Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Light Intensities and Temperatures on the Rate of Growth of Hapalosiphon sp. and Planktothricoides sp.
2.2. Effects of Light and Temperature on the Metabolites of Hapalosiphon sp. and Planktothricoides sp.
2.2.1. Effects of Light Intensity on the Metabolite Profiling of Hapalosiphon sp. and Planktothricoides sp. (Light Intensity and Time Point as Variables)
2.2.2. Effects of Temperature on the Metabolite Profiling of Hapalosiphon sp. and Planktothricoides sp. (Temperature and Time Point as Variables)
3. Discussion
3.1. Effects of Different Intensities of Light on the Growth and Metabolic Profiling of Hapalosiphon sp. MBR220 and Planktothricoides sp. SR001
3.2. Effects of Different Temperatures on the Growth and Metabolic Profiling of Hapalosiphon sp. MBR220 and Planktothricoides sp. SR001
4. Materials and Methods
4.1. Effects of Light Intensity and Temperature
4.2. Profiling of Metabolites
4.3. Data Analysis
4.4. Metabolic Pathway Mapping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Huisman, J. Climate Change: A Catalyst for Global Expansion of Harmful Cyanobacterial Blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating Toxic Planktonic Cyanobacterial Blooms in Aquatic Ecosystems Facing Increasing Anthropogenic and Climatic Pressures. Toxins 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, A.; Haakonsson, S.; Martin, M.V.; Salerno, G.L.; Echenique, R.O. Bloom-Forming cyanobacteria and Cyanotoxins in Argentina: A Growing Health and Environmental Concern. Limnologica 2018, 69, 103–114. [Google Scholar] [CrossRef]
- Lürling, M.; Roessink, I. On the Way to Cyanobacterial Blooms: Impact of the Herbicide Metribuzin on the Competition between a Green Alga (Scenedesmus) and a Cyanobacterium (Microcystis). Chemosphere 2006, 65, 618–626. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; E & FN Spon: London, UK; New York, NY, USA, 1999. [Google Scholar]
