Circulating PCSK9 Linked to Dyslipidemia in Lebanese Schoolchildren
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Population
2.2. Distribution of Lipid Parameters According to Age and Gender
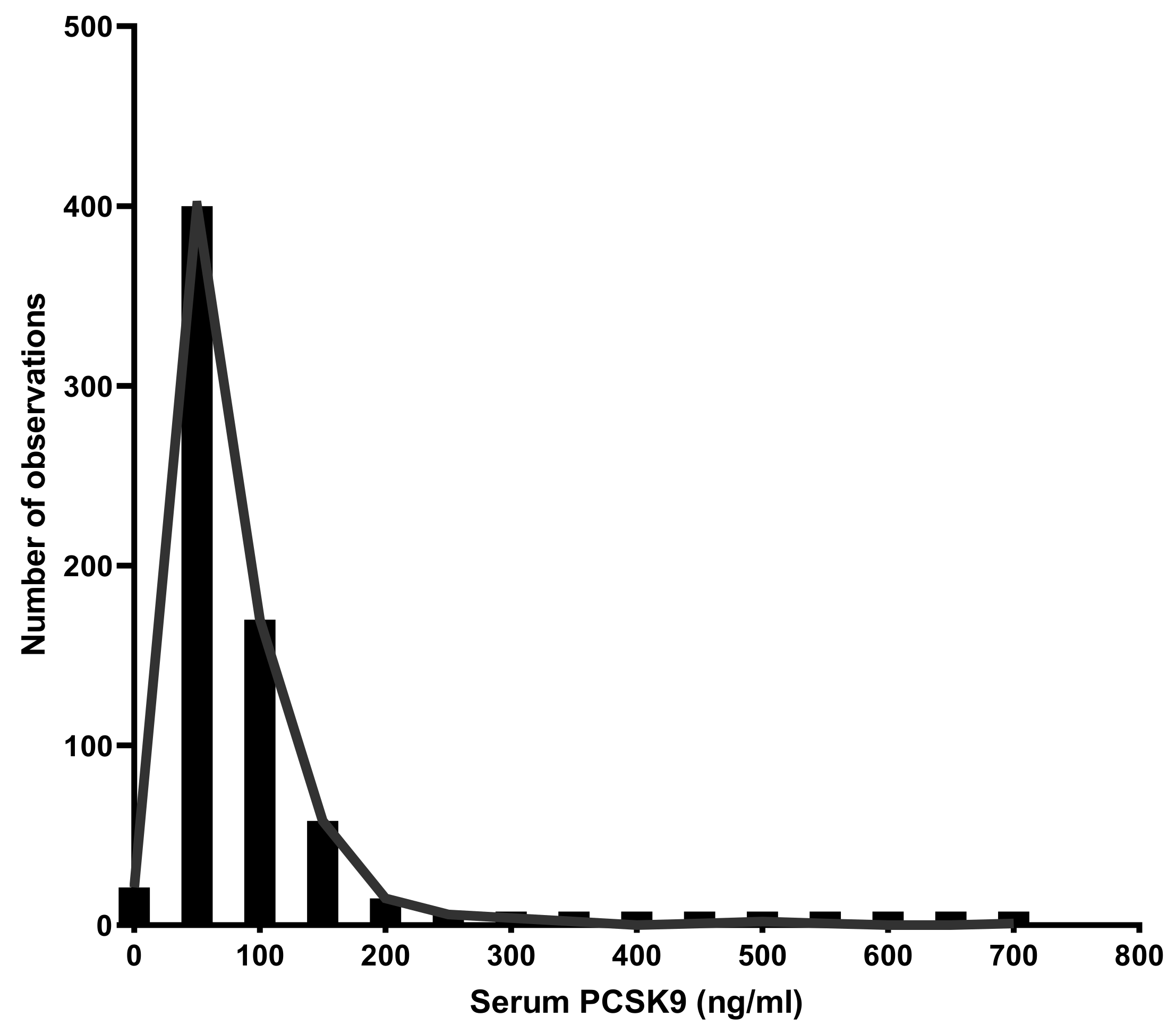
2.3. PCSK9 Levels Distribution in the Overall Population
2.4. PCSK9 Relationship with Lipid Parameters
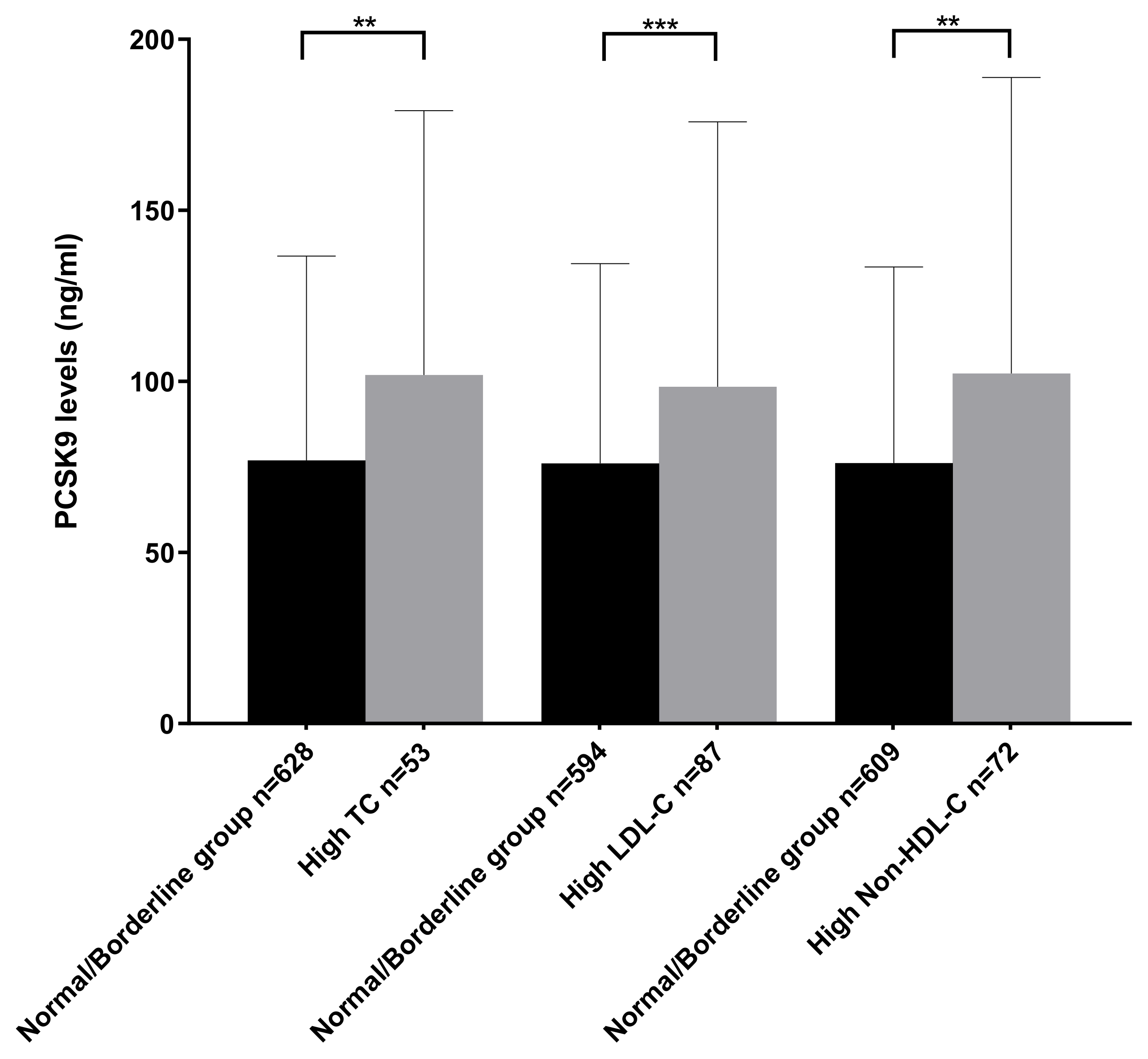
2.5. Comparison of PCSK9 Levels between Normolipidemic and Hyperlipidemic Subgroups
2.6. Relationship between PCSK9 and Menarche in Girls and Testosterone in Boys
2.7. Multiple Linear Regression Analysis with PCSK9 as the Dependent Variable
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Biological Parameters
4.2.1. Serum PCSK9 Assay
4.2.2. Lipid Profile
4.2.3. Lipoprotein (a)
4.2.4. Total Testosterone
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e603–e634. [Google Scholar] [CrossRef] [PubMed]
- Coakley, J.C. Lipids in Children and Links to Adult Vascular Disease. Clin. Biochem. Rev. 2018, 39, 65–76. [Google Scholar] [PubMed]
- Kwiterovich, P.O. Recognition and Management of Dyslipidemia in Children and Adolescents. J. Clin. Endocrinol. Metab. 2008, 93, 4200–4209. [Google Scholar] [CrossRef] [Green Version]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular Risk Factors in Childhood and Carotid Artery Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Miksenas, H.; Januzzi, J.L., Jr.; Natarajan, P. Lipoprotein(a) and Cardiovascular Diseases. JAMA 2021, 326, 352–353. [Google Scholar] [CrossRef]
- Ellis, K.L.; Watts, G.F. Is Lipoprotein(a) Ready for Prime-Time Use in the Clinic? Cardiol. Clin. 2018, 36, 287–298. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.-P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 Cause Autosomal Dominant Hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Abifadel, M.; Elbitar, S.; El Khoury, P.; Ghaleb, Y.; Chémaly, M.; Moussalli, M.-L.; Rabès, J.-P.; Varret, M.; Boileau, C. Living the PCSK9 Adventure: From the Identification of a New Gene in Familial Hypercholesterolemia towards a Potential New Class of Anticholesterol Drugs. Curr. Atheroscler. Rep. 2014, 16, 439. [Google Scholar] [CrossRef]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M.; Coggi, D.; Bonomi, A.; Amato, M.; Frigerio, B.; Sansaro, D.; Ravani, A.; Veglia, F.; Capra, N.; et al. Sex-Specific Predictors of PCSK9 Levels in a European Population: The IMPROVE Study. Atherosclerosis 2020, 309, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Xu, R.-X.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Qing, P.; Liu, G.; Dong, Q.; Li, J.-J. Proprotein Convertase Subtilisin-Kexin Type 9 as a Biomarker for the Severity of Coronary Artery Disease. Ann. Med. 2015, 47, 386–393. [Google Scholar] [CrossRef]
