Review on Multispectral Photoacoustic Analysis of Cancer: Thyroid and Breast
Abstract
:1. Introduction
2. Principles of Multispectral Photoacoustic Analysis
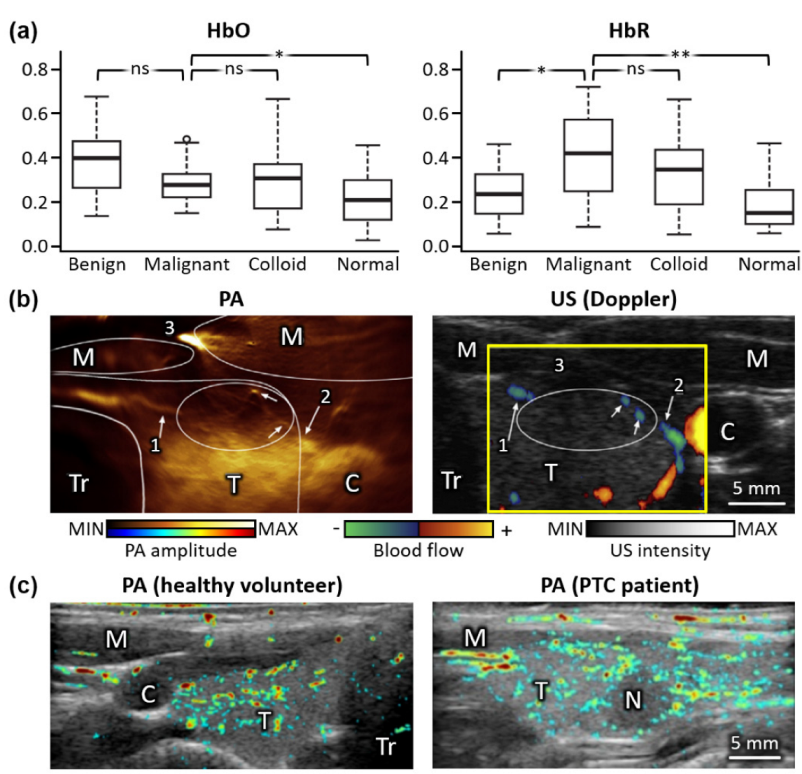
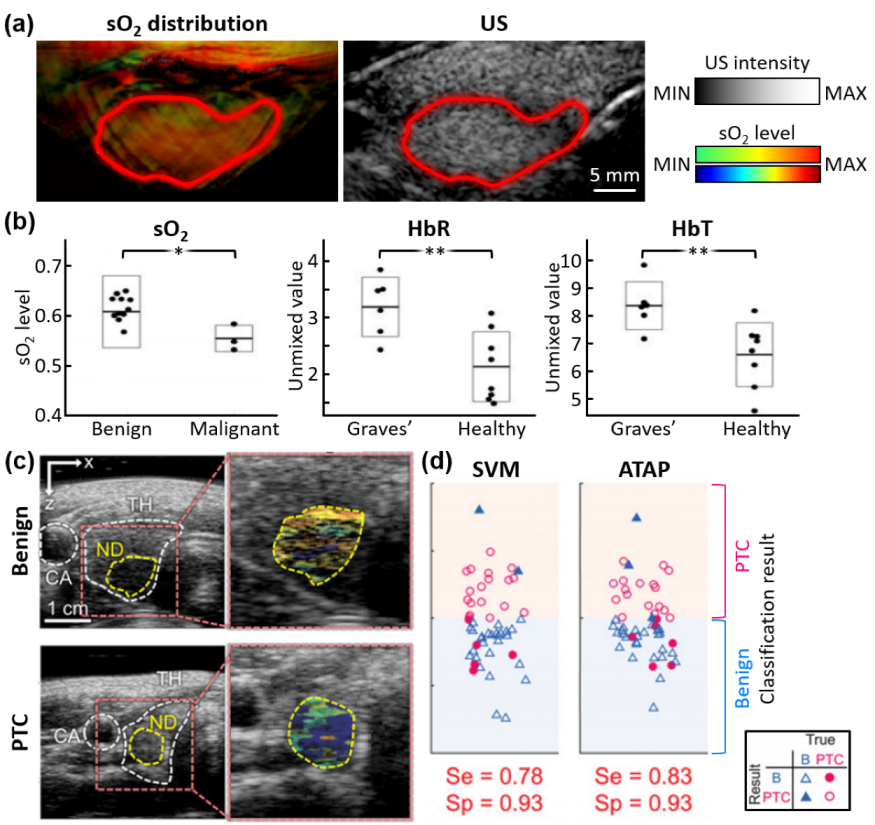
3. Multispectral Photoacoustic Analysis of Thyroid Gland
4. Multispectral Photoacoustic Analysis of Breast
5. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luker, G.D.; Luker, K.E. Optical Imaging: Current Applications and Future Directions. J. Nucl. Med. 2008, 49, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.W.; Kim, H.; Son, M.; Choi, J.; Kim, K.G.; Baek, J.H.; Park, Y.H.; An, J.; Choi, H.Y.; Ryu, S.Y.; et al. Intraoperative Label-Free Photoacoustic Histopathology of Clinical Specimens. Laser Photonics Rev. 2021, 15, 2100124. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, J.Y.; Choi, W.; Kim, C. High-Resolution Functional Photoacoustic Monitoring of Vascular Dynamics in Human Fingers. Photoacoustics 2021, 23, 100282. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Pramanik, M. Recent Advances in Photoacoustic Contrast Agents for In Vivo Imaging. Wires. Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- Wang, X.; Ku, G.; Wegiel, M.A.; Bornhop, D.J.; Stoica, G.; Wang, L.V. Noninvasive Photoacoustic Angiography of Animal Brains In Vivo with Near-Infrared Light and an Optical Contrast Agent. Opt. Lett. 2004, 29, 730–732. [Google Scholar] [CrossRef] [Green Version]
- Ku, G.; Wang, L.V. Deeply Penetrating Photoacoustic Tomography in Biological Tissues Enhanced with an Optical Contrast Agent. Opt. Lett. 2005, 30, 507–509. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-B.; Li, Y.; Tang, B.Z.; Yoon, J. Assembly Strategies of Organic-Based Imaging Agents for Fluorescence and Photoacoustic Bioimaging Applications. Chem. Soc. Rev. 2020, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Qu, E.; Dai, Z. Biocompatible Polypyrrole Nanoparticles as a Novel Organic Photoacoustic Contrast Agent for Deep Tissue Imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S. Gold Nanoparticles as a Contrast Agent for In Vivo Tumor Imaging with Photoacoustic Tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-W.; Liu, H.-L.; Li, M.-L.; Hsi, I.-W.; Fan, C.-T.; Huang, C.-Y.; Lu, Y.-J.; Hua, M.-Y.; Chou, H.-Y.; Liaw, J.-W. Magnetic Gold-Nanorod/PNIPAAmMA Nanoparticles for Dual Magnetic Resonance and Photoacoustic Imaging and Targeted Photothermal Therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Liu, M.; You, B.; Luo, G.; Chen, Y.; Liu, B.; Jiang, Z.; Chu, P.K.; Shao, J.; Yu, X.F. Biodegradable Bi2O2Se Quantum Dots for Photoacoustic Imaging-Guided Cancer Photothermal Therapy. Small 2020, 16, 1905208. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. Acs Appl. Mater. Interfaces 2020, 13, 306–311. [Google Scholar] [CrossRef]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1902279. [Google Scholar] [CrossRef]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep Tissue Photoacoustic Imaging of Nickel (II) Dithiolene-Containing Polymeric Nanoparticles in the Second Near-Infrared Window. Theranostics 2020, 10, 2509–2521. [Google Scholar] [CrossRef]