- Toporowska, M.; Pawlik-Skowronska, B.; Kalinowska, R. Accumulation and Effects of Cyanobacterial Microcystins and Anatoxin-a on Benthic Larvae of Chironomus Spp. (Diptera: Chironomidae). Eur. J. Entomol. 2014, 111, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Pearson, L.A.; Dittmann, E.; Mazmouz, R.; Ongley, S.E.; D’Agostino, P.M.; Neilan, B.A. The Genetics, Biosynthesis and Regulation of Toxic Specialized Metabolites of Cyanobacteria. Harmful Algae 2016, 54, 98–111. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Wan, Y.; Giesy, J.P.; Hu, J. Cyanobacteria Blooms Produce Teratogenic Retinoic Acids. Proc. Natl. Acad. Sci. USA 2012, 109, 9477–9482. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A Review of Cyanobacterial Odorous and Bioactive Metabolites: Impacts and Management Alternatives in Aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Watson, S.B.; Ridal, J.; Boyer, G.L. Taste and Odour and Cyanobacterial Toxins: Impairment, Prediction, and Management in the Great Lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 1779–1796. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. Engl. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Sorial, G.A. Treatment of Taste and Odor Causing Compounds 2-Methyl Isoborneol and Geosmin in Drinking Water: A Critical Review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Te, S.H.; Tan, B.F.; Boo, C.Y.; Thompson, J.R.; Gin, K.Y.-H. Genomics Insights into Production of 2-Methylisoborneol and a Putative Cyanobactin by Planktothricoides sp. SR001. Stand. Genom. Sci. 2017, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.F.; Te, S.H.; Boo, C.Y.; Gin, K.Y.-H.; Thompson, J.R. Insights from the draft genome of the subsection V (Stigonematales) cyanobacterium Hapalosiphon sp. Strain MRB220 associated with 2-MIB production. Stand. Genom. Sci. 2016, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Geider, R.J. Temperature and Algal Growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The Effects of Temperature and Nutrients on the Growth and Dynamics of Toxic and Non-Toxic Strains of Microcystis during Cyanobacteria Blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Helbling, E.W.; Banaszak, A.T.; Villafañe, V.E. Global Change Feed-Back Inhibits Cyanobacterial Photosynthesis. Sci. Rep. 2015, 5, 14514. [Google Scholar] [CrossRef] [Green Version]
