Obesity and Voiding Parameters in a Community-Based Population of Okinawa, Japan: Kumejima Digital Health Project (KDHP)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
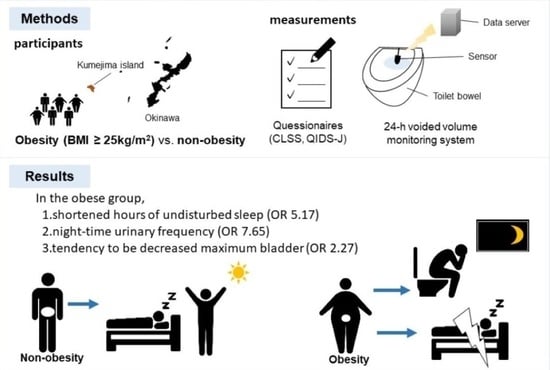
4.1. Study Design and Participants
4.2. Urinary Tract Symptoms Questionnaire
4.3. 24 h Voided Volume Monitoring System
4.4. Other Data Collection
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakagawa, H.; Niu, K.; Hozawa, A.; Ikeda, Y.; Kaiho, Y.; Ohmori-Matsuda, K.; Nakaya, N.; Kuriyama, S.; Ebihara, S.; Nagatomi, R.; et al. Impact of nocturia on bone fracture and mortality in older individuals: A Japanese longitudinal cohort study. J. Urol. 2010, 184, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.J.; Krambeck, A.E.; Jacobson, D.J.; McGree, M.E.; Jacobsen, S.J.; Lieber, M.M.; Roger, V.L.; Girman, C.J.; Sauver, J.L.S. Nocturia is associated with an increased risk of coronary heart disease and death. BJU Int. 2012, 110, 848–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupelian, V.; Fitzgerald, M.P.; Kaplan, S.A.; Norgaard, J.P.; Chiu, G.R.; Rosen, R.C. Association of nocturia and mortality: Results from the Third National Health and Nutrition Examination Survey. J. Urol. 2011, 185, 571–577. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Z.; Liu, G.; Daneshgari, F.; MacLennan, G.T.; Gupta, S. Metabolic syndrome, inflammation and lower urinary tract symptoms: Possible translational links. Prostate Cancer Prostatic Dis. 2016, 19, 7–13. [Google Scholar] [CrossRef]
- Melin, I.; Falconer, C.; Rössner, S.; Altman, D. Nocturia and overactive bladder in obese women: A case-control study. Obes. Res. Clin. Pract. 2007, 1, 187–193. [Google Scholar] [CrossRef]
- Gulur, D.M.; Mevcha, A.M.; Drake, M.J. Nocturia as a manifestation of systemic disease. BJU Int. 2011, 107, 702–713. [Google Scholar] [CrossRef]
- Rohrmann, S.; Smit, E.; Giovannucci, E.; Platz, E.A. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int. J. Obes. 2005, 29, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Bunn, F.; Kirby, M.; Pinkney, E.; Cardozo, L.; Chapple, C.; Chester, K.; Cruz, F.; Haab, F.; Kelleher, C.; Milsom, I.; et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int. J. Clin. Pract. 2015, 69, 199–217. [Google Scholar] [CrossRef] [Green Version]
- Gacci, M.; Corona, G.; Sebastianelli, A.; Serni, S.; De Nunzio, C.; Maggi, M.; Vignozzi, L.; Novara, G.; McVary, K.T.; Kaplan, S.A.; et al. Male Lower Urinary Tract Symptoms and Cardiovascular Events: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 788–796. [Google Scholar] [CrossRef]
- Yoshimura, K. Correlates for nocturia: A review of epidemiological studies. Int. J. Urol. 2012, 19, 317–329. [Google Scholar] [CrossRef]
- Van Doorn, B.; Bosch, J.L. Nocturia in older men. Maturitas 2012, 71, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.P.; Blaivas, J.G. Nocturnal polyuria versus overactive bladder in nocturia. Urology 2002, 60 (Suppl. S1), 28–32. [Google Scholar] [CrossRef]
- Miyazato, M.; Yonemoto, K.; Ashikari, A.; Saito, S.; Yamashiro, K.; Uehara, M.; Masuzaki, H.; Ishida, H.; Matsushita, M. Validation of a novel digital health monitoring system to measure the volume of voided urine. Neurourol. Urodyn. 2019, 38, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Ashikari, A.; Nakamura, K.; Nakamura, T.; Yamashiro, K.; Uema, T.; Uehara, M.; Masuzaki, H.; Saito, S.; Maeda, S.; et al. Effect of a mobile digital intervention to enhance physical activity in individuals with metabolic disorders on voiding patterns measured by 24-h voided volume monitoring system: Kumejima Digital Health Project (KDHP). Int. Urol. Nephrol. 2021, 53, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Natsume, O. A clinical investigation of nocturnal polyuria in patients with nocturia: A diurnal variation in arginine vasopressin secretion and its relevance to mean blood pressure. J. Urol. 2006, 176, 660–664. [Google Scholar] [CrossRef]
