Effects of Moderate–Intensity Physical Training on Skeletal Muscle Substrate Transporters and Metabolic Parameters of Ovariectomized Rats
Abstract
:1. Introduction
2. Results
2.1. FAT CD36 Quantification by Immunofluorescence
2.2. GLUT4 Quantification by Immunofluorescence
2.3. Muscular Glycogen, Muscular Triglyceride and Blood Glucose
2.4. Body Mass and Spontaneous Physical Activity
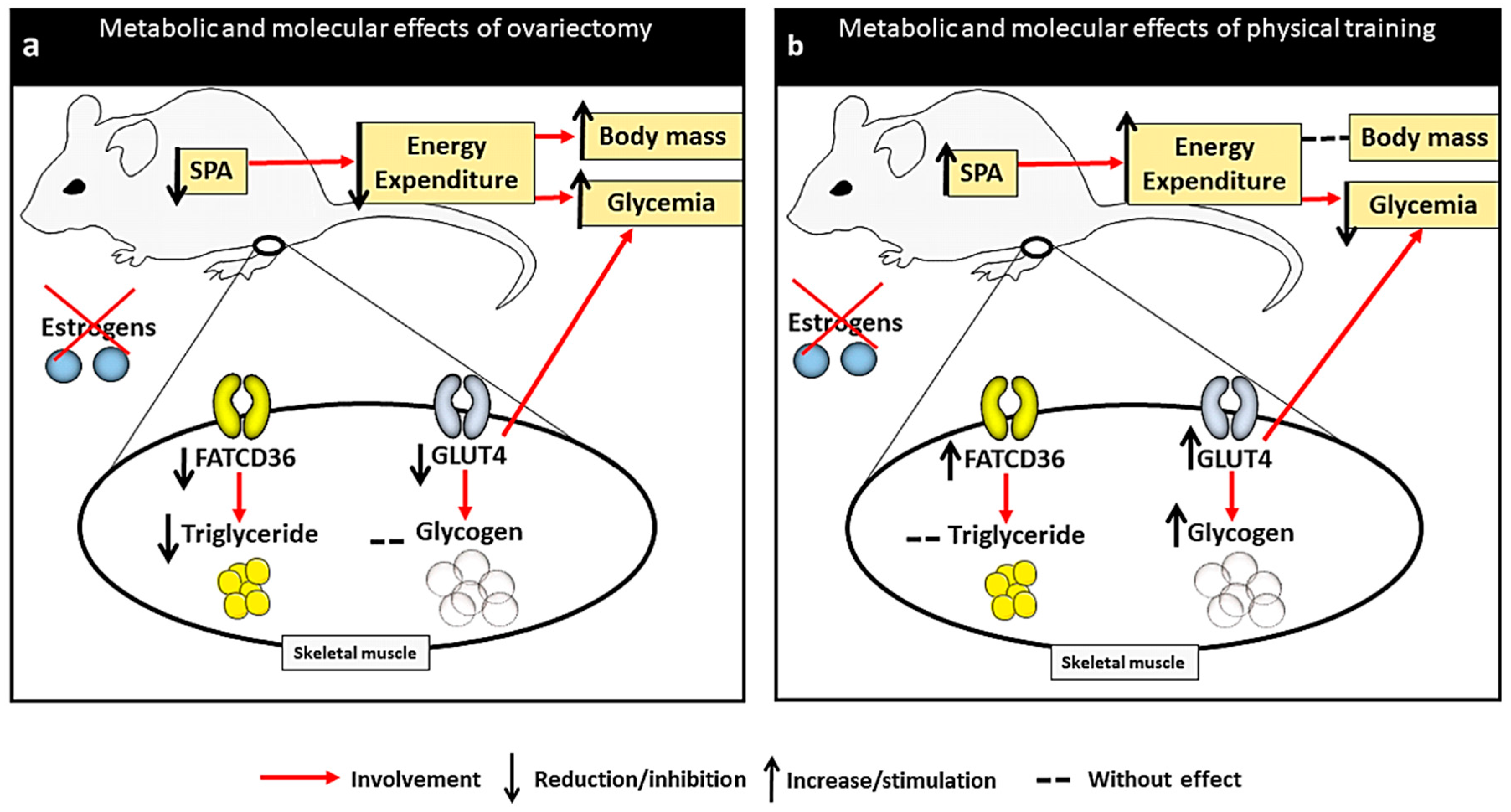
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Design
4.3. Surgical Procedure of Bilateral Ovariectomy
4.4. Aquatic Environment Adaptation
4.5. Critical Load Intensity Determination and Swimming Training Protocol
4.6. Spontaneous Physical Activity
4.7. Obtaining and Storing Biological Material
4.8. Histology and Immunofluorescence Procedures
4.9. Glycogen
4.10. Muscular Triglyceride
4.11. Blood Glucose Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoham, Z.; Schachter, M. Estrogen biosynthesis—Regulation, action, remote effects, and value of monitoring in ovarian stimulation cycles. Fertil. Steril. 1996, 65, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Sohrabji, F.; Okoreeh, A.; Panta, A. Sex hormones and stroke: Beyond estrogens. Horm. Behav. 2019, 111, 87–95. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Tena-Sempere, M.; López, M. Estradiol Regulation of Brown Adipose Tissue Thermogenesis. Adv. Exp. Med. Biol. 2017, 1043, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Fujii, N.L.; Inada, O.; Higaki, Y.; Furuichi, Y.; Nabeshima, Y.-I. Effects of 17β-estradiol and androgen on glucose metabolism in skeletal muscle. Endocrinology 2016, 157, 4691–4705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Yoon, H.M.; Kwon, O.; Lee, W.J. The effect of pueraria lobata/rehmannia glutinosa and exercise on fatty acid transporters expression in ovariectomized rats skeletal muscles. J. Exerc. Nutr. Biochem. 2016, 20, 32–38. [Google Scholar] [CrossRef]
- Pallottini, V.; Bulzomi, P.; Galluzzo, P.; Martini, C.; Marino, M. Estrogen regulation of adipose tissue functions: Involvement of estrogen receptor isoforms. Infect. Disord-Drug Targets (Former. Curr Drug Targets-Infect. Disord.) 2008, 8, 52–60. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 23, 15004. [Google Scholar] [CrossRef]
- Faulds, M.H.; Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.-A. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2012, 212, 3–12. [Google Scholar] [CrossRef]
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Lobo, R.A. Surgical menopause and cardiovascular risks. Menopause 2007, 4, 562–566. [Google Scholar] [CrossRef]