- Park, S.; Park, G.; Kim, J.; Choi, W.; Jeong, U.; Kim, C. Bi2Se3 Nanoplates for Contrast-Enhanced Photoacoustic Imaging at 1064 nm. Nanoscale 2018, 10, 20548–20558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Lyu, Y.; Zhang, L.; Xiong, Q.; Pramanik, M.; Pu, K. Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second Near-Infrared Window. Nano Lett. 2017, 17, 4964–4969. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; You, L.; Lan, L.; Lee, H.J.; Chaudhry, S.T.; Li, R.; Cheng, J.X.; Mei, J. Semiconducting Polymer Nanoparticles for Centimeters-Deep Photoacoustic Imaging in the Second Near-Infrared Window. Adv. Mater. 2017, 29, 1703403. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Molecular Fluorescence and Photoacoustic Imaging in the Second Near-Infrared Optical Window using Organic Contrast Agents. Adv. Biosyst. 2018, 2, 1700262. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Li, J.; Pu, K. Second Near-Infrared Absorbing Agents for Photoacoustic Imaging and Photothermal Therapy. Small Methods 2019, 3, 1900553. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging From Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Cho, S.-W.; Park, S.M.; Park, B.; Lee, T.G.; Kim, B.-M.; Kim, C.; Kim, J.; Lee, S.-W.; Kim, C.-S. High-Speed Photoacoustic Microscopy: A Review Dedicated on Light Sources. Photoacoustics 2021, 24, 100291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast Optical-Resolution Photoacoustic Microscopy using a 2-Axis Water-Proofing MEMS Scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-Resolution Photoacoustic Tomography of Resting-State Functional Connectivity in the Mouse Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Kim, J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Imaging of Small Animals In Vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J. Single-Impulse Panoramic Photoacoustic Computed Tomography of Small-Animal Whole-Body Dynamics at High Spatiotemporal Resolution. Nat. Biomed. 2017, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ermilov, S.; Su, R.; Conjusteau, A.; Anis, F.; Nadvoretskiy, V.; Anastasio, M.; Oraevsky, A. Three-Dimensional Optoacoustic and Laser-Induced Ultrasound Tomography System for Preclinical Research in Mice: Design and Phantom Validation. Ultrasonic Imaging 2016, 38, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, B.; Kim, T.; Jung, S.; Choi, W.; Ahn, J.; Yoon, D.; Kim, J.; Jeon, S.; Lee, D. Quadruple fusion imaging via transparent ultrasound transducer: Ultrasound, photoacoustic, optical coherence, and fluorescence imaging. Proc. Natl. Acad. Sci. USA 2021, 118, e1920879118. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, G.; Yu, Y.; Zhou, Y.; Carson, P.L.; Wang, X.; Liu, X. Real-Time Photoacoustic and Ultrasound Dual-Modality Imaging System Facilitated with Graphics Processing Unit and Code Parallel Optimization. J. Biomed. Opt. 2013, 18, 086001. [Google Scholar] [CrossRef] [Green Version]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous Transrectal Ultrasound and Photoacoustic Human Prostate Imaging. Sci. Transl. Med. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Needles, A.; Heinmiller, A.; Sun, J.; Theodoropoulos, C.; Bates, D.; Hirson, D.; Yin, M.; Foster, F.S. Development and initial application of a fully integrated photoacoustic micro-ultrasound system. IEEE T. Ultrason. Ferr. 2013, 60, 888–897. [Google Scholar] [CrossRef]
- Zafar, H.; Breathnach, A.; Subhash, H.M.; Leahy, M.J. Linear-Array-Based Photoacoustic Imaging of Human Microcirculation with a Range of High Frequency Transducer Probes. J. Biomed. Opt. 2015, 20, 051021. [Google Scholar] [CrossRef]
- Levi, J.; Sathirachinda, A.; Gambhir, S.S. A High-Affinity, High-Stability Photoacoustic Agent for Imaging Gastrin-Releasing Peptide Receptor in Prostate Cancer. Clin. Cancer Res. 2014, 20, 3721–3729. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Masthoff, M.; Claussen, J.; Ford, S.J.; Roll, W.; Burg, M.; Barth, P.J.; Heindel, W.; Schaefers, M.; Eisenblaetter, M. Multispectral Optoacoustic Tomography of the Human Breast: Characterisation of Healthy Tissue and Malignant Lesions using a Hybrid Ultrasound-Optoacoustic Approach. Eur. Radiol. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Park, E.-Y.; Park, B.; Choi, W.; Lee, K.J.; Kim, C. Towards Clinical Photoacoustic and Ultrasound Imaging: Probe Improvement and Real-Time Graphical User Interface. Exp. Biol. Med. 2020, 245, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Luís Deán-Ben, X.; Razansky, D. Adding Fifth Dimension to Optoacoustic Imaging: Volumetric Time-Resolved Spectrally Enriched Tomography. Light-Sci. Appl. 2014, 3, e137. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Choi, S.; Kim, C. Practical Review on Photoacoustic Computed Tomography using Curved Ultrasound Array Transducer. Biomed. Eng. Lett. 2022, 12, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Gateau, J.; Caballero, M.Á.A.; Dima, A.; Ntziachristos, V. Three-Dimensional Optoacoustic Tomography using a Conventional Ultrasound Linear Detector Array: Whole-Body Tomographic System for Small Animals. Med. Phys. 2013, 40, 013302. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Jankovic, L.; Wang, L.V. Performance Benchmarks of an Array-Based Hand-Held Photoacoustic Probe Adapted from a Clinical Ultrasound System for Non-Invasive Sentinel Lymph Node Imaging. Philos. Trans. R. Soc. A 2011, 369, 4644–4650. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Choi, W.; Kim, J.; Kim, C. Three-Dimensional Clinical Handheld Photoacoustic/Ultrasound Scanner. Photoacoustics 2020, 18, 100173. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, W.; Park, B.; Kim, C. A Deep Learning-Based Model that Reduces Speed of Sound Aberrations for Improved In Vivo Photoacoustic Imaging. IEEE T. Image Process 2021, 30, 8773–8784. [Google Scholar] [CrossRef]
