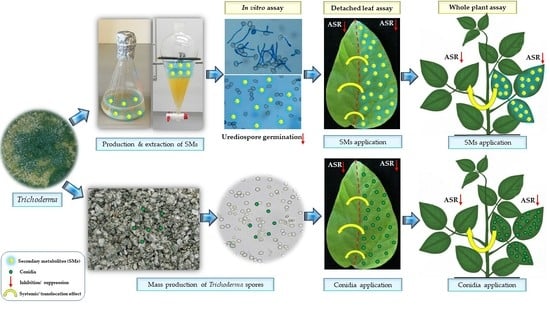
New Approaches to Manage Asian Soybean Rust (Phakopsora pachyrhizi) Using Trichoderma spp. or Their Antifungal Secondary Metabolites
Abstract
:1. Introduction
2. Results
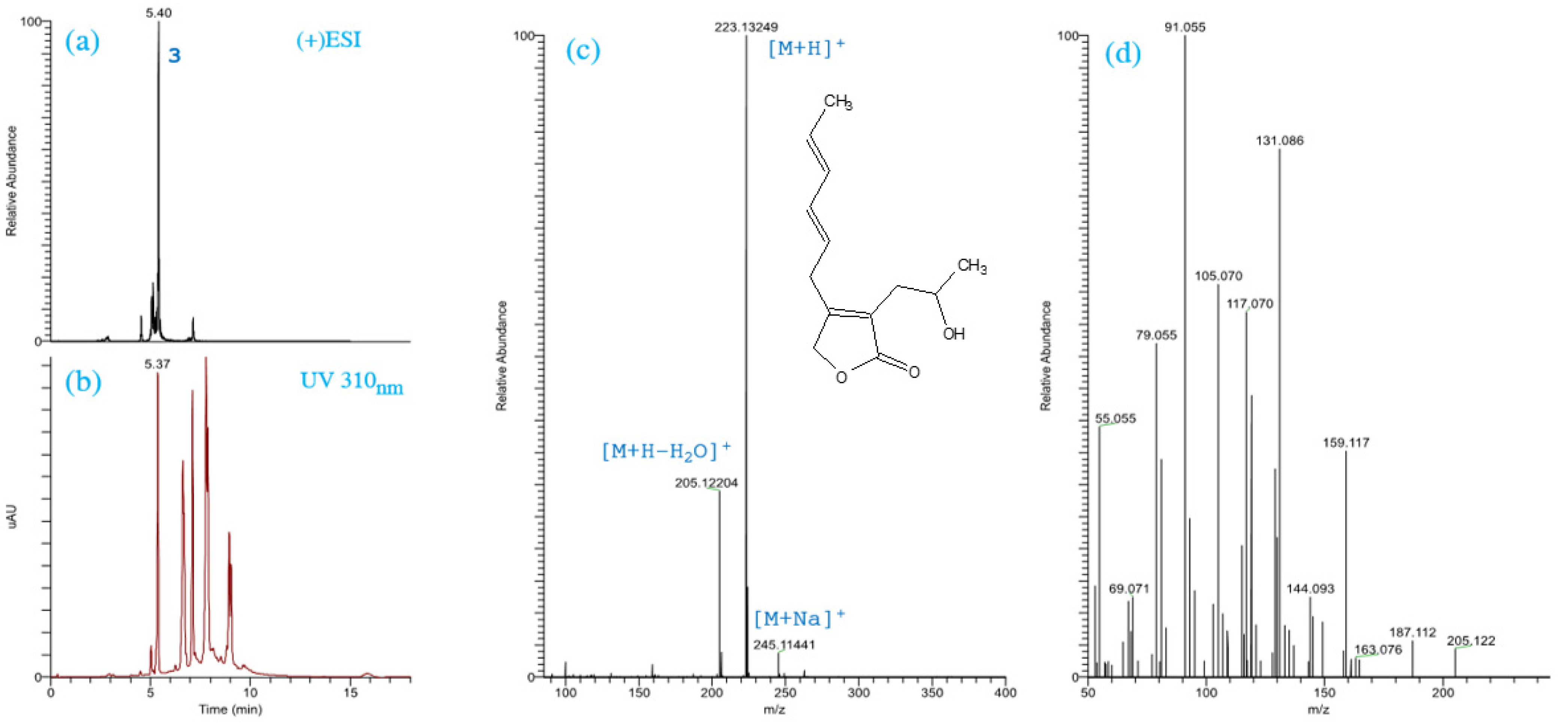
2.1. Identification of the Chemical Structures of SMs from Trichoderma spp.
2.2. In Vitro Efficacy of SMs on Urediospore Germination and Germ Tube Development
2.3. Diphasic Liquid–Solid Fermentation of Trichoderma Strains
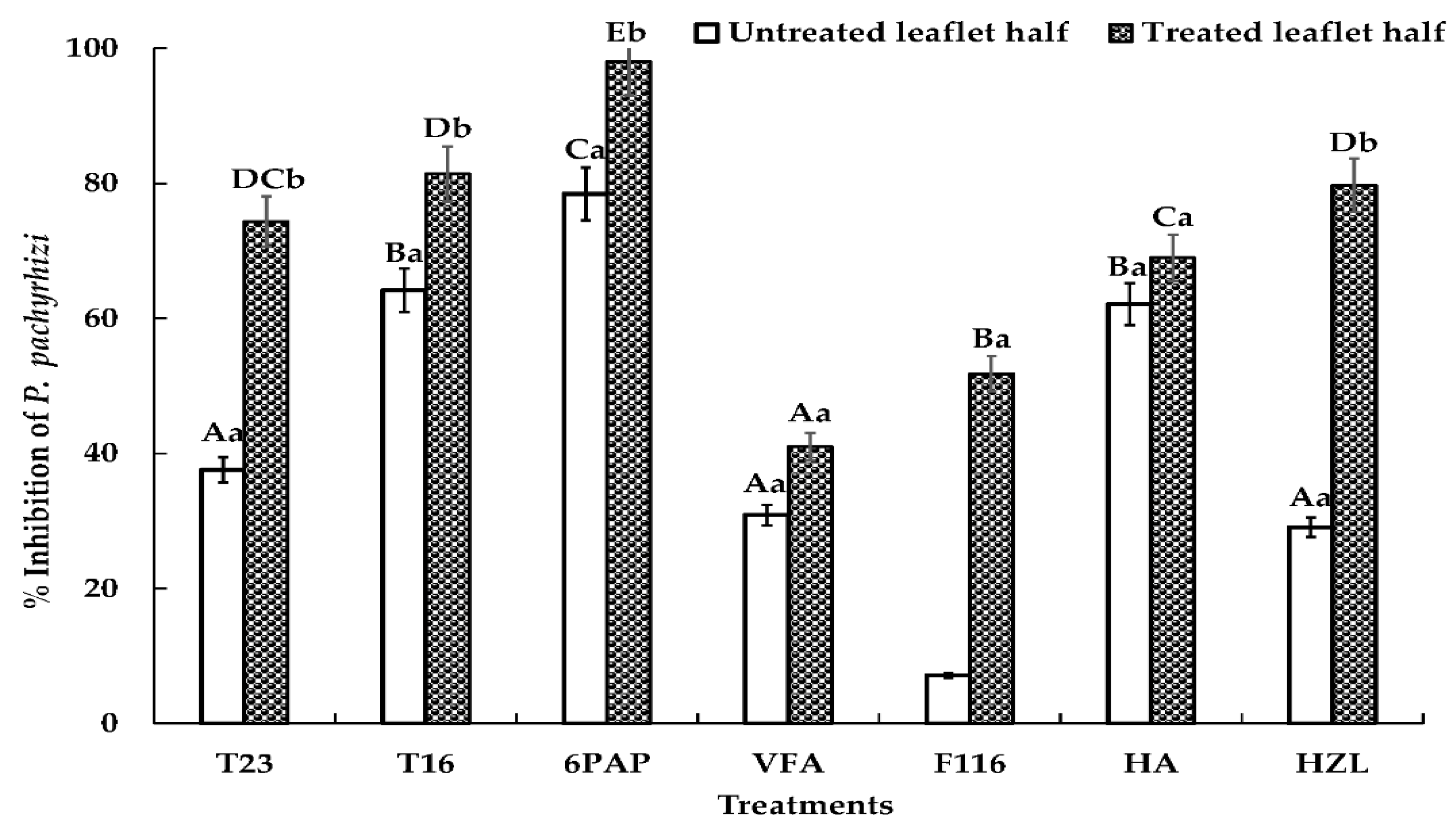
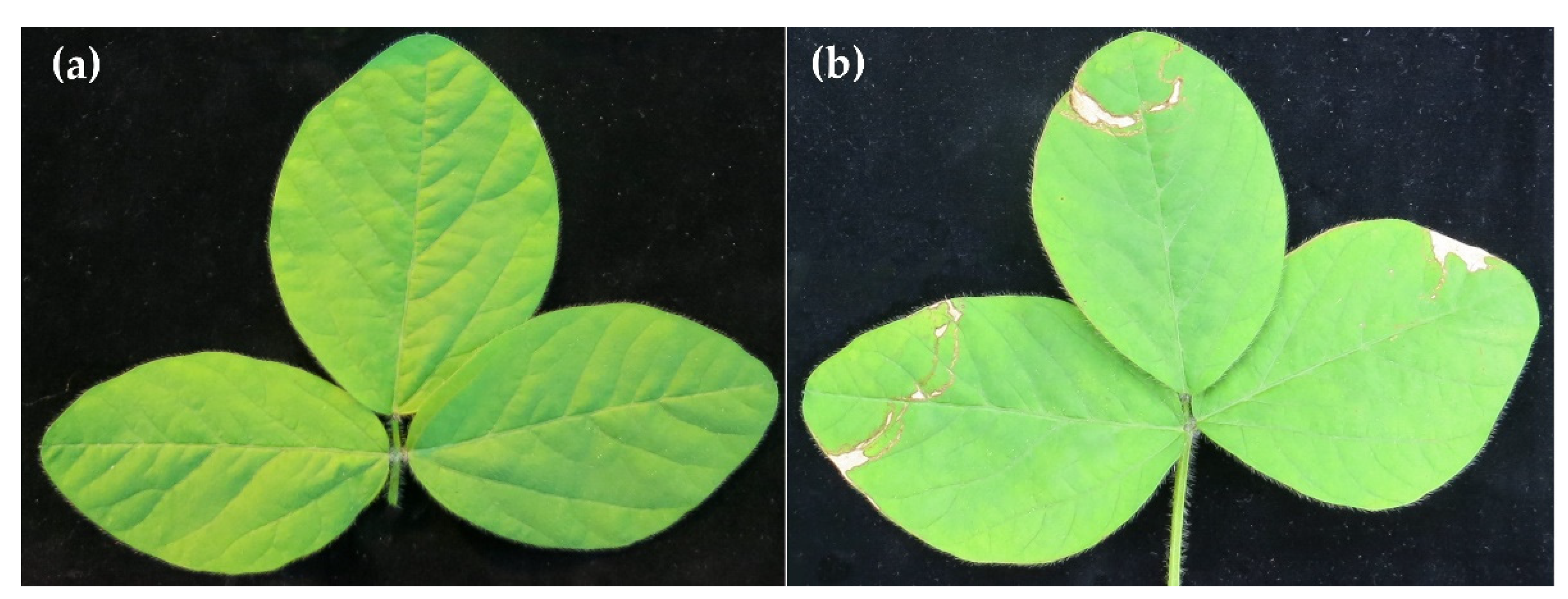
2.4. Effects of Trichoderma spp. or Their SMs on P. pachyrhizi on Detached Soybean Leaves
2.5. Validating the Efficiency of Trichoderma and Its Metabolites against P. pachyrhizi under Greenhouse Conditions
3. Discussion
4. Materials and Methods
4.1. Fungal Material
4.2. Production, Extraction, and Purification of the Secondary Metabolites from Trichoderma Cultures
4.3. Chemical Structure Elucidation of the Secondary Metabolites
4.4. Preparation of Urediospore Suspensions
4.5. Impact of Metabolites on Urediospore Germination and Germ Tube Elongation In Vitro
4.6. Starter Culture and Diphasic Liquid–Solid Fermentation of Trichoderma Strains
4.7. Effect of Trichoderma or Its SMs on P. pachyrhizi on Detached Leaves
4.8. Evaluating the Performance of Trichoderma or Its SMs against P. pachyrhizi under Greenhouse Conditions
4.9. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murithi, H.M.; Beed, F.; Tukamuhabwa, P.; Thomma, B.P.H.J.; Joosten, M.H.A.J. Soybean production in eastern and southern Africa and threat of yield loss due to soybean rust caused by Phakopsora pachyrhizi. Plant Pathol. 2016, 65, 176–188. [Google Scholar] [CrossRef]
- Koch, E.; Ebrahim-Nesbat, F.; Hoppe, H.H. Light and electron microscopic studies on the development of soybean rust (Phakopsora pachyrhizi Syd.) in susceptible soybean leaves. J. Phytopathol. 1983, 106, 302–320. [Google Scholar] [CrossRef]
- Chang, H.-X.; Miller, L.A.; Hartman, G.L. Melanin-independent accumulation of turgor pressure in appressoria of Phakopsora pachyrhizi. Phytopathology 2014, 104, 977–984. [Google Scholar] [CrossRef]
- Leonard, K.J.; Szabo, L.J. Stem rust of small grains and grasses caused by Puccinia graminis. Mol. Plant Pathol. 2005, 6, 99–111. [Google Scholar] [CrossRef]