- Brutemark, A.; Engstrom-Ost, J.; Vehmaa, A.; Gorokhova, E. Growth, Toxicity and Oxidative Stress of a Cultured Cyanobacterium (Dolichospermum Sp.) under Different CO2/PH and Temperature Conditions. Phycol. Res. 2015, 63, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Welker, M.; Von Döhren, H. Cyanobacterial Peptides—Nature’s Own Combinatorial Biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [Green Version]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental Conditions That Influence Toxin Biosynthesis in Cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Kaebernick, M.; Neilan, B.A. Ecological and Molecular Investigations of Cyanotoxin Production. FEMS Microbiol. Ecol. 2001, 35, 1–9. [Google Scholar] [CrossRef]
- Ojit, S.K.; Indrama, T.; Gunapati, O.; Avijeet, S.O.; Subhalaxmi, S.A.; Silvia, C.; Indira, D.W.; Romi, K.; Thadoi, D.A.; Tiwari, O.N.; et al. The Response of Phycobiliproteins to Light Qualities in Anabaena circinalis. J. Appl. Biol. Biotechnol. 2015, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, H.; Kok Leong, L.; Abdu Rahman, N.; Mian, S.; Begum, H.; Banerjee, S.; Endut, A. Effects of Different Light Source and Media on Growth and Production of Phycobiliprotein from Freshwater Cyanobacteria. Bioresour. Technol. 2018, 249, 652–658. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Golubic, S.; Abed, R.M.M.; Palińska, K.; Pauillac, S.; Chinain, M.; Laurent, D. Marine Toxic Cyanobacteria: Diversity, Environmental Responses and Hazards. Toxicon 2010, 56, 836–841. [Google Scholar] [CrossRef]
- Guidi-Rontani, C.; Jean, M.R.N.; Gonzalez-Rizzo, S.; Bolte-Kluge, S.; Gros, O. Description of New Filamentous Toxic Cyanobacteria (Oscillatoriales) Colonizing the Sulfidic Periphyton Mat in Marine Mangroves. FEMS Microbiol. Lett. 2014, 359, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins Produced in Cyanobacterial Water Blooms—Toxicity and Risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.; Brookes, J.D. Benthic Cyanobacteria: A Source of Cylindrospermopsin and Microcystin in Australian Drinking Water Reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Kudela, R.M.; Power, M.E. Widespread Anatoxin-a Detection in Benthic Cyanobacterial Mats throughout a River Network. PLoS ONE 2018, 13, e0197669. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humbert, J.F.; Laughinghouse, H.D.; Lazorchak, J.; McAllister, T.G.