- Huijgen, R.; Fouchier, S.W.; Denoun, M.; Hutten, B.A.; Vissers, M.N.; Lambert, G.; Kastelein, J.J.P. Plasma Levels of PCSK9 and Phenotypic Variability in Familial Hypercholesterolemia. J. Lipid Res. 2012, 53, 979–983. [Google Scholar] [CrossRef] [Green Version]
- Alborn, W.E.; Cao, G.; Careskey, H.E.; Qian, Y.-W.; Subramaniam, D.R.; Davies, J.; Conner, E.M.; Konrad, R.J. Serum Proprotein Convertase Subtilisin Kexin Type 9 Is Correlated Directly with Serum LDL Cholesterol. Clin. Chem. 2007, 53, 1814–1819. [Google Scholar] [CrossRef] [Green Version]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and Metabolic Determinants of Plasma PCSK9 Levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef]
- Baass, A.; Dubuc, G.; Tremblay, M.; Delvin, E.E.; O’Loughlin, J.; Levy, E.; Davignon, J.; Lambert, M. Plasma PCSK9 Is Associated with Age, Sex, and Multiple Metabolic Markers in a Population-Based Sample of Children and Adolescents. Clin. Chem. 2009, 55, 1637–1645. [Google Scholar] [CrossRef] [Green Version]
- Georges, N.; Simon, A.; Naim, B.; Georges, N.; Georges, A.F.; Tanios, A. Universal Screening Program for Lipid Disorders in 2–10 Years Old Lebanese Children: A New Approach. Int. J. Pediatr. Adolesc. Med. 2019, 6, 101–108. [Google Scholar] [CrossRef]
- Steiner, M.J.; Skinner, A.C.; Perrin, E.M. Fasting Might Not Be Necessary Before Lipid Screening: A Nationally Representative Cross-Sectional Study. Pediatrics 2011, 128, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Aydenian, H.; Fadel, M.A.; Baddoura, R. Epidemiological study of hyperlipemia in a Lebanese population. Ann. Biol. Clin. 1999, 57, 697–703. [Google Scholar]
- Hirbli, K.; Barakat-el Khoury, W.; Abi-Saab, M.; Bitar, H.; Najem, R. Blood cholesterol profile of the Lebanese population. Diabete Metab. 1990, 16, 435–440. [Google Scholar] [PubMed]