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilha, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Zheng, D.; Houmard, J.A.; Brault, J.J.; Hickner, R.C.; Cortright, R.N. Overexpression of PGC-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E253–E263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrup, C.M.; Egelund, J.; Nyberg, M.; Slingsby, M.H.L.; Andersen, C.B.; Logstrup, S.; Bangsbo, J.; Suetta, C.; Stallknecht, B.; Hellsten, Y. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am. J. Obs. Gynecol. 2017, 216, 384. [Google Scholar] [CrossRef] [Green Version]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of muscle glycogen metabolism during exercise: Implications for endurance performance and training adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, J.-H.; Hancock, C.R.; Han, D.-H.; Holloszy, J.O.; Nair, K.S.; Dasari, S. AMPK and PPARβ positive feedback loop regulates endurance exercise training-mediated GLUT4 expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E931–E939. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Rogers, N.H.; Witczak, C.A.; Hirshman, M.F.; Goodyear, L.J.; Greenberg, A.S. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem. Biophys Res. Commun. 2009, 382, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Huang, T.-H.; Cheng, T.-L.; Chang, C.-F.; Wang, C.-Z.; Wu, M.-H.; Kang, L. Exercise training ameliorates glucosamine-induced insulin resistance in ovariectomized rats. Menopause 2017, 24, 617–623. [Google Scholar] [CrossRef]
- MacDonald, T.L.; Ritchie, K.L.; Davies, S.; Hamilton, M.J.; Cervone, D.T.; Dyck, D.J. Exercise training is an effective alternative to estrogen supplementation for improving glucose homeostasis in ovariectomized rats. Physiol. Rep. 2015, 3, e12617. [Google Scholar] [CrossRef]
- Saengsirisuwan, V.; Pongseeda, S.; Prasannarong, M.; Vichaiwong, K.; Toskulkao, C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism 2009, 58, 38–47. [Google Scholar] [CrossRef]
- Ribas, V.; Nguyen, M.T.A.; Henstridge, D.C.; Nguyen, A.-K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloszy, J.O. Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr. Physiol. 2011, 1, 921–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Gorres, B.K.; Bomhoff, G.L.; Morris, J.K.; Geiger, P.C. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J. Physiol. 2011, 589, 2041–2054. [Google Scholar] [CrossRef]
- Turdi, S.; Huff, A.F.; Pang, J.; He, E.Y.; Chen, X.; Wang, S.; Cheng, Y.; Zhang, Y.; Ren, J. 17-β estradiol attenuates ovariectomy-induced changes in cardiomyocyte contractile function via activation of AMP-activated protein kinase. Toxicol. Lett. 2015, 232, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.-A. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef] [Green Version]
- Hansen, P.A.; McCarthy, T.J.; Pasia, N.E.; Spina, R.J.; Gulve, E.A. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J. Appl. Physiol. 1996, 80, 1605–1611. [Google Scholar] [CrossRef]
- Dresseno, L.P.; Lehnen, A.M.; Teló, G.; Silveira, A.; Markoski, M.M.; Machado, U.F.; Schaan, B.D. Impact of flaxseed and soy nuts as dietary supplements on lipid profile, insulin sensitivity, and GLUT4 expression in ovariectomized rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1282–1287. [Google Scholar] [CrossRef]
- Gobatto, C.A.; De Mello, M.A.R.; Sibuya, C.Y.; De Azevedo, J.R.M.; Dos Santos, L.A.; Kobubun, E. Maximal lactate steady state in rats submitted to swimming exercise. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 21–27. [Google Scholar] [CrossRef]
