston, B.W.; Doig, C.; Kubes, P.; Vogel, H.J.; Alberta Sepsis, N. Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit. Care 2015, 19, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennaro, T.S.; Puskarich, M.A.; McCann, M.R.; Gillies, C.E.; Pai, M.P.; Karnovsky, A.; Evans, C.R.; Jones, A.E.; Stringer, K.A. Using l-Carnitine as a Pharmacologic Probe of the Interpatient and Metabolic Variability of Sepsis. Pharmacotherapy 2020, 40, 913–923. [Google Scholar] [CrossRef]
- Su, L.; Huang, Y.; Zhu, Y.; Xia, L.; Wang, R.; Xiao, K.; Wang, H.; Yan, P.; Wen, B.; Cao, L.; et al. Discrimination of sepsis stage metabolic profiles with an LC/MS-MS-based metabolomics approach. BMJ Open Respir. Res. 2014, 1, e000056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szelig, L.; Kun, S.; Woth, G.; Molnar, G.A.; Zrinyi, Z.; Katai, E.; Lantos, J.; Wittmann, I.; Bogar, L.; Miseta, A.; et al. Time courses of changes of para-, meta-, and ortho-tyrosine in septic patients: A pilot study. Redox Rep. 2016, 21, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Mierzchala-Pasierb, M.; Lipinska-Gediga, M.; Fleszar, M.G.; Lewandowski, L.; Serek, P.; Placzkowska, S.; Krzystek-Korpacka, M. An analysis of urine and serum amino acids in critically ill patients upon admission by means of targeted LC-MS/MS: A preliminary study. Sci. Rep. 2021, 11, 19977. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef] [Green Version]
- Reisinger, A.C.; Posch, F.; Hackl, G.; Marsche, G.; Sourij, H.; Bourgeois, B.; Eller, K.; Madl, T.; Eller, P. Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients. Nutrients 2021, 13, 3106. [Google Scholar] [CrossRef]
- Antcliffe, D.; Jimenez, B.; Veselkov, K.; Holmes, E.; Gordon, A.C. Metabolic Profiling in Patients with Pneumonia on Intensive Care. EBioMedicine 2017, 18, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Li, G.B.; Hu, H.R.; Pan, W.F.; Li, B.; Ou, Z.Y.; Liang, H.Y.; Li, C. Plasma Metabolic Profiling of Pediatric Sepsis in a Chinese Cohort. Front. Cell Dev. Biol. 2021, 9, 643979. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Ferreira, B.L.; Tashima, A.K.; Brunialti, M.K.C.; Torquato, R.J.S.; Bafi, A.; Assuncao, M.; Azevedo, L.C.P.; Salomao, R. Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin. Proteom. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Tashima, A.K.; Brunialti, M.K.C.; Ferreira, E.R.; Torquato, R.J.S.; Mortara, R.A.; Machado, F.R.; Assuncao, M.; Rigato, O.; Salomao, R. Proteomic study revealed cellular assembly and lipid metabolism dysregulation in sepsis secondary to community-acquired pneumonia. Sci. Rep. 2017, 7, 15606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecatti, G.C.; Fernandes Messias, M.C.; Sant’Anna Paiola, R.M.; Figueiredo Angolini, C.F.; da Silva Cunha, I.B.; Eberlin, M.N.; de Oliveira Carvalho, P. Lipidomic Profiling of Plasma and Erythrocytes from Septic Patients Reveals Potential Biomarker Candidates. Biomark Insights 2018, 13, 1177271918765137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, K.A.; Serkova, N.J.; Karnovsky, A.; Guire, K.; Paine, R., 3rd; Standiford, T.J. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma (1)H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L4–L11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddnas, F.; Koskela, M.; Koivukangas, V.; Risteli, J.; Oikarinen, A.; Laurila, J.; Saarnio, J.; Ala-Kokko, T. Markers of collagen synthesis and degradation are increased in serum in severe sepsis: A longitudinal study of 44 patients. Crit. Care 2009, 13, R53. [Google Scholar] [CrossRef] [Green Version]
- Drobnik, W.; Liebisch, G.; Audebert, F.X.; Frohlich, D.; Gluck, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.S.; Nierhaus, A.; Rosler, G.; Lezius, S.; Harlandt, O.; Schwedhelm, E.; Boger, R.H.; Kluge, S. Symmetrical (SDMA) and asymmetrical dimethylarginine (ADMA) in sepsis: High plasma levels as combined risk markers for sepsis survival. Crit. Care 2018, 22, 216. [Google Scholar] [CrossRef] [Green Version]