- Gannagé-Yared, M.-H.; Farah, V.; Chahine, E.; Balech, N.; Ibrahim, T.; Asmar, N.; Barakett-Hamadé, V.; Jambart, S. Distribution and Correlates of Non-High-Density Lipoprotein Cholesterol and Triglycerides in Lebanese School Children. J. Clin. Lipidol. 2016, 10, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Saadé, C.; Sleilaty, G.; Gannagé-Yared, M.-H. Longitudinal Changes of Lipid Profile in the Lebanese Pediatric Population. Lipids Health Dis. 2019, 18, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial Hypercholesterolemias: Prevalence, Genetics, Diagnotics and Screening Recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S9–S17. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Tavori, H.; Fazio, S. PCSK9: From Basic Science Discoveries to Clinical Trials. Circ. Res. 2018, 122, 1420–1438. [Google Scholar] [CrossRef]
- Tavori, H.; Fan, D.; Blakemore, J.L.; Yancey, P.G.; Ding, L.; Linton, M.F.; Fazio, S. Serum Proprotein Convertase Subtilisin/Kexin Type 9 and Cell Surface Low-Density Lipoprotein Receptor: Evidence for a Reciprocal Regulation. Circulation 2013, 127, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Raal, F.; Panz, V.; Immelman, A.; Pilcher, G. Elevated PCSK9 Levels in Untreated Patients with Heterozygous or Homozygous Familial Hypercholesterolemia and the Response to High-Dose Statin Therapy. J. Am. Heart Assoc. 2013, 2, e000028. [Google Scholar] [CrossRef] [Green Version]
- Ruscica, M.; Ferri, N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and Arterial Stiffness in a Large Population Sample: Data from the Brisighella Heart Study. J. Am. Heart. Assoc. 2017, 6, e005764. [Google Scholar] [CrossRef] [Green Version]
- Leander, K.; Mälarstig, A.; Van’t Hooft, F.M.; Hyde, C.; Hellénius, M.-L.; Troutt, J.S.; Konrad, R.J.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef]
- Lambert, G.; Ancellin, N.; Charlton, F.; Comas, D.; Pilot, J.; Keech, A.; Patel, S.; Sullivan, D.R.; Cohn, J.S.; Rye, K.-A.; et al. Plasma PCSK9 Concentrations Correlate with LDL and Total Cholesterol in Diabetic Patients and Are Decreased by Fenofibrate Treatment. Clin. Chem. 2008, 54, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavori, H.; Christian, D.; Minnier, J.; Plubell, D.; Shapiro, M.D.; Yeang, C.; Giunzioni, I.; Croyal, M.; Duell, P.B.; Lambert, G.; et al. PCSK9 Association with Lipoprotein(a). Circ. Res. 2016, 119, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mba, C.M.; Mbacham, W.; Sobngwi, E.; Mbanya, J.C. Is PCSK9 Associated with Plasma Lipid Levels in a Sub-Saharan African Population of Patients with Obesity and Type 2 Diabetes? Diabetes Metab. Syndr. Obes. 2019, 12, 2791–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, L.; Gälman, C.; Angelin, B.; Rudling, M. Importance