- Edwards, H.H.; Bonde, M.R. Penetration and establishment of Phakopsora pachyrhizi in soybean leaves as observed by transmission electron microscopy. Phytopathology 2011, 101, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Yusnawan, E. Inhibition of spore germination of Phakopsora pachyrhizi using crude extracts of Amaranthus spinosus. Proc. Food Sci. 2015, 3, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Mendgen, K.; Wirsel, S.G.R.; Jux, A.; Hoffmann, J.; Boland, W. Volatiles modulate the development of plant pathogenic rust fungi. Planta 2006, 224, 1353–1361. [Google Scholar] [CrossRef] [Green Version]
- Langenbach, C.; Campe, R.; Beyer, S.F.; Mueller, A.N.; Conrath, U. Fighting Asian Soybean Rust. Front. Plant Sci. 2016, 7, 797. [Google Scholar] [CrossRef] [Green Version]
- Juliatti, F.C.; de Azevedo, L.A.S.; Juliatti, F.C. Strategies of chemical protection for controlling soybean rust. In Soybean: The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2017; pp. 35–62. [Google Scholar] [CrossRef] [Green Version]
- Erwin, D.C. Systemic fungicides: Disease control, translocation, and mode of action. Annu. Rev. Phytopathol. 1973, 11, 389–422. [Google Scholar] [CrossRef]
- Klittich, C.J.R. Fungicide mobility and the influence of physical properties. In Retention, Uptake, and Translocation of Agrochemicals in Plants; Myung, K., Satchivi, N.M., Kingston, C.K., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 95–109. ISBN 0-8412-2972-4. [Google Scholar]
- Klittich, C.J.R.; Ray, S.L. Effects of physical properties on the translaminar activity of fungicides. Pestic. Biochem. Physiol. 2013, 107, 351–359. [Google Scholar] [CrossRef]
- Godoy, C.V. Risk and management of fungicide resistance in the Asian soybean rust fungus Phakopsora pachyrhizi. In Fungicide Resistance in Crop Protection: Risk and Management; Thind, T.S., Ed.; CABI: Wallingford, UK, 2011; pp. 87–95. ISBN 9781845939052. [Google Scholar]