- Ploder, M.; Spittler, A.; Schroecksnadel, K.; Neurauter, G.; Pelinka, L.E.; Roth, E.; Fuchs, D. Accelerated Tryptophan Degradation in Trauma and Sepsis Patients is Related to Pro-inflammatory Response and to the Diminished in vitro Response of Monocytes. Pteridines 2009, 20, 54–61. [Google Scholar] [CrossRef]
- Sell, D.R.; Strauch, C.M.; Shen, W.; Monnier, V.M. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: Effects of diabetes, renal failure and sepsis. BioChem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Poeze, M.; Ramsay, G.; Deutz, N.E. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am. J. Clin. Nutr. 2009, 89, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, H.J.; Heezius, E.C.; Dallinga, G.M.; van Strijp, J.A.; Verhoef, J.; van Kessel, K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003, 31, 1359–1366. [Google Scholar] [CrossRef]
- Tanaka, S.; Couret, D.; Tran-Dinh, A.; Duranteau, J.; Montravers, P.; Schwendeman, A.; Meilhac, O. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 2020, 24, 134. [Google Scholar] [CrossRef] [Green Version]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in infectious diseases and sepsis. Handb Exp. Pharmacol. 2015, 224, 483–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, G.; Leeuwenburgh, C.; Brusko, T.; Moldawer, L.; Reddy, S.T.; Guirgis, F.W. Lipid and Lipoprotein Dysregulation in Sepsis: Clinical and Mechanistic Insights into Chronic Critical Illness. J. Clin. Med. 2021, 10, 1693. [Google Scholar] [CrossRef]
- Olofsson, S.O.; Boren, J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005, 258, 395–410. [Google Scholar] [CrossRef]
- Cirstea, M.; Walley, K.R.; Russell, J.A.; Brunham, L.R.; Genga, K.R.; Boyd, J.H. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J. Crit. Care 2017, 38, 289–294. [Google Scholar] [CrossRef]
- Berbee, J.F.; Havekes, L.M.; Rensen, P.C. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 2005, 11, 97–103. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Ahn, W.G.; Jung, J.S.; Kwon, H.Y.; Song, D.K. Alteration of Lysophosphatidylcholine-Related Metabolic Parameters in the Plasma of Mice with Experimental Sepsis. Inflammation 2017, 40, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Pelegrin, P. Cardiolipin in Immune Signaling and Cell Death. Trends Cell Biol. 2020, 30, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Synan, M.J.; Li, J.; Xiong, S.; Manni, M.L.; Liu, Y.; Chen, B.B.; Zhao, Y.; Shiva, S.; Tyurina, Y.Y.; et al. LPS impairs oxygen utilization in epithelia by triggering degradation of the mitochondrial enzyme Alcat1. J. Cell Sci. 2016, 129, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albeituni, S.; Stiban, J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. In The Role of Bioactive Lipids in Cancer, Inflammation and Related Diseases; Honn, K.V., Zeldin, D.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 169–191. [Google Scholar]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef] [Green Version]
- Yoshikai, Y. Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr. Opin. Infect. Dis. 2001, 14, 257–263. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of basic metabolic pathways in microbial sepsis: Therapeutic targets at last? EMBO Mol. Med. 2018, 10, e8712. [Google Scholar] [CrossRef]
- Zazula, R.; Moravec, M.; Pehal, F.; Nejtek, T.; Protus, M.; Muller, M. Myristic Acid Serum Levels and Their Significance for Diagnosis of Systemic Inflammatory Response, Sepsis, and Bacteraemia. J. Pers. Med. 2021, 11, 306. [Google Scholar] [CrossRef]