of Proprotein Convertase Subtilisin/Kexin Type 9 in the Hormonal and Dietary Regulation of Rat Liver Low-Density Lipoprotein Receptors. Endocrinology 2009, 150, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, L.; Henriksson, P.; Westerlund, E.; Hovatta, O.; Angelin, B.; Rudling, M. Endogenous Estrogens Lower Plasma PCSK9 and LDL Cholesterol but Not Lp(a) or Bile Acid Synthesis in Women. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 810–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Gälman, C.; Rudling, M.; Angelin, B. Influence of Physiological Changes in Endogenous Estrogen on Circulating PCSK9 and LDL Cholesterol. J. Lipid Res. 2015, 56, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Ooi, T.C.; Raymond, A.; Cousins, M.; Favreau, C.; Taljaard, M.; Gavin, C.; Jolly, E.E.; Malone, S.; Eapen, L.; Chretien, M.; et al. Relationship between Testosterone, Estradiol and Circulating PCSK9: Cross-Sectional and Interventional Studies in Humans. Clinica Chimica Acta 2015, 446, 97–104. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Terentes-Printzios, D.; Georgiopoulos, G.; Skoumas, I.; Koutagiar, I.; Ioakeimidis, N.; Stefanadis, C.; Tousoulis, D. Prediction of Cardiovascular Events with Levels of Proprotein Convertase Subtilisin/Kexin Type 9: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 252, 50–60. [Google Scholar] [CrossRef]
- Haney, E.M.; Huffman, L.H.; Bougatsos, C.; Freeman, M.; Steiner, R.D.; Nelson, H.D. Screening and Treatment for Lipid Disorders in Children and Adolescents: Systematic Evidence Review for the US Preventive Services Task Force. Pediatrics 2007, 120, e189–e214. [Google Scholar] [CrossRef]
- Webber, L.S.; Srinivasan, S.R.; Wattigney, W.A.; Berenson, G.S. Tracking of Serum Lipids and Lipoproteins from Childhood to Adulthood. The Bogalusa Heart Study. Am. J. Epidemiol. 1991, 133, 884–899. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and Inflammation: A Review of Experimental and Clinical Evidence. Eur. Heart. J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef]
- Cariou, B.; Langhi, C.; Le Bras, M.; Bortolotti, M.; Lê, K.-A.; Theytaz, F.; Le May, C.; Guyomarc’h-Delasalle, B.; Zaïr, Y.; Kreis, R.; et al. Plasma PCSK9 Concentrations during an Oral Fat Load and after Short Term High-Fat, High-Fat High-Protein and High-Fructose Diets. Nutr. Metab. 2013, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; Ciccone, M.M. Nutraceuticals and Dyslipidaemia: Beyond the Common Therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Gannagé-Yared, M.-H.; Lahoud, C.; Younes, N.; Chedid, R.; Sleilaty, G. Prevalence and Status of Lipoprotein (a) among Lebanese School Children. Sci. Rep. 2020, 10, 20620. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