- Kregel, K.C.; Allen, D.L.; Booth, F.W.; Fleshner, M.R.; Henriksen, E.J.; Musch, T.I.; O’Leary, D.S.; Parks, C.M.; Poole, D.C.; Ra’anan, A.W.; et al. Resource Book for the Design of Animal Exercise Protocols; American Physiological Society: Rockville, MD, USA, 2006; pp. 35–41. [Google Scholar]
- Beck, W.R.; De Araujo, D.D.; Scariot, P.P.M.; Dos Reis, I.G.M.; Gobatto, C.A. Time to exhaustion at anaerobic threshold in swimming rats: Metabolic investigation. Bratisl. Lek. Listy 2014, 115, 617–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.B.; Laughlin, M.H. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J. Exp. Biol. 1985, 115, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Cartee, G.D. Contraction-stimulated glucose transport in rat skeletal muscle is sustained despite reversal of increased PAS-phosphorylation of AS160 and TBC1D1. J. Appl. Physiol. 2008, 105, 1788–1795. [Google Scholar] [CrossRef] [Green Version]
- Ojuka, E.O.; Jones, T.E.; Nolte, L.A.; Chen, M.; Wamhoff, B.R.; Sturek, M.O.; Holloszy, J.O. Regulation of GLUT4 biogenesis in muscle: Evidence for involvement of AMPK and Ca2+. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1008–E1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudry, K.M.; Devries, M.C. Sex-based differences in hepatic and skeletal muscle triglyceride storage and metabolism. Appl. Physiol. Nutr. Metab. 2019, 44, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Cheng, Y. Triglyceride metabolism in exercising muscle. Biochim. Biophys Acta Mol. Cell Biol. Lipids 2017, 1862, 1250–1259. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. Oestrogen’s regulation of fat metabolism during exercise and gender specific effects. Curr. Opin. Pharmacol. 2012, 12, 363–371. [Google Scholar] [CrossRef]
- Barbosa, M.R.; Shiguemoto, G.E.; Tomaz, L.M.; Ferreira, F.C.; Rodrigues, M.F.C.; Domingues, M.M.; Master, M.V.C.S.; Canevazzi, G.H.R.; Silva-Magosso, N.S.; Selistre-De-Araujo, H.S.; et al. Resistance training and ovariectomy: Antagonic effects in mitochondrial biogenesis markers in rat skeletal muscle. Int. J. Sports Med. 2016, 37, 841–848. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.C.; Luiken, J.J.F.P. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie 2016, 136, 21–26. [Google Scholar] [CrossRef]
- Kusuhara, K.; Madsen, K.; Jensen, L.; Hellsten, Y.; Pilegaard, H. Calcium signalling in the regulation of PGC-1α, PDK4 and HKII mRNA expression. Biol. Chem. 2007, 388, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherk, V.D.; Jackman, M.R.; Higgins, J.A.; Giles, E.D.; Foright, R.M.; Presby, D.M.; Carpenter, R.D.; Johnson, G.C.; Oljira, R.; Houck, J.A.; et al. Impact of Exercise and Activity on Weight Regain and Musculoskeletal Health PostOVX. Med. Sci. Sports Exerc. 