- Langley, R.J.; Tsalik, E.L.; van Velkinburgh, J.C.; Glickman, S.W.; Rice, B.J.; Wang, C.; Chen, B.; Carin, L.; Suarez, A.; Mohney, R.P.; et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013, 5, 195ra195. [Google Scholar] [CrossRef] [Green Version]
- Seymour, C.W.; Yende, S.; Scott, M.J.; Pribis, J.; Mohney, R.P.; Bell, L.N.; Chen, Y.F.; Zuckerbraun, B.S.; Bigbee, W.L.; Yealy, D.M.; et al. Metabolomics in pneumonia and sepsis: An analysis of the GenIMS cohort study. Intensive Care Med. 2013, 39, 1423–1434. [Google Scholar] [CrossRef]
- Rogers, A.J.; McGeachie, M.; Baron, R.M.; Gazourian, L.; Haspel, J.A.; Nakahira, K.; Fredenburgh, L.E.; Hunninghake, G.M.; Raby, B.A.; Matthay, M.A.; et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS ONE 2014, 9, e87538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yin, P.; Amathieu, R.; Savarin, P.; Xu, G. Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal. Bioanal. Chem. 2016, 408, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef]
- Liu, Z.; Triba, M.N.; Amathieu, R.; Lin, X.; Bouchemal, N.; Hantz, E.; Le Moyec, L.; Savarin, P. Nuclear magnetic resonance-based serum metabolomic analysis reveals different disease evolution profiles between septic shock survivors and non-survivors. Crit. Care 2019, 23, 169. [Google Scholar] [CrossRef] [Green Version]
- Beloborodova, N.V.; Sarshor, Y.N.; Bedova, A.Y.; Chernevskaya, E.A.; Pautova, A.K. Involvement of Aromatic Metabolites in the Pathogenesis of Septic Shock. Shock 2018, 50, 273–279. [Google Scholar] [CrossRef]
- Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [CrossRef] [Green Version]
- Van den Berghe, G.; Wilmer, A.; Hermans, G.; Meersseman, W.; Wouters, P.J.; Milants, I.; Van Wijngaerden, E.; Bobbaers, H.; Bouillon, R. Intensive Insulin Therapy in the Medical ICU. N. Engl. J. Med. 2006, 354, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Garcia-Simon, M.; Morales, J.M.; Modesto-Alapont, V.; Gonzalez-Marrachelli, V.; Vento-Rehues, R.; Jorda-Minana, A.; Blanquer-Olivas, J.; Monleon, D. Prognosis Biomarkers of Severe Sepsis and Septic Shock by 1H NMR Urine Metabolomics in the Intensive Care Unit. PLoS ONE 2015, 10, e0140993. [Google Scholar] [CrossRef] [Green Version]
- Roth, E.; Funovics, J.; Mühlbacher, F.; Schemper, M.; Mauritz, W.; Sporn, P.; Fritsch, A. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin. Nutr. 1982, 1, 25–41. [Google Scholar] [CrossRef]
- Spustová, V.; Dzúrik, R.; Geryková, M. Hippurate participation in the inhibition of glucose utilization in renal failure. Czech. Med. 1987, 10, 79–89. [Google Scholar] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Kamisoglu, K.; Haimovich, B.; Calvano, S.E.; Coyle, S.M.; Corbett, S.A.; Langley, R.J.; Kingsmore, S.F.; Androulakis, I.P. Human metabolic response to systemic inflammation: Assessment of the concordance between experimental endotoxemia and clinical cases of sepsis/SIRS. Crit Care 2015, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, D.; Hu, Q.; Wu, J.; Wang, Y.; Feng, J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br. J. Nutr. 2015, 113, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Deen, C.P.J.; Veen, A.V.; Gomes-Neto, A.W.; Geleijnse, J.M.; Berg, K.; Heiner-Fokkema, M.R.; Kema, I.P.; Bakker, S.J.L. Urinary Excretion of N(1)-Methylnicotinamide and N(1)-Methyl-2-Pyridone-5-Carboxamide and Mortality in Kidney Transplant Recipients. Nutrients 2020, 12, 2059. [Google Scholar] [CrossRef]
- Slominska, E.M.; Smolenski, R.T.; Szolkiewicz, M.; Leaver, N.; Rutkowski, B.; Simmonds, H.A.; Swierczynski, J. Accumulation of plasma N-methyl-2-pyridone-5-carboxamide in patients with chronic renal failure. Mol. Cell Biochem. 2002, 231, 83–88. [Google Scholar] [CrossRef]
- Jones, T.N.; Janani, L.; Gordon, A.C.; Al-Beidh, F.; Antcliffe, D.B. A Novel Role for Cytochrome P450 Epoxygenase Metabolites in Septic Shock. Crit. Care Explor. 2022, 4, e0622. [Google Scholar] [CrossRef]