- Furlan, S.H.; Carvalho, F.K.; Antuniassi, U.R. Strategies for the control of Asian soybean rust (Phakopsora pachyrhizi) in Brazil: Fungicide resistance and application efficacy. Outlook Pest Manag. 2018, 29, 120–123. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Ward, N.A.; Schneider, R.W.; Aime, M.C. Colonization of soybean rust sori by Simplicillium lanosoniveum. Fungal Ecol. 2011, 4, 303–308. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.; Rashad, Y.M.; Ibrahim, S.A. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp. Physiol. Mol. Plant Pathol. 2018, 103, 84–91. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Grabski, G.C.; Mendgen, K. Einsatz von V. lecanii als biologisches Schädlingsbekämpfungsmittel gegen den Bohnenrostpilz U. appendiculatus var. appendiculatus im Feld und im Gewächshaus. J. Phytopathol. 1985, 113, 243–251. [Google Scholar] [CrossRef]
- Saksirirat, W.; Hoppe, H.-H. Degradation of uredospores of the soybean rust fungus (Phakopsora pachyrhizi Syd.) by cell-free culture filtrates of the mycoparasite Verticillium psalliotae Treschow. J. Phytopathol. 1991, 132, 33–45. [Google Scholar] [CrossRef]
- Kumar, S.; Jha, D.K. Trichothecium roseum: A potential agent for the biological control of soybean rust. Indian Phytopathol. 2002, 55, 232–234. [Google Scholar]
- Ward, N.A.; Robertson, C.L.; Chanda, A.K.; Schneider, R.W. Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the soybean rust pathogen, and its use as a biological control agent. Phytopathology 2012, 102, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Wagacha, J.M.; Muthomi, J.W.; Mutitu, E.W.; Mwaura, F.B. Control of bean rust using antibiotics produced by Bacillus and Streptomyces species-translocation and persistence in snap beans. J. Appl. Sci. Environ. Manag. 2007, 11, 165–168. [Google Scholar] [CrossRef]
- Abeysinghe, S. Systemic resistance induced by Trichoderma harzianum RU01 against Uromyces appendiculatus on Phaseolus vulgaris. J. Natl. Sci. Found. Sri Lanka 2009, 37, 203–207. [Google Scholar] [CrossRef]
- Burmeister, L.; Hau, B. Control of the bean rust fungus Uromyces appendiculatus by means of Trichoderma harzianum: Leaf disc assays on the antibiotic effect of spore suspensions and culture filtrates. BioControl 2009, 54, 575–585. [Google Scholar] [CrossRef]
- Dey, U.; Harlapur, S.I.; Dhutraj, D.N.; Suryawanshi, A.P.; Jagtap, G.P.; Apet, K.T.; Badgujar, S.L.; Gholve, V.M.; Kamble, H.N.; Kuldhar, D.P.; et al. Effect of fungicides, botanicals, bioagents and Indigenous Technology Knowledge (ITKs) on germination of urediniospores of Puccinia sorghi in vitro. Afr. J. Agric. Res. 2013, 8, 4960–4971. [Google Scholar] [CrossRef]