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic Benthic Freshwater Cyanobacterial Proliferations: Challenges and Solutions for Enhancing Knowledge and Improving Monitoring and Mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and Cyanotoxins in a Changing Environment: Concepts, Controversies, Challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Deng, P.; Li, X.; Petriello, M.C.; Wang, C.; Morris, A.J.; Hennig, B. Application of Metabolomics to Characterize Environmental Pollutant Toxicity and Disease Risks. Rev. Environ. Health 2019, 34, 251–259. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Qian, L.; Ding, L.-Y.; Wang, L.; Wong, M.H.; Tao, H.-C. Ecological and Toxicological Assessments of Anthropogenic Contaminants Based on Environmental Metabolomics. Environ. Sci. Ecotechnol. 2021, 5, 100081. [Google Scholar] [CrossRef]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental Metabolomics: A Critical Review and Future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Schwarz, D.; Nodop, A.; Hüge, J.; Purfürst, S.; Forchhammer, K.; Michel, K.-P.; Bauwe, H.; Kopka, J.; Hagemann, M. Metabolic and Transcriptomic Phenotyping of Inorganic Carbon Acclimation in the Cyanobacterium Synechococcus elongatus PCC 7942. Plant Physiol. 2011, 155, 1640–1655. [Google Scholar] [CrossRef] [Green Version]
- Hasunuma, T.; Kikuyama, F.; Matsuda, M.; Aikawa, S.; Izumi, Y.; Kondo, A. Dynamic Metabolic Profiling of Cyanobacterial Glycogen Biosynthesis under Conditions of Nitrate Depletion. J. Exp. Bot. 2013, 64, 2943–2954. [Google Scholar] [CrossRef] [Green Version]
- Le Manach, S.; Duval, C.; Marie, A.; Djediat, C.; Catherine, A.; Edery, M.; Bernard, C.; Marie, B. Global Metabolomic Characterizations of Microcystis Spp. Highlights Clonal Diversity in Natural Bloom-Forming Populations and Expands Metabolite Structural Diversity. Front. Microbiol. 2019, 10, 791. [Google Scholar] [CrossRef] [Green Version]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [Green Version]
- Kleinteich, J.; Wood, S.A.; Küpper, F.C.; Camacho, A.; Quesada, A.; Frickey, T.; Dietrich, D.R. Temperature-Related Changes in Polar Cyanobacterial Mat Diversity and Toxin Production. Nat. Clim. Change 2012, 2, 356–360. [Google Scholar] [CrossRef]