- Park, E.-Y.; Lee, H.; Han, S.; Kim, C.; Kim, J. Photoacoustic Imaging Systems Based on Clinical Ultrasound Platform. Exp. Biol. Med. 2022, 247, 551–560. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical Photoacoustic Imaging Platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.H.; Lee, C.; Han, J.H.; Choi, W.; Kim, J.; Park, G.S.; Rhie, J.W.; Lee, J.H.; Kim, C. 3D Wide-Field Multispectral Photoacoustic Imaging of Human Melanomas In Vivo: A Pilot Study. J. Eur. Acad. Dermatol. 2020, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.H.; Park, B.; Seo, H.M.; Bang, C.H.; Park, G.S.; Park, Y.M.; Rhie, J.W.; Lee, J.H.; Kim, C. Multispectral Ex Vivo Photoacoustic Imaging of Cutaneous Melanoma for Better Selection of the Excision Margin. Br. J. Dermatol. 2018, 179, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In Vitro Photoacoustic Measurement of Hemoglobin Oxygen Saturation using a Single Pulsed Broadband Supercontinuum Laser Source. Appl. Opt. 2014, 53, 3884–3889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of Hemoglobin Oxygen Saturation Variations in Single Vessels In Vivo using Photoacoustic Microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Stoica, G.; Xie, X.; Ku, G.; Wang, L.V. Noninvasive Imaging of Hemoglobin Concentration and Oxygenation in the Rat Brain using High-Resolution Photoacoustic Tomography. J. Biomed. Opt. 2006, 11, 024015. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Morgounova, E.; Jiang, C.; Choi, J.-H.; Bischof, J.C.; Ashkenazi, S. In Vivo Photoacoustic Lifetime Imaging of Tumor Hypoxia in Small Animals. J. Biomed. Opt. 2013, 18, 076019. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jhang, D.-F.; Tsai, C.-H.; Chiang, N.-J.; Tsao, C.-H.; Chuang, C.-C.; Chen, L.-T.; Chang, W.-S.W.; Liao, L.-D. In Vivo Assessment of Hypoxia Levels in Pancreatic Tumors using a Dual-Modality Ultrasound/Photoacoustic Imaging System. Micromachines 2021, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-L.; Oh, J.-T.; Xie, X.; Ku, G.; Wang, W.; Li, C.; Lungu, G.; Stoica, G.; Wang, L.V. Simultaneous Molecular and Hypoxia Imaging of Brain Tumors In Vivo using Spectroscopic Photoacoustic Tomography. Proc. IEEE 2008, 96, 481–489. [Google Scholar]
- Steinberg, I.; Kim, J.; Schneider, M.K.; Hyun, D.; Zlitni, A.; Hopper, S.M.; Klap, T.; Sonn, G.A.; Dahl, J.J.; Kim, C. Superiorized Photo-Acoustic Non-NEgative Reconstruction (SPANNER) for Clinical Photoacoustic Imaging. IEEE Trans. Med. Imaging 2021, 40, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Olefir, I.; Tzoumas, S.; Restivo, C.; Mohajerani, P.; Xing, L.; Ntziachristos, V. Deep Learning-Based Spectral Unmixing for Optoacoustic Imaging of Tissue Oxygen Saturation. IEEE Trans. Med. Imaging 2020, 39, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Dal Maso, L.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Megwalu, U.