- Twizeyimana, M.; Hartman, G.L. Effect of selected biopesticides in reducing soybean rust (Phakopsora pachyrhizi) development. Plant Dis. 2019, 103, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Dorighello, D.V.; Forner, C.; de Campos Leite, R.M.V.B.; Bettiol, W. Management of Asian soybean rust with Bacillus subtilis in sequential and alternating fungicide applications. Australas. Plant Pathol. 2020, 49, 79–86. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Antagonistic effect of 6-pentyl-alpha-pyrone produced by Trichoderma harzianum toward Fusarium moniliforme. J. Plant Dis. Prot. 2007, 114, 62–68. [Google Scholar] [CrossRef]
- El-Hasan, A.; Buchenauer, H. Actions of 6-pentyl-alpha-pyrone in controlling seedling blight incited by Fusarium moniliforme and inducing defence responses in maize. J. Phytopathol. 2009, 157, 697–707. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Detection of viridiofungin A and other antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- El-Hasan, A.; Schöne, J.; Höglinger, B.; Walker, F.; Voegele, R.T. Assessment of the antifungal activity of selected biocontrol agents and their secondary metabolites against Fusarium graminearum. Eur. J. Plant Pathol. 2018, 150, 91–103. [Google Scholar] [CrossRef]
- Geraldine, A.M.; Lopes, F.A.C.; Carvalho, D.D.C.; Barbosa, E.T.; Rodrigues, A.R.; Brandão, R.S.; Ulhoa, C.J.; Lobo Jun, M. Cell wall-degrading enzymes and parasitism of sclerotia are key factors on field biocontrol of white mold by Trichoderma spp. Biol. Control 2013, 67, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Gomes, E.V.; do Nascimento Costa, M.; de Paula, R.G.; de Azevedo, R.R.; da Silva, F.L.; Noronha, E.F.; Ulhoa, C.J.; Monteiro, V.N.; Cardoza, R.E.; Gutiérrez, S.; et al. The Cerato-Platanin protein Epl-1 from Trichoderma harzianum is involved in mycoparasitism, plant resistance induction and self cell wall protection. Sci. Rep. 2015, 5, 17998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Chet, I. The possible role of competition between Trichoderma harzianum and Fusarium oxysporum on rhizosphere colonization. Phytopathology 1989, 79, 198–203. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Howell, C.R.; Stipanovic, R.D. Gliovirin, a new antibiotic from Gliocladium virens, and its role in the biological control of Pythium ultimum. Can. J. Microbiol. 1983, 29, 321–324. [Google Scholar] [CrossRef]