- Eilers, L.; Zachariah, J.P. Lipid Screening in Youth. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Emerging Risk Factors Collaboration. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic Variants Associated with Lp(a) Lipoprotein Level and Coronary Disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, R.; Boekholdt, S.M.; Stoekenbroek, R.M.; Hovingh, G.K.; Witztum, J.L.; Wareham, N.J.; Sandhu, M.S.; Khaw, K.-T.; Tsimikas, S. Population and Assay Thresholds for the Predictive Value of Lipoprotein (a) for Coronary Artery Disease: The EPIC-Norfolk Prospective Population Study. J. Lipid Res. 2016, 57, 697–705. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, P.; Roussel, R.; Fumeron, F.; Abou-Khalil, Y.; Velho, G.; Mohammedi, K.; Jacob, M.-P.; Steg, P.G.; Potier, L.; Ghaleb, Y.; et al. Plasma Proprotein-Convertase-Subtilisin/Kexin Type 9 (PCSK9) and Cardiovascular Events in Type 2 Diabetes. Diabetes Obes. Metab. 2018, 20, 943–953. [Google Scholar] [CrossRef]
- Frontini, M.G.; Srinivasan, S.R.; Xu, J.; Tang, R.; Bond, M.G.; Berenson, G.S. Usefulness of Childhood Non-High Density Lipoprotein Cholesterol Levels versus Other Lipoprotein Measures in Predicting Adult Subclinical Atherosclerosis: The Bogalusa Heart Study. Pediatrics 2008, 121, 924–929. [Google Scholar] [CrossRef]
| Total Population (n = 681) | Boys (n = 347) | Girls (n = 334) | |
|---|---|---|---|
| Age | 12.94 (10.68–14.73) | 13.21 (10.80–14.73) | 12.54 (10.52–14.73) |
| Age groups | |||
| 8–11 years (n = 279) | 40.97% | 37.18% | 44.91% |
| 12–14 years (n = 243) | 35.68% | 39.19% | 32.04% |
| 15–18 years (n = 159) | 23.35% | 23.63% | 23.05% |
| BMI percentiles | |||
| Obese (n = 77) | 11.31% | 13.26% | 9.28% |
| Overweight (n = 130) | 19.09% | 21.90% | 16.17% |
| Normal or thinness (n = 474) | 69.60% | 64.84% | 74.55% |
| Lipid parameters | |||
| TC (mmol/L) | 4.10 (3.60–4.60) | 4.00 (3.54–4.50) | 4.19 (3.60–4.60) |
| LDL-C (mmol/L) | 2.50 (2.08–2.96) | 2.45 (2.05–2.94) | 2.56 (2.14–3.01) |
| TG (mmol/L) | 1.08 (0.80–1.56) | 1.05 (0.75–1.48) | 1.14 (0.84–1.61) |
| HDL-C (mmol/L) | 1.30 (1.10–1.50) | 1.25 (1.10–1.50) | 1.30 (1.10–1.50) |