- Khaliq, W.; Grossmann, P.; Neugebauer, S.; Kleyman, A.; Domizi, R.; Calcinaro, S.; Brealey, D.; Graler, M.; Kiehntopf, M.; Schauble, S.; et al. Lipid metabolic signatures deviate in sepsis survivors compared to non-survivors. Comput. Struct. Biotechnol. J. 2020, 18, 3678–3691. [Google Scholar] [CrossRef]
- Evans, C.R.; Karnovsky, A.; Puskarich, M.A.; Michailidis, G.; Jones, A.E.; Stringer, K.A. Untargeted Metabolomics Differentiates l-Carnitine Treated Septic Shock 1-Year Survivors and Nonsurvivors. J. Proteome Res. 2019, 18, 2004–2011. [Google Scholar] [CrossRef]
- Chung, K.P.; Chen, G.Y.; Chuang, T.Y.; Huang, Y.T.; Chang, H.T.; Chen, Y.F.; Liu, W.L.; Chen, Y.J.; Hsu, C.L.; Huang, M.T.; et al. Increased Plasma Acetylcarnitine in Sepsis Is Associated with Multiple Organ Dysfunction and Mortality: A Multicenter Cohort Study. Crit. Care Med. 2019, 47, 210–218. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, J.Y.; Chen, W.S.; Liu, M.H.; Cheng, C.W.; Cheng, M.L.; Wang, C.H. Phenylalanine- and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int. J. Infect. Dis. 2019, 85, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Cambiaghi, A.; Diaz, R.; Martinez, J.B.; Odena, A.; Brunelli, L.; Caironi, P.; Masson, S.; Baselli, G.; Ristagno, G.; Gattinoni, L.; et al. An Innovative Approach for The Integration of Proteomics and Metabolomics Data In Severe Septic Shock Patients Stratified for Mortality. Sci. Rep. 2018, 8, 6681. [Google Scholar] [CrossRef] [Green Version]
- Dalli, J.; Colas, R.A.; Quintana, C.; Barragan-Bradford, D.; Hurwitz, S.; Levy, B.D.; Choi, A.M.; Serhan, C.N.; Baron, R.M. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations with Survival and Clinical Outcomes. Crit. Care Med. 2017, 45, 58–68. [Google Scholar] [CrossRef]
- Wang, L.; Ko, E.R.; Gilchrist, J.J.; Pittman, K.J.; Rautanen, A.; Pirinen, M.; Thompson, J.W.; Dubois, L.G.; Langley, R.J.; Jaslow, S.L.; et al. Human genetic and metabolite variation reveals that methylthioadenosine is a prognostic biomarker and an inflammatory regulator in sepsis. Sci. Adv. 2017, 3, e1602096. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, M.; Cambiaghi, A.; Brunelli, L.; Giordano, S.; Caironi, P.; Guatteri, L.; Raimondi, F.; Gattinoni, L.; Latini, R.; Masson, S.; et al. Mortality prediction in patients with severe septic shock: A pilot study using a target metabolomics approach. Sci. Rep. 2016, 6, 20391. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.S.; Park, B.H.; Jung, W.J.; Lee, I.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S.; et al. Prognostic Implications of Serum Lipid Metabolism over Time during Sepsis. Biomed. Res. Int. 2015, 2015, 789298. [Google Scholar] [CrossRef] [Green Version]
- Chien, J.Y.; Jerng, J.S.; Yu, C.J.; Yang, P.C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005, 33, 1688–1693. [Google Scholar] [CrossRef]
- Vermont, C.L.; den Brinker, M.; Kakeci, N.; de Kleijn, E.D.; de Rijke, Y.B.; Joosten, K.F.; de Groot, R.; Hazelzet, J.A. Serum lipids and disease severity in children with severe meningococcal sepsis. Crit. Care Med. 2005, 33, 1610–1615. [Google Scholar] [CrossRef]
- Sprung, C.L.; Cerra, F.B.; Freund, H.R.; Schein, R.M.H.; Konstantinides, F.N.; Marcial, E.H.; Pena, M. Amino acid alterations and encephalopathy in the sepsis syndrome. Crit. Care Med. 1991, 19, 753–757. [Google Scholar] [CrossRef]