- Boopathi, T.; Ki, J.-S. Impact of Environmental Factors on the Regulation of Cyanotoxin Production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Su, M.; Liu, T.; Guo, Q.; Wang, Q.; Burch, M.; Yu, J.; Yang, M. Light as a Possible Regulator of MIB-Producing Planktothrix in Source Water Reservoir, Mechanism and in-situ Verification. Harmful Algae 2019, 88, 101658. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Pratelli, R.; Pilot, G. Regulation of Amino Acid Metabolic Enzymes and Transporters in Plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Samek, D.; Mišurcová, L.; Machu, L.; Buňka, F.; Fišera, M. Influencing of Amino Acid Composition of Green Freshwater Algae and Cyanobacterium by Methods of Cultivation. Turkish J. Biochem. 2013, 38, 360–368. [Google Scholar] [CrossRef]
- Patelou, M.; Infante, C.; Dardelle, F.; Randewig, D.; Kouri, E.D.; Udvardi, M.K.; Tsiplakou, E.; Mantecón, L.; Flemetakis, E. Transcriptomic and Metabolomic Adaptation of Nannochloropsis gaditana Grown under Different Light Regimes. Algal Res. 2020, 45, 101735. [Google Scholar] [CrossRef]
- Bolay, P.; Muro-Pastor, M.I.; Florencio, F.J.; Klähn, S. The Distinctive Regulation of Cyanobacterial Glutamine Synthetase. Life 2018, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Larkum, A.W.D.; Grossmann, A.R.; Raven, J.A. Photosynthesis in Algae: Biochemical and Physiological Mechanisms, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Good, A.G.; Zaplachinski, S.T. The Effects of Drought Stress on Free Amino Acid Accumulation and Protein Synthesis in Brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar] [CrossRef]
- Díaz, P.; Borsani, O.; Marquez, A. Nitrogen Metabolism in Relation to Drought Stress Responses in Cultivated and Model Lotus Species. Lotus Newslr. 2005, 35, 83–92. [Google Scholar]
- Angeleri, M.; Muth-Pawlak, D.; Aro, E.-M.; Battchikova, N. Study of O-Phosphorylation Sites in Proteins Involved in Photosynthesis-Related Processes in Synechocystis Sp. Strain PCC 6803: Application of the SRM Approach. J. Proteome Res. 2016, 15, 4638–4652. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Plavsic, M.; Terzic, S.; Ahel, M.; van den Berg, C.M.G. Folic Acid in Coastal Waters of the Adriatic Sea. Mar. Freshw. Res. 2003, 53, 1245–1252. [Google Scholar] [CrossRef]