- Weindling, R.; Emerson, O.H. The isolation of a toxic substance from the culture filtrate of Trichoderma. Phytopathology 1936, 26, 1068–1070. [Google Scholar]
- Collins, R.P.; Halim, A.F. Characterization of the major aroma constituent of the fungus Trichoderma viride. J. Agric. Food Chem. 1972, 20, 437–438. [Google Scholar] [CrossRef]
- Cutler, H.G.; Cuttler, S.J.; Ross, S.A.; El Sayed, K.; Dugan, F.M.; Bartlett, M.G.; Hill, A.A.; Hill, R.A.; Parker, S.R. Koninginin G, a new metabolite from Trichoderma aureoviride. J. Nat. Prod. 1999, 62, 137–139. [Google Scholar] [CrossRef]
- Sawa, R.; Mori, Y.; Iinuma, H.; Naganawa, H.; Hamada, M.; Yoshida, S.; Furutani, H.; Kajimura, Y.; Fuwa, T.; Takeuchi, T. Harzianic acid, a new antimicrobial antibiotic from a fungus. J. Antibiot. 1994, 47, 731–732. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Vinale, F.; Ghisalberti, E.L.; Sivasithamparam, K.; Marra, R.; Ritieni, A.; Ferracane, R.; Woo, S.; Lorito, M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009, 48, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Bottiglieri, A.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; et al. Bivalent Metal-Chelating Properties of Harzianic Acid Produced by Trichoderma pleuroticola Associated to the Gastropod Melarhaphe neritoides. Molecules 2020, 25, 2147. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lanzuise, S.; et al. A novel fungal metabolite with beneficial properties for agricultural applications. Molecules 2014, 19, 9760–9772. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Hoeksma, J.; Beenker, W.A.; van der Beek, S.; den Hertog, J. Harzianic Acid has multi-target antimicrobial activity against Gram-Positive Bacteria. bioRxiv 2021. [Google Scholar] [CrossRef]
- Xie, L.; Zang, X.; Cheng, W.; Zhang, Z.; Zhou, J.; Chen, M.; Tang, Y. Harzianic acid from Trichoderma afroharzianum is a natural product inhibitor of acetohydroxyacid synthase. J. Am. Chem. Soc. 2021, 143, 9575–9584. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; Andolfi, A. Coordination Properties of the Fungal Metabolite Harzianic Acid toward Toxic Heavy Metals. Toxics 2021, 9, 19. [Google Scholar] [CrossRef]