2019, 51, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.M.; Caliman, I.F.; Molosh, A.I.; Fitz, S.D.; Truitt, W.A.; Bonaventure, P.; Carpenter, J.S.; Shekhar, A.; Johnson, P.L. Hypothalamic orexin’s role in exacerbated cutaneous vasodilation responses to an anxiogenic stimulus in a surgical menopause model. Psychoneuroendocrinology 2016, 65, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Proietto, J. Obesity and weight management at menopause. Aust. Fam. Physician 2017, 46, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Kotz, C.M.; Perez-Leighton, C.E.; Teske, J.A.; Billington, C.J. Spontaneous physical activity defends against obesity. Curr. Obes. Rep. 2017, 6, 362–370. [Google Scholar] [CrossRef]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221, jeb161570. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayan, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef] [Green Version]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts. Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- Zarrow, M.X.; Yochim, J.M.; McCarthy, J.L. Experimental Endocrinology: A Sourcebook of Basic Techniques; Academic Press: New York, NY, USA; London, UK, 1964; pp. 39–40. [Google Scholar]
- Gobatto, C.A.; Scariot, P.P.M.; Ribeiro, L.F.P.; Manchado-Gobatto, F.B. Critical load estimation in young swimming rats using hyperbolic and linear models. Comp. Exerc. Physiol. 2013, 9, 85–91. [Google Scholar] [CrossRef]
- De Lima, A.A.; Gobatto, C.A.; Messias, L.H.D.; Scariot, P.P.M.; Forte, L.D.M.; Santin, J.O.; Manchado-Gobatto, F.B. Two water environment adaptation models enhance motor behavior and improve the success of the lactate minimum test in swimming rats. Mot. Rev. Ed. Fis. 2017, 23, 101607. [Google Scholar] [CrossRef] [Green Version]
- Beck, W.R.; Gobatto, C.A. Effects of maximum intensity aerobic swimming exercise until exhaustion at different times of day on the hematological parameters in rats. Acta Physiol. Hung. 2013, 100, 427–434. [Google Scholar] [CrossRef]
- Beck, W.R.; Scariot, P.P.M.; Gobatto, C.A. Melatonin is an Ergogenic Aid for Exhaustive Aerobic Exercise only during the Wakefulness. Int. J. Sports Med. 2016, 37, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, W.R.; Scariot, P.P.M.; Carmo, S.S.; Manchado-Gobatto, F.B.; Gobatto, C.A. Metabolic profile and spontaneous physical activity modulation under short-term food restriction in young rats. Mot. Rev. Ed. Fis. 2017, 23, e101606. [Google Scholar] [CrossRef] [Green Version]
- Chausse, B.; Solon, C.; Da Silva, C.C.C.; Dos Reis, I.G.M.; Manchado-Gobatto, F.B.; Gobatto, C.A.; Velloso, L.A.; Kowaltowski, A.J. Intermittent fasting induces hypothalamic modifications resulting in low feeding efficiency, low body mass and overeating. Endocrinology 2014, 155, 2456–2466. [Google Scholar] [CrossRef] [Green Version]