- Bonnet, S.; Webb, E.A.; Panzeca, C.; Karl, D.M.; Capone, D.G.; Sañudo-Wilhelmy, S.A. Vitamin B12 Excretion by Cultures of the Marine Cyanobacteria Crocosphaera and Synechococcus. Limnol. Oceanogr. 2010, 55, 1959–1964. [Google Scholar] [CrossRef] [Green Version]
- Lau, N.-S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. Biomed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef]
- Kultschar, B. Secondary Metabolites in Cyanobacteria; Vijakumar, R., Raja, S.S.S., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 2. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Krajewska, M.; Szymczak-Żyła, M.; Kobos, J.; Witak, M.; Kowalewska, G. Canthaxanthin in Recent Sediments as an Indicator of Heterocystous Cyanobacteria in Coastal Waters. Oceanologia 2019, 61, 78–88. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and Carotenogenesis in Cyanobacteria: Unique Ketocarotenoids and Carotenoid Glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Cegłowska, M.; Toruńska-Sitarz, A.; Stoń-Egiert, J.; Mazur-Marzec, H.; Kosakowska, A. Characteristics of Cyanobacterium Pseudanabaena galeata CCNP1313 from the Baltic Sea. Algal Res. 2020, 47, 101861. [Google Scholar] [CrossRef]
- Maršálek, B.; Zahradníčková, H.; Hronková, M. Extracellular Abscisic Acid Produced by Cyanobacteria under Salt Stress. J. Plant Physiol. 1992, 139, 506–508. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Nicolle, A.; Granéli, W.; Hallgren, P.; Kritzberg, E.; Persson, A.; Björk, J.; Nilsson, P.A.; Brönmark, C. Food Chain Length Alters Community Response to Global Change in Aquatic Systems. Nat. Clim. Change 2013, 3, 228–233. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.-A. Local Food Web Management Increases Resilience and Buffers Against Global Change Effects on Freshwaters. Sci. Rep. 2016, 6, 29542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate Change and Harmful Benthic Microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, M.; Montgomery, B.L. Survival Strategies in the Aquatic and Terrestrial World: The Impact of Second Messengers on Cyanobacterial Processes. Life 2014, 4, 745–769. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Haase, F.; Hartmann, M.D.; Hagemann, M.; Forchhammer, K. PII-like Signaling Protein SbtB Links CAMP Sensing with Cyanobacterial Inorganic Carbon Response. Proc. Natl. Acad. Sci. USA 2018, 115, E4861–E4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forchhammer, K.; Selim, K.A. Carbon/Nitrogen Homeostasis Control in Cyanobacteria. FEMS Microbiol. Rev. 2020, 44, 33–53. [Google Scholar] [CrossRef]