- Kang, D.; Kim, J.; Choi, J.N.; Liu, K.H.; Lee, C.H. Chemotaxonomy of Trichoderma spp. Using mass spectrometry-based metabolite profiling. J. Microbiol. Biotechnol. 2011, 21, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Claydon, N.; Allan, M.; Hanson, J.R.; Avent, A.G. Antifungal alkyl pyrones of Trichoderma harzianum. Trans. Br. Mycol. Soc. 1987, 88, 503–513. [Google Scholar] [CrossRef]
- Scarselletti, R.; Faull, J.L. In vitro activity of 6-pentyl-α-pyrone, a metabolite of Trichoderma harzianum, in the inhibition of Rhizoctonia solani and Fusarium oxysporum f.sp. lycopersici. Mycol. Res. 1994, 98, 1207–1209. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol 2008, 72, 80–86. [Google Scholar] [CrossRef]
- DeMaggio, A.E.; Stetler, D.A. Mobilisation of storage reserves during fern spore germination. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1985, 86, 195–202. [Google Scholar] [CrossRef]
- De la Cruz-Quiroz, R.; Robledo-Padilla, F.; Aguilar, C.N.; Roussos, S. Forced aeration influence on the production of spores by Trichoderma strains. Waste Biomass Valorization 2017, 8, 2263–2270. [Google Scholar] [CrossRef]
- De la Cruz-Quiroz, R.D.; Roussos, S.; Hernandez-Castillo, D.; Rodríguez-Herrera, R.; López-López, L.I.; Castillo, F.; Aguilar, C.N. Solid-state fermentation in a bag bioreactor effect of corn cob mixed with phytopathogen biomass on spore and cellulase production by Trichoderma asperellum. In Fermentation Processes; Jozala, A.F., Ed.; IntechOpen: London, UK, 2017; pp. 43–56. ISBN 978-953-51-2927-1. [Google Scholar]
- Niranjana, S.R.; Lalitha, S.; Hariprasad, P. Mass multiplication and formulations of biocontrol agents for use against fusarium wilt of pigeonpea through seed treatment. Int. J. Pest. Manag. 2009, 55, 317–324. [Google Scholar] [CrossRef]
- Franceschi, V.T.; Alves, K.S.; Mazaro, S.M.; Godoy, C.V.; Duarte, H.S.S.; Del Ponte, E.M. A new standard area diagram set for assessment of severity of soybean rust improves accuracy of estimates and optimizes resource use. Plant Pathol. 2020, 69, 495–505. [Google Scholar] [CrossRef]