- Englert, J.A.; Rogers, A.J. Metabolism, Metabolomics, and Nutritional Support of Patients with Sepsis. Clin. Chest Med. 2016, 37, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Ploder, M.; Neurauter, G.; Spittler, A.; Schroecksnadel, K.; Roth, E.; Fuchs, D. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 2008, 35, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Changsirivathanathamrong, D.; Wang, Y.; Rajbhandari, D.; Maghzal, G.J.; Mak, W.M.; Woolfe, C.; Duflou, J.; Gebski, V.; dos Remedios, C.G.; Celermajer, D.S.; et al. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit. Care Med. 2011, 39, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Pandhare, J.; Liu, Y. The metabolism of proline as microenvironmental stress substrate. J. Nutr. 2008, 138, 2008s–2015s. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline Precursors and Collagen Synthesis: Biochemical Challenges of Nutrient Supplementation and Wound Healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chiarla, C.; Giovannini, I.; Siegel, J.H. High phosphoserine in sepsis: Panel of clinical and plasma amino acid correlations. Springerplus 2014, 3, 279. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.W.; Boldogh, I.; Popov, V.L.; Saini, S.S.; Chopra, A.K. Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Lab. Invest. 1998, 78, 523–534. [Google Scholar]
- Long, C.L.; Birkhahn, R.H.; Geiger, J.W.; Betts, J.E.; Schiller, W.R.; Blakemore, W.S. Urinary excretion of 3-methylhistidine: An assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism 1981, 30, 765–776. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. MicroBiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef] [Green Version]
- Levels, J.H.; Abraham, P.R.; van den Ende, A.; van Deventer, S.J. Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 2001, 69, 2821–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis—Risk factor and therapeutic approach. Front. Pharmacol. 2015, 6, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dong, J.B.; Wu, M.P. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur. J. Pharmacol. 2008, 590, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.A.; García-Palmieri, M.R. Cholesterol, Triglycerides, and Associated Lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworth Publishers: Boston, MA, USA, 1990. [Google Scholar]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, A.; Böttcher, A.; Orsó, E.; Kapinsky, M.; Nagy, P.; Bodnár, A.; Spreitzer, I.; Liebisch, G.; Drobnik, W.; Gempel, K.; et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 2001, 31, 3153–3164. [Google Scholar] [CrossRef]
- Opal, S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [CrossRef]
- Klein, M.E.; Rieckmann, M.; Sedding, D.; Hause, G.; Meister, A.; Mader, K.; Lucas, H. Towards the Development of Long Circulating Phosphatidylserine (PS)- and Phosphatidylglycerol (PG)-Enriched Anti-Inflammatory Liposomes: Is PEGylation Effective? Pharmaceutics 2021, 13, 282. [Google Scholar] [CrossRef]
- Frasch, S.C.; Bratton, D.L. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. 2012, 51, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Misheva, M.; Kotzamanis, K.; Davies, L.C.; Tyrrell, V.J.; Rodrigues, P.R.S.; Benavides, G.A.; Hinz, C.; Murphy, R.C.; Kennedy, P.; Taylor, P.R.; et al. Oxylipin metabolism is controlled by mitochondrial beta-oxidation during bacterial inflammation. Nat. Commun. 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. BioChem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bellien, J.; Joannides, R. Epoxyeicosatrienoic Acid Pathway in Human Health and Diseases. J. Cardiovasc. Pharmacol. 2013, 61, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Gragasin, F.S.; Wu, X.; Wang, S.; McMurtry, S.; Kim, D.H.; Platonov, M.; Koshal, A.; Hashimoto, K.; Campbell, W.B.; et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation 2003, 107, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Fleming, I.; Rueben, A.; Popp, R.; Fisslthaler, B.; Schrodt, S.; Sander, A.; Haendeler, J.; Falck, J.R.; Morisseau, C.; Hammock, B.D.; et al. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arter. Thromb Vasc. Biol. 2007, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Sun, D.; Jacobson, A.; Carroll, M.A.; Falck, J.R.; Kaley, G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ. Res. 2005, 96, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Black, J.L.; Armour, C.L.; Vincenc, K.S.; Johnson, P.R. A comparison of the contractile activity of PGD2 and PGF2 alpha on human isolated bronchus. Prostaglandins 1986, 32, 25–31. [Google Scholar] [CrossRef]