- Ohmori, M.; Okamoto, S. Photoresponsive CAMP Signal Transduction in Cyanobacteria. Photochem. Photobiol. Sci. 2004, 3, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Frain, K.M.; Gangl, D.; Jones, A.; Zedler, J.A.Z.; Robinson, C. Protein Translocation and Thylakoid Biogenesis in Cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine Residues as Endogenous Antioxidants in Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Levine, R.L. Methionine in Proteins Defends against Oxidative Stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine Metabolism and Methyltransferases in the Regulation of Aging and Lifespan Extension across Species. Aging Cell 2019, 18, e13034. [Google Scholar] [CrossRef] [Green Version]
- Cerbin, S.; Pérez, G.; Rybak, M.; Wejnerowski, Ł.; Konowalczyk, A.; Helmsing, N.; Naus-Wiezer, S.; Meima-Franke, M.; Pytlak, Ł.; Raaijmakers, C.; et al. Methane-Derived Carbon as a Driver for Cyanobacterial Growth. Front. Microbiol. 2022, 13, 837198. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Filatova, D.; Jones, M.R.; Haley, J.A.; Núñez, O.; Farré, M.; Janssen, E.M. Cyanobacteria and their secondary metabolites in three freshwater reservoirs in the United Kingdom. Environ. Sci. Eur. 2021, 33, 29. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and Purification of Australian Isolates of the Toxic Cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]

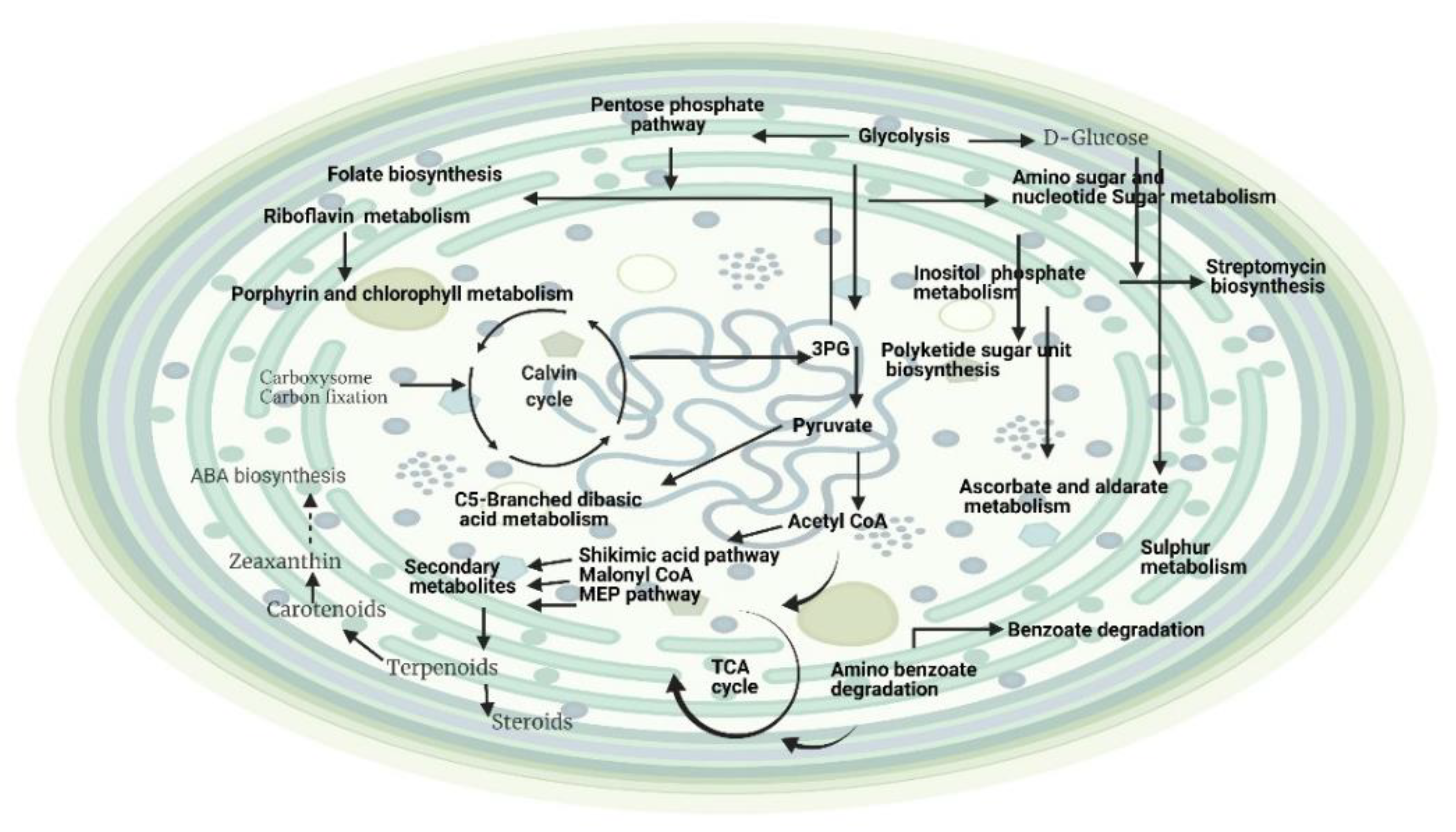
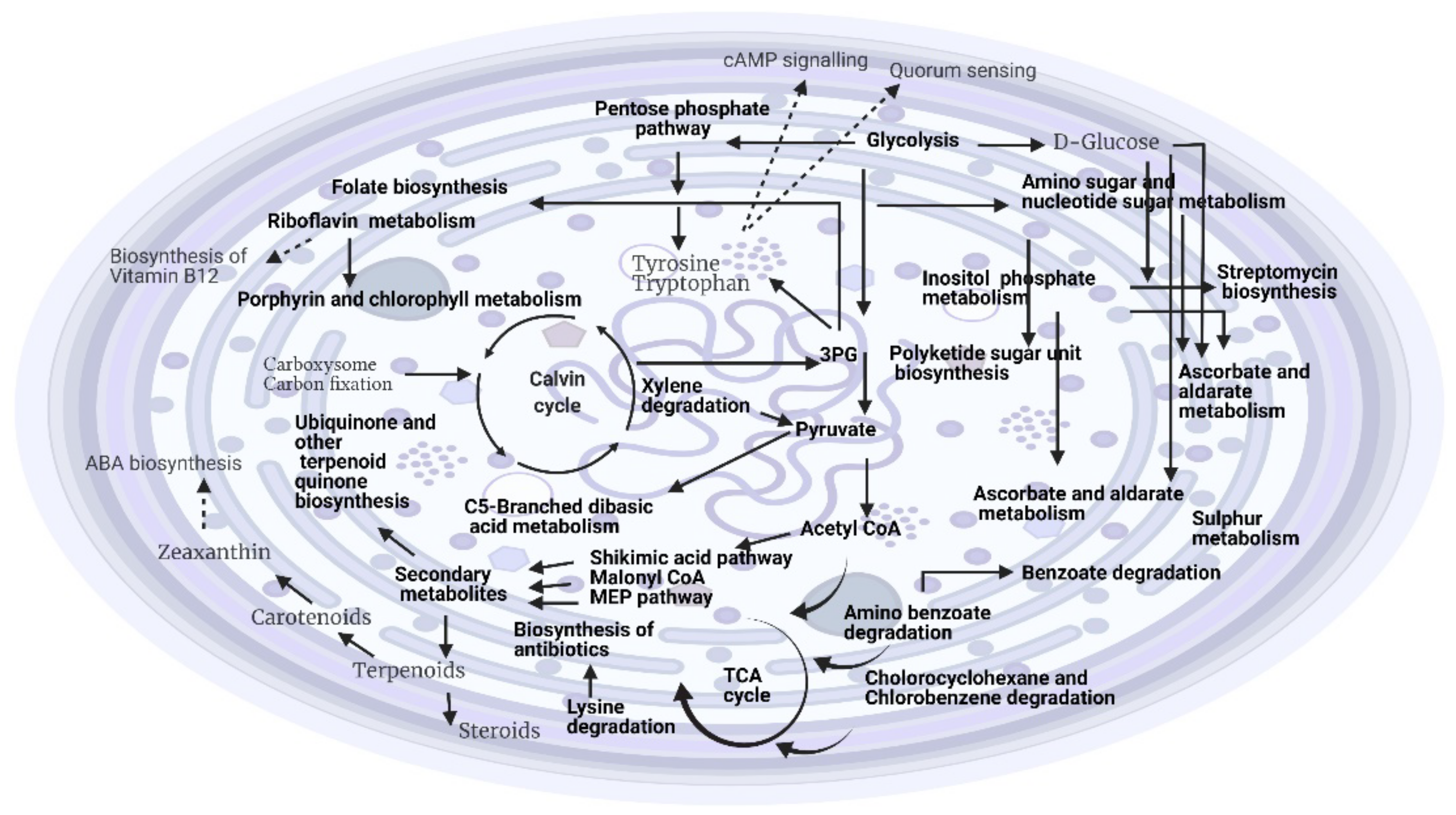
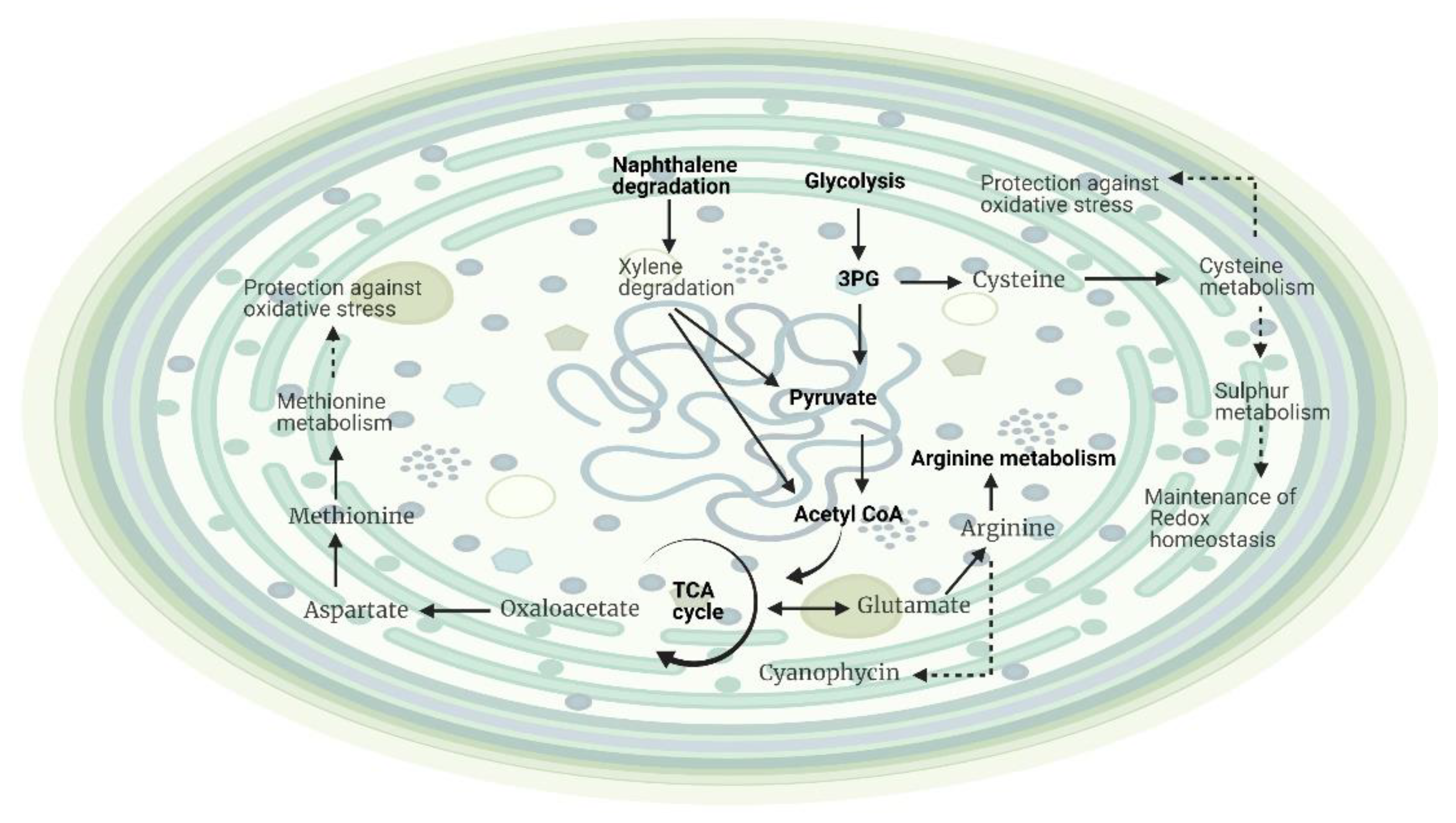
| Light Intensity (µmol Photons m−2s−1) | Growth Rate (Day−1) | |
|---|---|---|
| Hapalosiphon sp. (MRB 220) | Planktothricoides sp. (SR001) | |
| 10 ± 3 | 0.067 ± 0.004 | 0.090 ± 0.008 |
| 25 ± 3 | 0.055 ± 0.005 | 0.200 ± 0.021 |
| 50 ± 3 | 0.068 ± 0.005 | 0.260 ± 0.025 |
| 100 ± 3 | 0.073 ± 0.007 | 0.320 ± 0.038 |
| Temperature (°C) | Growth Rate (Day−1) | |
|---|---|---|
| Hapalosiphon sp. (MRB 220) | Planktothricoides sp. (SR001) | |
| 25 | 0.064 ± 0.005 | 0.100 ± 0.009 |
| 28 | 0.057 ± 0.006 | 0.120 ± 0.014 |
| 33 | 0.070 ± 0.009 | 0.160 ± 0.018 |
| 38 | 0.060 ± 0.006 | 0.130 ± 0.014 |