- Reis, E.M.; Deuner, E.; Zanatta, M. In vivo sensitivity of Phakopsora pachyrhizi to DMI and QoI fungicides. Summa Phytopathol. 2015, 41, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Godoy, C.V.; Koga, L.J.; Canteri, M.G. Diagrammatic scale for assessment of soybean rust severity. Fitopatol. Bras. 2006, 31, 63–68. [Google Scholar] [CrossRef]
- Govindasamy, V.; Balasubramanian, R. Biological control of groundnut rust, Puccinia arachidis, by Trichoderma harzianum. J. Plant Dis. Prot. 1989, 96, 337–345. [Google Scholar]
- Zuntini, B.; Alvarez, R.d.C.F.; Theodoro, G.d.F.; Zuffo, A.M. Effect of adding fungicide to mixtures of triazoles and strobilurins in the control of downy mildew and Asian soybean rust. Pesqui. Agropecuária Trop. 2019, 49, e53688. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, G.A.H. Method for Reducing Phytotoxicity of Fungicides. 2017 (20170367337). Available online: https://www.freepatentsonline.com/y2017/0367337.html (accessed on 4 March 2022).
- Dang, X.; Chen, B.; Liu, F.; Ren, H.; Liu, X.; Zhou, J.; Qin, Y.; Lin, D. Auxin signaling-mediated apoplastic pH modification functions in petal conical cell shaping. Cell Rep. 2020, 30, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N. Dynamic function and regulation of apoplast in the plant body. J. Plant Res. 1998, 111, 133–148. [Google Scholar] [CrossRef]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The apoplast: A key player in plant survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Inguagiato, J.C.; Miele, K.M. Influence of simulated rainfall on efficacy of fluazinam, chlorothalonil and iprodione for dollar spot control in creeping bentgrass. Crop Prot. 2016, 83, 48–55. [Google Scholar] [CrossRef]
- Schepers, H.T.A.M. Effect of rain on efficacy of fungicide deposits on potato against Phytophthora infestans. Potato Res. 1996, 39, 541–550. [Google Scholar] [CrossRef]
- Hirschburger, D.; Müller, M.; Voegele, R.T.; Link, T. Reference genes in the pathosystem Phakopsora pachyrhizi/ soybean suitable for normalization in transcript profiling. Int. J. Mol. Sci. 2015, 16, 23057–23075. [Google Scholar] [CrossRef] [Green Version]
- Finney, D.J. The estimation of the ED50 for a logistic response curve. Sankhyā Indian J. Stat. 1952, 12, 121–136. [Google Scholar]
- Bonner, F.T. Measurement and Management of Tree Seed Moisture; Research Paper SO-177; United States Department of Agriculture, Forest Service: New Orleans, LA, USA, 1981.