- Moes, J.R.; Holden, J.E. Characterizing activity and muscle atrophy changes in rats with neuropathic pain: A pilot study. Biol. Res. Nurs. 2014, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Scariot, P.P.; Manchado-Gobatto, F.B.; Torsoni, A.S.; Dos Reis, I.G.M.; Beck, W.R.; Gobatto, C.A. Continuous Aerobic Training in Individualized Intensity Avoids Spontaneous Physical Activity Decline and Improves MCT1 Expression in Oxidative Muscle of Swimming Rats. Front. Physiol. 2016, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Scariot, P.P.M.; Manchado-Gobatto, F.B.; Prolla, T.A.; Dos Reis, I.G.M.; Gobatto, C.A. Housing conditions modulate spontaneous physical activity, feeding behavior, aerobic running capacity and adiposity in C57BL/6J mice. Horm. Behav. 2019, 115, 104556. [Google Scholar] [CrossRef]
- Biesiadecki, B.J.; Brand, P.H.; Koch, L.G.; Britton, S.L. A gravimetric method for the measurement of total spontaneous activity in rats. Proc. Soc. Exp. Biol. Med. 1999, 222, 65–69. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. AVMA Guidelines on Euthanasia; AVMA: Schaumber, IL, USA, 2013; pp. 44–45. [Google Scholar]
- Bradley, H.; Shaw, C.S.; Worthington, P.L.; Shepherd, S.O.; Cocks, M.; Wagenmakers, A.J.M. Quantitative immunofluorescence microscopy of subcellular GLUT 4 distribution in human skeletal muscle: Effects of endurance and sprint interval training. Physiol. Rep. 2014, 2, e12085. [Google Scholar] [CrossRef] [PubMed]
- Faria, V.S.; Pejon, T.M.M.; Gobatto, C.A.; De Araujo, G.G.; Cornachione, A.S.; Beck, W.R. Acute melatonin administration improves exercise tolerance and the metabolic recovery after exhaustive effort. Sci. Rep. 2021, 11, 19228. [Google Scholar] [CrossRef] [PubMed]
- Pejon, T.M.M.; Faria, V.S.; Gobatto, C.A.; Manchado-Gobatto, F.B.; Scariot, P.P.M.; Cornachione, A.S.; Beck, W.R. 12-wk of physical training in ovariectomised rats on PGC-1α, NRF-1 and energy substrates. Int. J. Sports Med. 2022, 43. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 1988. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejon, T.M.M.; Scariot, P.P.M.; Selistre-de-Araujo, H.S.; Gobatto, C.A.; Cornachione, A.S.; Beck, W.R. Effects of Moderate–Intensity Physical Training on Skeletal Muscle Substrate Transporters and Metabolic Parameters of Ovariectomized Rats. Metabolites 2022, 12, 402. https://doi.org/10.3390/metabo12050402
Pejon TMM, Scariot PPM, Selistre-de-Araujo HS, Gobatto CA, Cornachione AS, Beck WR. Effects of Moderate–Intensity Physical Training on Skeletal Muscle Substrate Transporters and Metabolic Parameters of Ovariectomized Rats. Metabolites. 2022; 12(5):402. https://doi.org/10.3390/metabo12050402
Chicago/Turabian StylePejon, Taciane Maria Melges, Pedro Paulo Menezes Scariot, Heloísa Sobreiro Selistre-de-Araujo, Claudio Alexandre Gobatto, Anabelle Silva Cornachione, and Wladimir Rafael Beck. 2022. "Effects of Moderate–Intensity Physical Training on Skeletal Muscle Substrate Transporters and Metabolic Parameters of Ovariectomized Rats" Metabolites 12, no. 5: 402. https://doi.org/10.3390/metabo12050402
APA StylePejon, T. M. M., Scariot, P. P. M., Selistre-de-Araujo, H. S., Gobatto, C. A., Cornachione, A. S., & Beck, W. R. (2022). Effects of Moderate–Intensity Physical Training on Skeletal Muscle Substrate Transporters and Metabolic Parameters of Ovariectomized Rats. Metabolites, 12(5), 402. https://doi.org/10.3390/metabo12050402