- Blanker, M.H.; Bernsen, R.M.D.; Bosch, J.L.H.R.R.; Thomas, S.; Groeneveld, F.P.M.J.; Prins, A.; Bohnen, A.M. Normal values and determinants of circadian urine production in older men: A population based study. J. Urol. 2002, 168 Pt 1, 1453–1457. [Google Scholar] [CrossRef]
- Miyazato, M.; Tohyama, K.; Touyama, M.; Nakamura, H.; Oshiro, T.; Ueda, S.; Saito, S. Effect of continuous positive airway pressure on nocturnal urine production in patients with obstructive sleep apnea syndrome. Neurourol. Urodyn. 2017, 36, 376–379. [Google Scholar] [CrossRef]
- Takayama, M.; Omori, S.; Iwasaki, K.; Shiomi, E.; Takata, R.; Sugimura, J.; Abe, T.; Obara, W. Relationship between nocturnal polyuria and non-dipping blood pressure in male patients with lower urinary tract symptoms. Low Urin Tract. Symptoms 2019, 11, O98–O102. [Google Scholar] [CrossRef]
- Kimura, G.; Dohi, Y.; Fukuda, M. Salt sensitivity and circadian rhythm of blood pressure: The keys to connect CKD with cardiovascular events. Hypertens. Res. 2010, 33, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, O.; Hiro, S.; Hotta, S.; Mogami, S.; Yamagami, H. Efficacy of fesoterodine on nocturia and quality of sleep in Asian patients with overactive bladder. Urology 2014, 83, 750–755. [Google Scholar] [CrossRef]
- Leger, D.; Debellemaniere, E.; Rabat, A.; Bayon, V.; Benchenane, K.; Chennaoui, M. Slow-wave sleep: From the cell to the clinic. Sleep Med. Rev. 2018, 41, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N. The physiology of sleep and the impact of ageing. Eur. Urol. Suppl. 2005, 3, 17–23. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doorn, B.; Kok, E.T.; Blanker, M.H.; Martens, E.P.; Bohnen, A.M.; Bosch, J.R. The natural history and predictive factors of voided volume in older men: The Krimpen Study. J. Urol. 2011, 185, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Nocturnal polyuria in older people: Pathophysiology and clinical implications. J. Am. Geriatr. Soc. 2000, 48, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Kendig, D.M.; Ets, H.K.; Moreland, R.S. Effect of type II diabetes on male rat bladder contractility. Am. J. Physiol. Renal. Physiol. 2016, 310, F909–F922. [Google Scholar] [CrossRef] [Green Version]
- Nomiya, M.; Sagawa, K.; Yazaki, J.; Takahashi, N.; Kushida, N.; Haga, N.; Aikawa, K.; Matsui, T.; Oka, M.; Fukui, T.; et al. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol. Urodyn. 2012, 31, 185–189. [Google Scholar] [CrossRef]
- Zhang, Q.; Siroky, M.; Yang, J.-H.; Zhao, Z.; Azadzoi, K. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology 2014, 84, e1–e7. [Google Scholar] [CrossRef]
- Inoue, S.; Saito, M.; Tsounapi, P.; Dimitriadis, F.; Ohmasa, F.; Kinoshita, Y.; Satoh, K.; Takenaka, A. Effect of silodosin on detrusor overactivity in the male spontaneously hypertensive rat. BJU Int. 2012, 110 Pt 2, E118–E124. [Google Scholar] [CrossRef]
- Tarcan, T.; Azadzoi, K.M.; Siroky, M.B.; Goldstein, I.; Krane, R.J. Age-related erectile and voiding dysfunction: The role of arterial insufficiency. Br. J. Urol. 1998, 82 (Suppl. S1), 26–33. [Google Scholar] [CrossRef]
- Watanabe, J.; Kotani, K. Metabolic Syndrome for Cardiovascular Disease Morbidity and Mortality Among General Japanese People: A Mini Review. Vasc. Health Risk Manag. 2020, 16, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Examination Committee of Criteria for ’Obesity Disease’ in Japan and J.S.f.t.S.o. Obesity, New Criteria for ‘Obesity Disease’ in Japan. Circ. J. 2002, 66, 987–992.