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid, 2022; in press. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Sherman, S.I.; Tuttle, R.M. Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Taskforce. Thyroid 2006, 16, 109–142. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard BA, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E. Guidelines for the Management of Thyroid Cancer. Clin. Endocrinol. 2014, 81, 1–122. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.-K. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Chng, C.L.; Tan, H.C.; Too, C.W.; Lim, W.Y.; Chiam, P.P.S.; Zhu, L.; Nadkarni, N.V.; Lim, A.Y.Y. Diagnostic Performance of ATA, BTA and TIRADS Sonographic Patterns in the Prediction of Malignancy in Histologically Proven Thyroid Nodules. Singap. Med. J. 2018, 59, 578. [Google Scholar] [CrossRef] [Green Version]
- Dogra, V.S.; Chinni, B.K.; Valluru, K.S.; Moalem, J.; Giampoli, E.J.; Evans, K.; Rao, N.A. Preliminary Results of Ex Vivo Multispectral Photoacoustic Imaging in the Management of Thyroid Cancer. Am. J. Roentgenol. 2014, 202, W552–W558. [Google Scholar] [CrossRef]
- Dima, A.; Ntziachristos, V. In-Vivo Handheld Optoacoustic Tomography of the Human Thyroid. Photoacoustics 2016, 4, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhao, L.; He, X.; Su, N.; Zhao, C.; Tang, H.; Hong, T.; Li, W.; Yang, F.; Lin, L. Photoacoustic/Ultrasound Dual Imaging of Human Thyroid Cancers: An Initial Clinical Study. Biomed. Opt. Express 2017, 8, 3449–3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roll, W.; Markwardt, N.A.; Masthoff, M.; Helfen, A.; Claussen, J.; Eisenblätter, M.; Hasenbach, A.; Hermann, S.; Karlas, A.; Wildgruber, M. Multispectral Optoacoustic Tomography of Benign and Malignant Thyroid Disorders—A Pilot Study. J. Nucl. Med. 2019, 60, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Porter, P.L. Global Trends in Breast Cancer Incidence and Mortality. Salud Publica Mexico 2009, 51, s141–s146. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA-Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Pediconi, F.; Catalano, C.; Roselli, A.; Dominelli, V.; Cagioli, S.; Karatasiou, A.; Pronio, A.; Kirchin, M.A.; Passariello, R. The Challenge of Imaging Dense Breast Parenchyma: Is Magnetic Resonance Mammography the Technique of Choice? A Comparative Study with X-ray Mammography and Whole-Breast Ultrasound. Investig. Radiol. 2009, 44, 412–421. [Google Scholar] [CrossRef]
- Nazari, S.S.; Mukherjee, P. An Overview of Mammographic Density and Its Association with Breast Cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-l.; Zhou, J.-q.; Chen, Q.; Deng, Y.-c. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤ 2 cm) breast cancer. Medicine 2021, 100, e26531. [Google Scholar] [CrossRef]
- Murphy, K.J.; Brunberg, J.A.; Cohan, R.H. Adverse Reactions to Gadolinium Contrast Media: A Review of 36 Cases. Am. J. Roentgenol. 1996, 167, 847–849. [Google Scholar] [CrossRef] [Green Version]