- Kang, K.H.; Shim, J.J.; Banerjee, M.; Newman, J.H. PGF2 alpha causes bronchoconstriction and pulmonary vasoconstriction via thromboxane receptors in rat lung. Korean J. Intern. Med. 1996, 11, 74–81. [Google Scholar] [CrossRef]
- Samuelsson, B. Role of basic science in the development of new medicines: Examples from the eicosanoid field. J. Biol. Chem. 2012, 287, 10070–10080. [Google Scholar] [CrossRef] [Green Version]
- Tam, V.C.; Quehenberger, O.; Oshansky, C.M.; Suen, R.; Armando, A.M.; Treuting, P.M.; Thomas, P.G.; Dennis, E.A.; Aderem, A. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 2013, 154, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients with Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; Leligdowicz, A.; Contrepois, K.; Jauregui, A.; Vessel, K.; Deiss, T.J.; Belzer, A.; Liu, T.; Lippi, M.; Ke, S.; et al. Plasma Metabolites in Early Sepsis Identify Distinct Clusters Defined by Plasma Lipids. Crit. Care Explor. 2021, 3, e0478. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014, 55, 2401–2407. [Google Scholar] [CrossRef] [Green Version]
- Jennaro, T.S.; Viglianti, E.M.; Ingraham, N.E.; Jones, A.E.; Stringer, K.A.; Puskarich, M.A. Serum Levels of Acylcarnitines and Amino Acids Are Associated with Liberation from Organ Support in Patients with Septic Shock. J. Clin. Med. 2022, 11, 627. [Google Scholar] [CrossRef]
- Giacominelli-Stuffler, R.; Angelucci, C.B.; Liberatoscioli, L.; Cortese, C.; Dainese, E.; Maccarrone, M. Relationships between paraoxon and 2-coumaranone hydrolytic activities in sera genotyped for PON1 Q192R polymorphism. Clin. BioChem. 2009, 42, 1512–1516. [Google Scholar] [CrossRef]
- Chubarov, A.S. Homocysteine Thiolactone: Biology and Chemistry. Encyclopedia 2021, 1, 37. [Google Scholar] [CrossRef]
- Stebbing, J.; Sharma, A.; North, B.; Athersuch, T.J.; Zebrowski, A.; Pchejetski, D.; Coombes, R.C.; Nicholson, J.K.; Keun, H.C. A metabolic phenotyping approach to understanding relationships between metabolic syndrome and breast tumour responses to chemotherapy. Ann. Oncol. 2012, 23, 860–866. [Google Scholar] [CrossRef]
- Cambiaghi, A.; Pinto, B.B.; Brunelli, L.; Falcetta, F.; Aletti, F.; Bendjelid, K.; Pastorelli, R.; Ferrario, M. Characterization of a metabolomic profile associated with responsiveness to therapy in the acute phase of septic shock. Sci. Rep. 2017, 7, 9748. [Google Scholar] [CrossRef] [Green Version]
- Puskarich, M.A.; Finkel, M.A.; Karnovsky, A.; Jones, A.E.; Trexel, J.; Harris, B.N.; Stringer, K.A. Pharmacometabolomics of l-carnitine treatment response phenotypes in patients with septic shock. Ann. Am. Thorac. Soc. 2015, 12, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puskarich, M.A.; Kline, J.A.; Krabill, V.; Claremont, H.; Jones, A.E. Preliminary safety and efficacy of L-carnitine infusion for the treatment of vasopressor-dependent septic shock: A randomized control trial. JPEN J. Parenter Enter. Nutr. 2014, 38, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gando, S.; Nanzaki, S.; Sasaki, S.; Aoi, K.; Kemmotsu, O. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit. Care Med. 1998, 26, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhao, J.; Liu, J.; Gou, W. Fibrinopeptide A Induces Expression of C-Reactive Protein via the ROS-ERK1/2/P38-NF-kappaB Signal Pathway in Vascular Smooth Muscle Cells. Cell Physiol. BioChem. 2018, 47, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.H.; Peng, Y.C.; Chien, H.Y.; Lu, M.L.; Du, H.I.; Wu, Y.L. Attenuation of LPS-induced lung inflammation by glucosamine in rats. Am. J. Respir Cell Mol. Biol. 2013, 49, 1110–1119. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Eyre, D.R.; Paz, M.A.; Gallop, P.M. Cross-linking in collagen and elastin. Annu. Rev. BioChem. 1984, 53, 717–748. [Google Scholar] [CrossRef]