| Non-HDL-C (mmol/L) | 2.70 (2.30–3.20) | 2.61(2.27–3.20) | 2.80 (2.32–3.30) |
| Lp(a) (nmol/L) | 26 (10–48) | 25 (10–46) | 26 (10–55) |
| Characteristics | Total Population | Boys | Girls | p-Value (Gender) |
|---|---|---|---|---|
| Total population | 63.22 (44.59–91.80) | 65.14 (42.40–95.80) | 62.05 (46.48–88.45) | 0.60 |
| Age groups | ||||
| 8–11 years (n = 279) | 64.30 (46.95–93.38) | 71.08 (47.55–109.0) | 59.63 (46.26–86.75) | 0.07 |
| 12–14 years (n = 243) | 63.22 (42.82–86.83) | 63.62 (40.53–85.91) | 63.16 (46.36–88.18) | 0.91 |
| 15–18 years (n = 159) | 60.55 (44.51–93.96) | 58.70 (40.69–86.03) | 61.74 (47.30–95.34) | 0.35 |
| p-value (age groups) | 0.49 | 0.13 | 0.71 | |
| BMI percentiles | ||||
| Obese | 64.86 (46.60–90.45) | 67.41 (48.34–99.83) | 58.84 (44.76–85.71) | 0.55 |
| Overweight | 68.30 (49.17–100.10) | 73.80 (48.97–102.60) | 62.67 (48.94–96.08) | 0.39 |
| Normal or thinness | 61.56 (43.26–89.69) | 60.02 (40.61–94.41) | 62.25 (46.36–88.70) | 0.75 |
| p-value (BMI group) | 0.13 | 0.15 | 0.76 |
| Normal/ Borderline Group (n = 628) | High TC (n = 53) | Normal/ Borderline Group (n = 594) | High LDL-C (n = 87) | Normal/ Borderline Group (n = 609) | High Non-HDL-C n = 72) | Normal/ Borderline Group (n = 593) | High Lp(a) (n = 88) | Normal/ Borderline Group (n = 483) | High TG (n = 198) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 12.95 (10.74–14.71) | 12.72 (10.15–15.15) | 12.95 (10.67–14.71) | 12.94 (10.92–15.28) | 12.94 (10.72–14.69) | 13.00 (10.32–15.40) | 12.90 (10.59–14.71) | 13.31 (11.32–14.95) | 13.18 (10.88–15.23) | 12.33 (10.34–14.24) |
| PCSK9 value (ng/mL) | 62.29 (44.05–89.17) | 82.10 (55.72–120.6) | 61.30 (43.98–88.25) | 77.25 (53.60–119.10) | 61.85 (43.99–89.31) | 73.40 (57.18–118.60) | 63.85 (45.31–92.13) | 58.09 (41.15–84.92) | 63.39 (45.07–90.96) | 62.95 (43.85–96.05) |
| Lipid parameters | ||||||||||
| TC (mmol/L) | 4.00 (3.57–4.40) | 5.60 (5.43–5.90) | 4.00 (3.50–4.380) | 5.30 (4.90–5.70) | 4.00 (3.52–4.40) | 5.48 (5.00–5.80) | 4.10 (3.60–4.59) | 4.10 (3.73–4.78) | 4.00 (3.50–4.47) | 4.30 (3.89–4.80) |
| LDL–C (mmol/L) | 2.44 (2.06–2.84) | 3.94 (3.59–4.20) | 2.40 (2.04–2.79) | 3.62 (3.45–4.05) | 2.41 (2.05–2.81) | 3.77 (3.55–4.10) | 2.48 (2.07–2.95) | 2.59 (2.19–3.10) | 2.40 (2.04–2.86) | 2.77 (2.29–3.25) |
| TG (mmol/L) | 1.05 (0.78–1.50) | 1.38 (1.09–1.91) | 1.025 (0.77–1.47) | 1.43 (1.10–1.92) | 1.02 (0.77–1.44) | 1.74 (1.22–2.45) | 1.07 (0.79–1.57) | 1.20 (0.84–1.54) | 0.89 (0.71–1.13) | 1.92 (1.63–2.46) |