- Burkett, B.J.; Hanemann, C.W. A Review of Supplemental Screening Ultrasound for Breast Cancer: Certain Populations of Women with Dense Breast Tissue May Benefit. Acad. Radiol. 2016, 23, 1604–1609. [Google Scholar] [CrossRef]
- Heijblom, M.; Steenbergen, W.; Manohar, S. Clinical Photoacoustic Breast Imaging: The Twente Experience. IEEE Pulse 2015, 6, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Kharine, A.; van Hespen, J.C.; Steenbergen, W.; van Leeuwen, T.G. The Twente Photoacoustic Mammoscope: System Overview and Performance. Phys. Med. Biol. 2005, 50, 2543. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wang, W.; Luo, D.; Chitgupi, U.; Geng, J.; Zhou, Y.; Wang, L.; Lovell, J.F.; Xia, J. Deep Tissue Photoacoustic Computed Tomography with a Fast and Compact Laser System. Biomed. Opt. Express 2017, 8, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, R.A.; Kuzmiak, C.M.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Steed, D. Dedicated 3D Photoacoustic Breast Imaging. Med. Phys. 2013, 40, 113301. [Google Scholar] [CrossRef] [Green Version]
- Schoustra, S.M.; Piras, D.; Huijink, R.; Op’t Root, T.J.; Alink, L.; Kobold, W.M.F.; Steenbergen, W.; Manohar, S. Twente Photoacoustic Mammoscope 2: System Overview and Three-Dimensional Vascular Network Images in Healthy Breasts. J. Biomed. Opt. 2019, 24, 121909. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-Breath-Hold Photoacoustic Computed Tomography of the Breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Lim, R.; Zhang, H.; Zheng, W.; Wang, Y.; Tiao, M.; Oh, K.W.; Fan, X.C.; Bonaccio, E.; Takabe, K. Dual scan mammoscope (DSM)—a new portable photoacoustic breast imaging system with scanning in craniocaudal plane. IEEE Trans. Bio-Med. Eng. 2019, 67, 1321–1327. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Zhang, H.; Zheng, E.; Nagarajan, S.; Bonaccio, E.; Takabe, K.; Fan, X.C.; Xia, J. Photoacoustic dual-scan mammoscope: Results from 38 patients. Biomed. Eng. Lett. 2021, 12, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M. Visualization of Tumor-Related Blood Vessels in Human Breast by Photoacoustic Imaging System with a Hemispherical Detector Array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Menezes, G.L.; Pijnappel, R.M.; Meeuwis, C.; Bisschops, R.; Veltman, J.; Lavin, P.T.; Van De Vijver, M.J.; Mann, R.M. Downgrading of Breast Masses Suspicious for Cancer by using Optoacoustic Breast Imaging. Radiology 2018, 288, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Kothapalli, S.-R.; Liu, H.; Koh, A.L.; Jokerst, J.V.; Jiang, H.; Yang, M.; Li, J.; Levi, J.; Wu, J.C. Construction and Validation of Nano Gold Tripods for Molecular Imaging of Living Subjects. J. Am. Chem. Soc. 2014, 136, 3560–3571. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef]
- Cavigli, L.; Khlebtsov, B.N.; Centi, S.; Khlebtsov, N.G.; Pini, R.; Ratto, F. Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods. Nanomaterials 2021, 11, 116. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A.; Höckel, M. Tumor Hypoxia and Malignant Progression. Method. Enzymol. 2004, 381, 335–354. [Google Scholar]