- Fujisawa, D.; Nakagawa, A.; Tajima, M.; Sado, M.; Kikuchi, T.; Iba, M.; Hanaoka, M.; Ono, Y. Reliability and validity of quick inventory of depressive symptomatology Japanese version. Seishin Shinkeigaku Zasshi 2008, 2008, S-324. [Google Scholar]
- Miyauchi, Y.; Okazoe, H.; Okujyo, M.; Inada, F.; Kakehi, T.; Kikuchi, H.; Ichikawa, H.; Arakawa, Y.; Mori, Y.; Kakehi, Y. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015, 85, 333–336. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 59) | Non-Obesity (N = 29) | Obesity (N = 30) | p-Value | |
|---|---|---|---|---|
| Age (years) | 54.2 ± 12.9 | 56.1 ± 12.9 | 52.3 ± 12.7 | 0.2552 |
| Sex, n (%) | 0.5221 | |||
| Female | 26 (44.1) | 14 (48.3) | 12 (40.0) | |
| Male | 33 (55.9) | 15 (51.7) | 18 (60.0) | |
| Body mass index (kg/m2) | 25.5 ± 3.77 | 22.9 ± 1.88 | 28.0 ± 3.39 | <0.0001 |
| Abdominal circumference (cm) | 88.8 ± 10.00 | 82.3 ± 6.73 | 95.1 ± 8.4 | <0.0001 |
| Systolic blood pressure (mmHg) | 135.0 ± 19.8 | 128.2 ± 19.6 | 141.8 ± 17.9 | 0.0079 |
| Diastolic blood pressure (mmHg) | 80.2 ± 13.5 | 73.0 ± 10.8 | 87.3 ± 12.3 | <0.0001 |
| Fasting glucose (mg/dL) | 84.5 (80–92) | 84 (80–89) | 85 (79.5–92.5) | 0.5743 |
| HbA1c (%) | 5.6 ± 0.35 | 5.6 ± 0.40 | 5.6 ± 0.31 | 0.4177 |
| Triglyceride (mg/dL) | 104 (81–153) | 101 (68–123.5) | 119.5 (86.25–244) | 0.0243 |
| Total cholesterol (mg/dL) | 200.3 ± 25.8 | 196.2 ± 28.4 | 204.3 ± 22.7 | 0.2334 |
| HDL-C (mg/dL) | 59.4 ± 16.3 | 63.0 ± 17.7 | 56.0 ± 14.3 | 0.1013 |
| LDL-C (mg/dL) | 111.8 ± 28.6 | 112.4 ± 25.6 | 111.2 ± 31.6 | 0.8678 |
| Creatinine (mg/dL) | 0.73 ± 0.17 | 0.72 ± 0.14 | 0.73 ± 0.19 | 0.8624 |
| Non-Obesity (N = 29) | Obesity (N = 30) | p-Value | |
|---|---|---|---|
| Daytime urinary frequency, CLSS Q1 | 0 (0–1) | 0 (0–1) | 0.6890 |
| Nighttime urinary frequency, CLSS Q2 | 0 (0–1) | 1 (0.75–1) | 0.012 |
| Urinary urgency, CLSS Q3 | 0 (0–1) | 0 (0–1) | 0.7072 |
| Urinary urgency incontinence, CLSS Q4 | 0 (0) | 0 (0) | 0.7078 |
| Stress urinary incontinence, CLSS Q5 | 0 (0) | 0 (0–0.25) | 0.6193 |
| Weak urinary stream, CLSS Q6 | 0 (0–1) | 0 (0–1) | 0.9229 |
| Straining, CLSS Q7 | 0 (0) | 0 (0) | 0.3713 |
| Feeling of incomplete emptying, CLSS Q8 | 0 (0–1) | 0 (0–1) | 0.7612 |
| Bladder pain, CLSS Q9 | 0 (0) | 0 (0) | 0.2918 |
| Urethral pain, CLSS Q10 | 0 (0) | 0 (0) | 0.1325 |
| Mid-nocturnal insomnia, QIDS-J Q2 | 2 (1–2) | 3 (1–3) | 0.0058 |
| Non-Obesity (N = 29) | Obesity (N = 30) | p-Value | |
|---|---|---|---|
| Water intake (mL/day) | 1708.3 ± 705.9 | 1925.5 ± 765.8 | 0.2624 |
| Daytime urinary frequency (times) | 6.0 (5–7) | 6.0 (4.75–8) | 0.6789 |
| Nighttime urinary frequency (times) | 1.0 (0–1) | 1.0 (0–2) | 0.2414 |