| Factor | Df | Sums of Squares | Mean of Squares | F-Value | R2 |
|---|---|---|---|---|---|
| Time point | 2.00 | 0.29 | 0.15 | 10.50 | 0.17 * |
| Light | 3.00 | 0.34 | 0.11 | 8.17 | 0.19 * |
| Time point * Light | 6.00 | 0.33 | 0.05 | 3.93 | 0.18 * |
| Residuals | 58.00 | 0.81 | 0.01 | 0.46 * | |
| Total | 69.00 | 1.77 | 1.00 |
| Factor | Df | Sums Of Squares | Mean of Squares | F-Value | R2 |
|---|---|---|---|---|---|
| Time point | 1.00 | 0.001 | 0.001 | 2.526 | 0.069 * |
| Light | 3.00 | 0.003 | 0.001 | 2.952 | 0.24 * |
| Residuals | 25.00 | 0.009 | 0.000 | 0.687 | 0.68 * |
| Total | 29.00 | 0.014 | 1.000 |
| Factor | Df | Sums Of Squares | Mean of Squares | F-Value | R2 |
|---|---|---|---|---|---|
| Time point | 2.00 | 0.06 | 0.03 | 14.09 | 0.19 * |
| Temperature | 3.00 | 0.08 | 0.03 | 14.01 | 0.28 * |
| Time point * Temperature | 6.00 | 0.04 | 0.01 | 3.26 | 0.13 * |
| Residuals | 60.00 | 0.12 | 0.00 | 0.40 | |
| Total | 71.00 | 0.30 | 1.00 |
| Factor | Df | Sums Of Squares | Mean of Squares | F-Value | R2 |
|---|---|---|---|---|---|
| Time point | 3 | 0.050999 | 0.0169997 | 15.5196 | 0.3 * |
| Temperature | 3 | 0.012636 | 0.0042119 | 3.8452 | 0.07 * |
| Time point * Temperature | 9 | 0.022259 | 0.0024732 | 2.2579 | 0.13 * |
| Residuals | 78 | 0.085439 | 0.0010954 | 0.5 * | |
| Total | 93 | 0.171332 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, B.; Majedi, S.M.; Pavagadhi, S.; Te, S.H.; Boo, C.Y.; Gin, K.Y.-H.; Swarup, S. Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites 2022, 12, 406. https://doi.org/10.3390/metabo12050406
Mohanty B, Majedi SM, Pavagadhi S, Te SH, Boo CY, Gin KY-H, Swarup S. Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites. 2022; 12(5):406. https://doi.org/10.3390/metabo12050406
Chicago/Turabian StyleMohanty, Bijayalaxmi, Seyed Mohammad Majedi, Shruti Pavagadhi, Shu Harn Te, Chek Yin Boo, Karina Yew-Hoong Gin, and Sanjay Swarup. 2022. "Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria" Metabolites 12, no. 5: 406. https://doi.org/10.3390/metabo12050406
APA StyleMohanty, B., Majedi, S. M., Pavagadhi, S., Te, S. H., Boo, C. Y., Gin, K. Y.-H., & Swarup, S. (2022). Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites, 12(5), 406. https://doi.org/10.3390/metabo12050406