- Horiguchi, A.; Tsujita, K.; Irisawa, K.; Kasamatsu, T.; Hirota, K.; Kawaguchi, M.; Shinchi, M.; Ito, K.; Asano, T.; Shinmoto, H. A Pilot Study of Photoacoustic Imaging System for Improved Real-Time Visualization of Neurovascular Bundle During Radical Prostatectomy. Prostate 2016, 76, 307–315. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Yang, Y.; Park, B.; Ahn, J.; Cho, S.; Lee, C.; Seo, D.-K.; Cho, J.-H. Three-Dimensional Multistructural Quantitative Photoacoustic and US Imaging of Human Feet In Vivo. Radiology, 2022; in press. [Google Scholar] [CrossRef]


| Imaging Method | Imaging System | Transducer | Imaging Depth [mm] | Frame Rate [fps] | λ [nm] | # of Subjects | Accuracy [%] | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Type | [MHz] | ||||||||
| ex vivo | n/a | Linear | 5 | 5 | 10 | 760, 850, 930, 970 | 13 malignant 30 benign 13 colloid 32 normal | Se. 69.2 Sp. 96.9 | [71] |
| in vivo | Terason 2000+, Teratech | Arc | 7.5 | ~20 | 10 | 800 | 2 healthy | n/a | [72] |
| Resona7, Mindray Bio-Medical Electronics | Linear | 5.8 | 3.5 | 10 | 1064 | 10 PTC 3 normal | n/a | [73] | |
| MSOT Acuity Echo, iThera Medical | Arc | 3 | ~20 | 25 | 700, 730, 760, 800, 850, 900, 920, 950 | 6 Graves’ disease 3 malignant 13 benign 8 healthy | n/a | [74] | |
| EC-12R, Alpinion Medical Systems | Linear | 7.5 | ~30 | 5 | 700, 756, 796, 866, 900 | 23 PTC 29 benign | Se. 83 Sp. 93 | [75] | |
| Imaging System | Transducer | Imaging Depth [mm] | Imaging Time [min] | λ [nm] | # of Subjects | Accuracy [%] | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | [MHz] | |||||||
| Custom | Hemi- spherical | 2 | 53 | 3.2 | 756 | 4 healthy | n/a | [88] |
| Custom | Arc | 1 | 22 | 4 | 755, 1064 | 2 healthy | n/a | [89] |
| SonixDAQ, Ultrasonix Medical | Ring | 2.25 | ~40 | ~15 s | 1064 | 7 malignant 1 healthy | n/a | [90] |
| Vintage 256, (Verasonics) | Linear | 2.25 | ~70 | 1 | 1064 | 38 malignant | n/a | [92] |
| PAM-03, Canon-Optosonics | Hemi- spherical | 2 | 27 | 4 | 755, 795 | 22 malignant | n/a | [93] |
| Custom | Arc | 5 | ~20 | 2–4 | 700–970 (10 nm interval) | 10 malignant 3 healthy | n/a | [94] |
| Imagio, Seno Medical Instruments | Linear | 10 | ~30 | Real-time | 755, 1064 | 1079 benign 678 malignant | Se. 98.6 Sp. 43.0 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Lee, H.; Kim, C.; Kim, J. Review on Multispectral Photoacoustic Analysis of Cancer: Thyroid and Breast. Metabolites 2022, 12, 382. https://doi.org/10.3390/metabo12050382
Han S, Lee H, Kim C, Kim J. Review on Multispectral Photoacoustic Analysis of Cancer: Thyroid and Breast. Metabolites. 2022; 12(5):382. https://doi.org/10.3390/metabo12050382
Chicago/Turabian StyleHan, Seongyi, Haeni Lee, Chulhong Kim, and Jeesu Kim. 2022. "Review on Multispectral Photoacoustic Analysis of Cancer: Thyroid and Breast" Metabolites 12, no. 5: 382. https://doi.org/10.3390/metabo12050382