- Seiler, N.; Knödgen, B.; Haegele, K. N-(3-aminopropyl)pyrrolidin-2-one, a product of spermidine catabolism in vivo. BioChem. J. 1982, 208, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Kullberg, R.F.J.; Wiersinga, W.J.; Haak, B.W. Gut microbiota and sepsis: From pathogenesis to novel treatments. Curr. Opin. Gastroenterol. 2021, 37, 578–585. [Google Scholar] [CrossRef]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef] [Green Version]
- Beloborodova, N.V.; Olenin, A.Y.; Pautova, A.K. Metabolomic findings in sepsis as a damage of host-microbial metabolism integration. J. Crit. Care 2018, 43, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Vladimirovna, B.N. Interaction of Host-Microbial Metabolism in Sepsis. In Sepsis; InTechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Fedotcheva, N.; Olenin, A.; Beloborodova, N. Influence of Microbial Metabolites on the Nonspecific Permeability of Mitochondrial Membranes under Conditions of Acidosis and Loading with Calcium and Iron Ions. Biomedicines 2021, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Wiersinga, W.J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2017, 2, 135–143. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Negoro, T.; Okayasu, H.; Sakagami, H.; Fujisawa, S. Inhibition of NO production by activated macrophages by phenolcarboxylic acid monomers and polymers with radical scavenging activity. Anticancer Res. 2003, 23, 1317–1323. [Google Scholar]
- Jankowski, J.; van der Giet, M.; Jankowski, V.; Schmidt, S.; Hemeier, M.; Mahn, B.; Giebing, G.; Tolle, M.; Luftmann, H.; Schluter, H.; et al. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J. Clin. Invest. 2003, 112, 256–264. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Burnham, K.L.; Davenport, E.E.; Radhakrishnan, J.; Humburg, P.; Gordon, A.C.; Hutton, P.; Svoren-Jabalera, E.; Garrard, C.; Hill, A.V.S.; Hinds, C.J.; et al. Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia. Am. J. Respir. Crit. Care Med. 2017, 196, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Davenport, E.E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Fjell, C.D.; Thair, S.; Hsu, J.L.; Walley, K.R.; Russell, J.A.; Boyd, J. Cytokines and signaling molecules predict clinical outcomes in sepsis. PLoS ONE 2013, 8, e79207. [Google Scholar] [CrossRef] [Green Version]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Wasyluk, W.; Zwolak, A. Metabolic Alterations in Sepsis. J. Clin. Med. 2021, 10, 2412. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Vutipongsatorn, K.; Jiménez, B.; Antcliffe, D.B. Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites 2022, 12, 376. https://doi.org/10.3390/metabo12050376
Hussain H, Vutipongsatorn K, Jiménez B, Antcliffe DB. Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites. 2022; 12(5):376. https://doi.org/10.3390/metabo12050376
Chicago/Turabian StyleHussain, Humma, Kritchai Vutipongsatorn, Beatriz Jiménez, and David B. Antcliffe. 2022. "Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response" Metabolites 12, no. 5: 376. https://doi.org/10.3390/metabo12050376
APA StyleHussain, H., Vutipongsatorn, K., Jiménez, B., & Antcliffe, D. B. (2022). Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites, 12(5), 376. https://doi.org/10.3390/metabo12050376