| HDL-C (mmol/L) | 1.30 (1.10–1.50) | 1.30 (1.15–1.65) | 1.30 (1.10–1.50) | 1.20 (1.00–1.40) | 1.30 (1.10–1.50) | 1.10 (1.00–1.32) | 1.30 (1.10–1.50) | 1.30 (1.1–1.50) | 1.30 (1.20–1.50) | 1.10 (1.00–1.30) |
| Non-HDL-C (mmol/L) | 2.64 (2.30–3.10) | 4.30 (3.90–4.55) | 2.60 (2.26–3.00) | 4.00 (3.72–4.38) | 2.60 (2.30–3.10) | 4.10 (3.90–4.40) | 2.70 (2.30–3.20) | 2.90 (2.40–3.30) | 2.60 (2.20–3.00) | 3.11 (2.70–3.70) |
| Lp(a) (nmol/L) | 24 (10–46.75) | 44 (20–104) | 24 (10–46.25) | 35 (15–65) | 25 (10–48) | 32.50 (14.25–62) | 21 (8–40) | 130 (94–175) | 25 (10–48) | 29 (10–52) |
| Variable | Β | Std. Error | p-Value |
|---|---|---|---|
| A-Boys | |||
| Intercept | 4.07 | 0.28 | <0.0001 |
| Age | −0.007 | 01 | 0.62 |
| Ln (BMI) | −0.03 | 0.04 | 0.43 |
| Ln (Non-HDL-C) | 0.48 | 0.13 | 0.0003 |
| Ln (TG) | 0.02 | 0.08 | 0.84 |
| Ln (HDL-C) | 0.25 | 0.17 | 0.16 |
| Ln (Lp(a)) | −0.06 | 0.03 | 0.07 |
| B-Girls | |||
| Intercept | 3.55 | 0.23 | <0.0001 |
| Age | 0.02 | 0.01 | 0.11 |
| Ln (BMI) | −0.02 | 0.03 | 0.52 |
| Ln (Non-HDL-C) | 0.48 | 0.12 | 0.0001 |
| Ln (TG) | −0.01 | 0.07 | 0.86 |
| Ln (HDL-C) | 0.04 | 0.14 | 0.78 |
| Ln (Lp(a)) | −0.006 | 0.03 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azar, Y.; Gannagé-Yared, M.-H.; Naous, E.; Ayoub, C.; Abou Khalil, Y.; Chahine, E.; Elbitar, S.; Ghaleb, Y.; Boileau, C.; Varret, M.; et al. Circulating PCSK9 Linked to Dyslipidemia in Lebanese Schoolchildren. Metabolites 2022, 12, 504. https://doi.org/10.3390/metabo12060504
Azar Y, Gannagé-Yared M-H, Naous E, Ayoub C, Abou Khalil Y, Chahine E, Elbitar S, Ghaleb Y, Boileau C, Varret M, et al. Circulating PCSK9 Linked to Dyslipidemia in Lebanese Schoolchildren. Metabolites. 2022; 12(6):504. https://doi.org/10.3390/metabo12060504
Chicago/Turabian StyleAzar, Yara, Marie-Hélène Gannagé-Yared, Elie Naous, Carine Ayoub, Yara Abou Khalil, Elise Chahine, Sandy Elbitar, Youmna Ghaleb, Catherine Boileau, Mathilde Varret, and et al. 2022. "Circulating PCSK9 Linked to Dyslipidemia in Lebanese Schoolchildren" Metabolites 12, no. 6: 504. https://doi.org/10.3390/metabo12060504
APA StyleAzar, Y., Gannagé-Yared, M.-H., Naous, E., Ayoub, C., Abou Khalil, Y., Chahine, E., Elbitar, S., Ghaleb, Y., Boileau, C., Varret, M., El Khoury, P., & Abifadel, M. (2022). Circulating PCSK9 Linked to Dyslipidemia in Lebanese Schoolchildren. Metabolites, 12(6), 504. https://doi.org/10.3390/metabo12060504