- Fisher, R.A. Statistical methods for research workers. In Breakthroughs in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 66–70. ISBN 978-0-387-94039-7. [Google Scholar]



| Metabolite | Producing Organism | Strain | LD50 (ppm) | |
|---|---|---|---|---|
| Germination | LGT | |||
| 6PAP | T. asperellum | T23 | 58 a | 33 a |
| VFA | T. asperellum | T23 | 299 b | 542 b |
| HA | T. harzianum | T16 | 712 c | 346 c |
| HZL | T. harzianum | T16 | 222 d | 394 d |
| F116 | T. harzianum | T16 | 2118 e | 609 e |
| Substrate | Strain | CFU (Spore g−1) |
|---|---|---|
| Whole rye kernels | T16 | 3.4 × 107 ± 0.57 × 107 Ba |
| T23 | 2.6 × 106 ± 2.15 × 106 Aa | |
| Crushed rye kernels | T16 | 1.5 × 108 ± 0.43 × 108 Bc |
| T23 | 1.3 × 107 ± 1.31 × 107 Ab | |
| Whole wheat kernels | T16 | 9.2 × 107 ± 0.64 × 107 Bb |
| T23 | 1.7 × 107 ± 2.07 × 107 Ab | |
| Crushed wheat kernels | T16 | 8.1 × 108 ± 3.12 × 108 Bd |
| T23 | 2.5 × 107 ± 0.86 × 107 Ac |
| Treatment | Treated Trifoliates | Untreated Trifoliates | ||
|---|---|---|---|---|
| % UCA | % Inhibition | % UCA | % Inhibition | |
| T16 | 27.70 ± 2.21 | 26.31 a | 16.37 ± 2.03 | 41.41 c |
| T23 | 23.25 ± 4.72 | 38.16 b | 13.03 ± 3.87 | 53.36 d |
| 6PAP | 11.90 ± 1.48 | 84.18 e | 49.70 ± 2.31 | 31.74 b |
| HA | 25.70 ± 2.86 | 65.82 d | 59.20 ± 0.96 | 18.68 a |
| HZL | 18.95 ± 3.07 | 50.39 c | 16.28 ± 5.08 | 41.73 c |
| Osiris® | 00.00 ± 0.00 | 100.00 f | 11.50 ± 0.76 | 84.20 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hasan, A.; Walker, F.; Klaiber, I.; Schöne, J.; Pfannstiel, J.; Voegele, R.T. New Approaches to Manage Asian Soybean Rust (Phakopsora pachyrhizi) Using Trichoderma spp. or Their Antifungal Secondary Metabolites. Metabolites 2022, 12, 507. https://doi.org/10.3390/metabo12060507
El-Hasan A, Walker F, Klaiber I, Schöne J, Pfannstiel J, Voegele RT. New Approaches to Manage Asian Soybean Rust (Phakopsora pachyrhizi) Using Trichoderma spp. or Their Antifungal Secondary Metabolites. Metabolites. 2022; 12(6):507. https://doi.org/10.3390/metabo12060507
Chicago/Turabian StyleEl-Hasan, Abbas, Frank Walker, Iris Klaiber, Jochen Schöne, Jens Pfannstiel, and Ralf T. Voegele. 2022. "New Approaches to Manage Asian Soybean Rust (Phakopsora pachyrhizi) Using Trichoderma spp. or Their Antifungal Secondary Metabolites" Metabolites 12, no. 6: 507. https://doi.org/10.3390/metabo12060507
APA StyleEl-Hasan, A., Walker, F., Klaiber, I., Schöne, J., Pfannstiel, J., & Voegele, R. T. (2022). New Approaches to Manage Asian Soybean Rust (Phakopsora pachyrhizi) Using Trichoderma spp. or Their Antifungal Secondary Metabolites. Metabolites, 12(6), 507. https://doi.org/10.3390/metabo12060507