| Daytime urinary volume (mL) | 587.3 (408.4–861.5) | 535.1 (355.5–662.1) | 0.3958 |
| Nocturnal urinary volume (mL) | 190.4 (124.1–405.8) | 195.6 (93.3–283.7) | 0.2955 |
| NPi (%) | 24.1 (18.0–38.0) | 25.2 (12.9–32.9) | 0.6712 |
| MBC (mL) | 226.9 (171.6–315.6) | 186.1 (128.6–293.6) | 0.1541 |
| HUS (min) | 330 (198.0–416.0) | 211.5 (45.75–411.0) | 0.1541 |
| Average urine flow rate (mL/s) | 23.6 (19.0–25.4) | 18.8 (14.0–25.2) | 0.5956 |
| Cases in | Cases in | Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| Non-Obesity | Obesity | for Obesity (vs. Non-Obesity) | ||||
| (N = 29) | (N = 30) | Crude | p-Value | Age and Sex-Adjusted | p-Value | |
| Nighttime frequency (≥score 1), CLSS Q2 | 12 | 23 | 4.11 (1.32–12.80) | 0.0149 | 7.65 (1.88–31.14) | 0.0045 |
| Mid-nocturnal insomnia (≥score 2), QIDS-J Q2 | 13 | 20 | 1.73 (0.53–5.64) | 0.3626 | 1.82 (0.55–6.08) | 0.3301 |
| Nighttime frequency (≥1 time), s-HMSU | 18 | 22 | 1.68 (0.56–5.07) | 0.3565 | 2.45 (0.67–9.14) | 0.1724 |
| Increased Npi (>33%), s-HMSU | 11 | 7 | 0.50 (0.16–1.54) | 0.2269 | 0.57 (0.17–1.89) | 0.3602 |
| Decreased MBC (<212 mL), s-HMSU | 12 | 17 | 1.85 (0.66–5.21) | 0.2422 | 2.27 (0.76–6.78) | 0.1780 |
| Shortened HUS (<302 min), s-HMSU | 11 | 18 | 2.45 (0.86–7.00) | 0.0928 | 5.17 (1.33–20.03) | 0.0175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashikari, A.; Miyazato, M.; Nakamura, K.; Yamashiro, K.; Nakamura, T.; Uema, T.; Uehara, M.; Masuzaki, H.; Saito, S.; Maeda, S.; et al. Obesity and Voiding Parameters in a Community-Based Population of Okinawa, Japan: Kumejima Digital Health Project (KDHP). Metabolites 2022, 12, 468. https://doi.org/10.3390/metabo12050468
Ashikari A, Miyazato M, Nakamura K, Yamashiro K, Nakamura T, Uema T, Uehara M, Masuzaki H, Saito S, Maeda S, et al. Obesity and Voiding Parameters in a Community-Based Population of Okinawa, Japan: Kumejima Digital Health Project (KDHP). Metabolites. 2022; 12(5):468. https://doi.org/10.3390/metabo12050468
Chicago/Turabian StyleAshikari, Asuka, Minoru Miyazato, Koshi Nakamura, Kiyoto Yamashiro, Takehiro Nakamura, Tsugumi Uema, Moriyuki Uehara, Hiroaki Masuzaki, Seiichi Saito, Shiro Maeda, and et al. 2022. "Obesity and Voiding Parameters in a Community-Based Population of Okinawa, Japan: Kumejima Digital Health Project (KDHP)" Metabolites 12, no. 5: 468. https://doi.org/10.3390/metabo12050468
APA StyleAshikari, A., Miyazato, M., Nakamura, K., Yamashiro, K., Nakamura, T., Uema, T., Uehara, M., Masuzaki, H., Saito, S., Maeda, S., Ishida, H., & Matsushita, M. (2022). Obesity and Voiding Parameters in a Community-Based Population of Okinawa, Japan: Kumejima Digital Health Project (KDHP). Metabolites, 12(5), 468. https://doi.org/10.3390/metabo12050468