Reconstruction of the Genome-Scale Metabolic Model of Saccharopolyspora erythraea and Its Application in the Overproduction of Erythromycin
Abstract
:1. Introduction
2. Results
2.1. Properties of the Constructed GSMM
2.2. Model Validation
2.2.1. Verification of Carbon and Nitrogen Source Availability
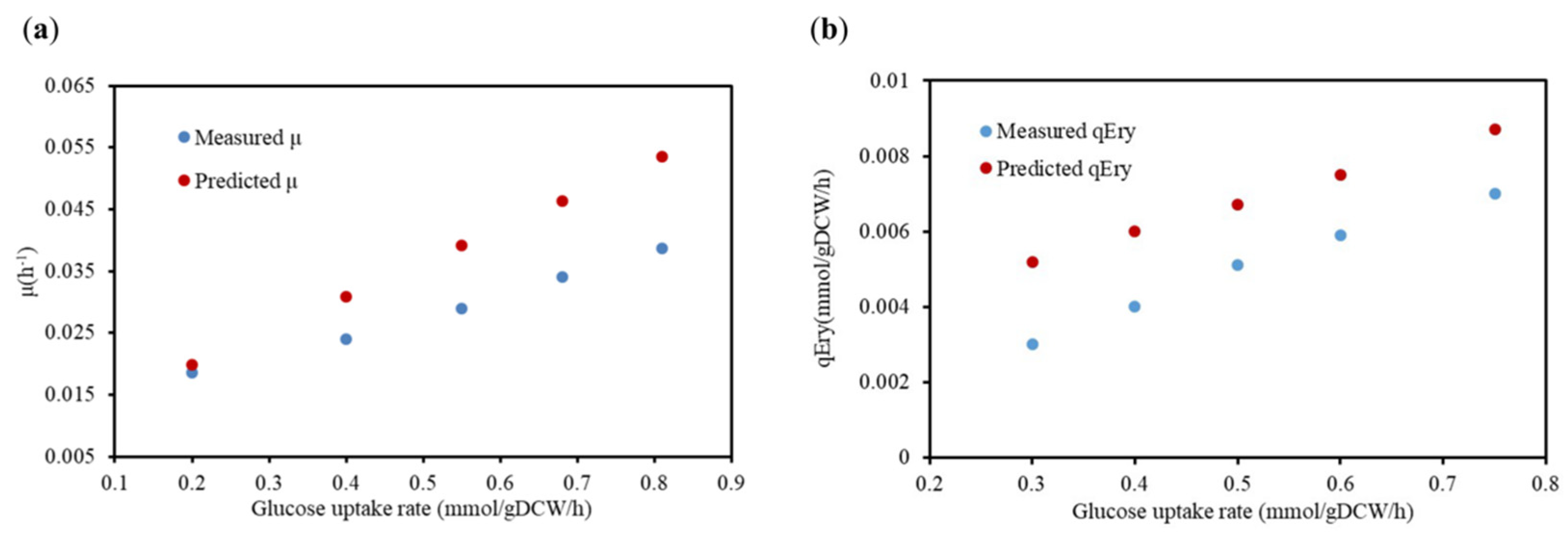
2.2.2. Verification of Physiological Metabolic Parameters
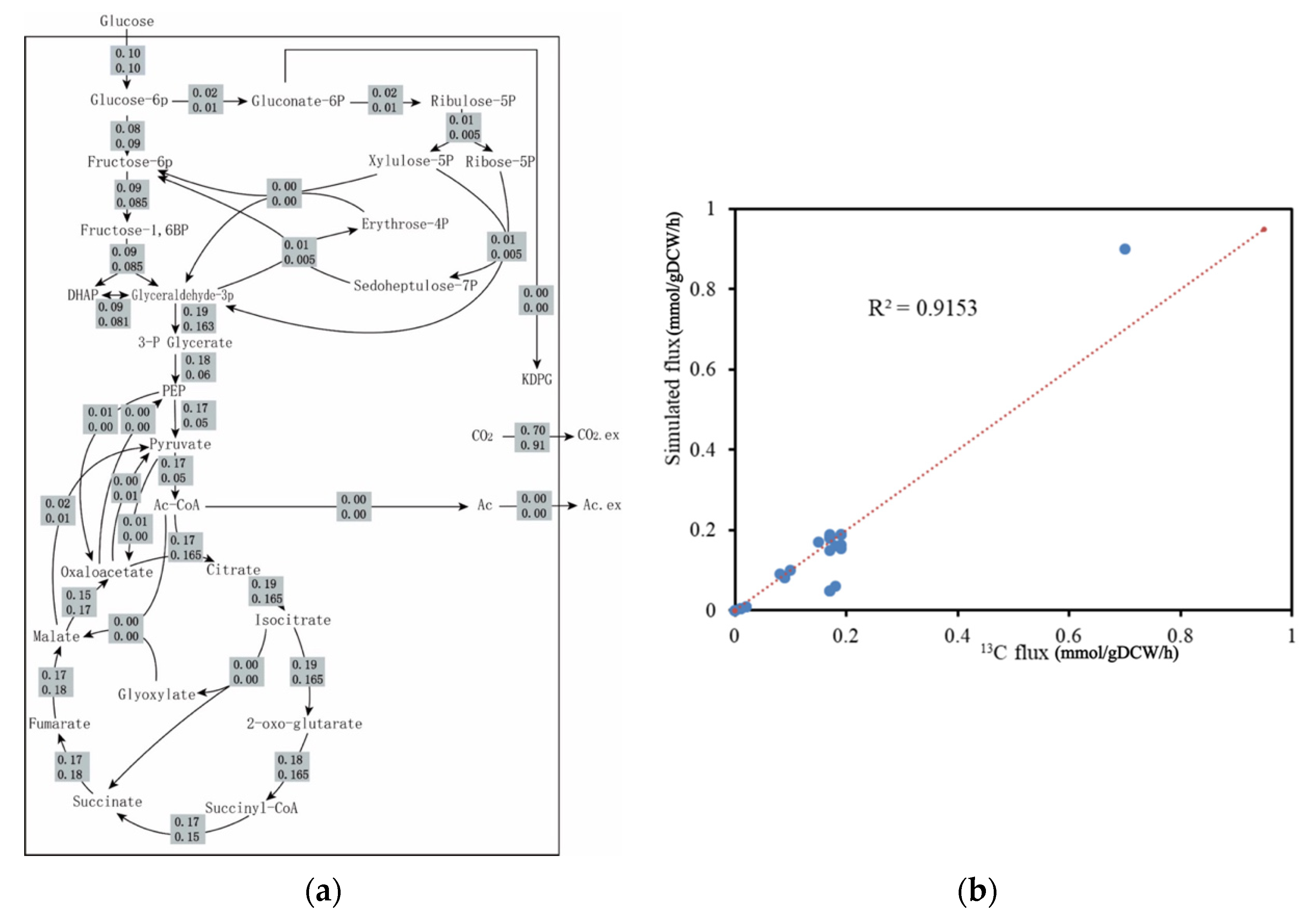
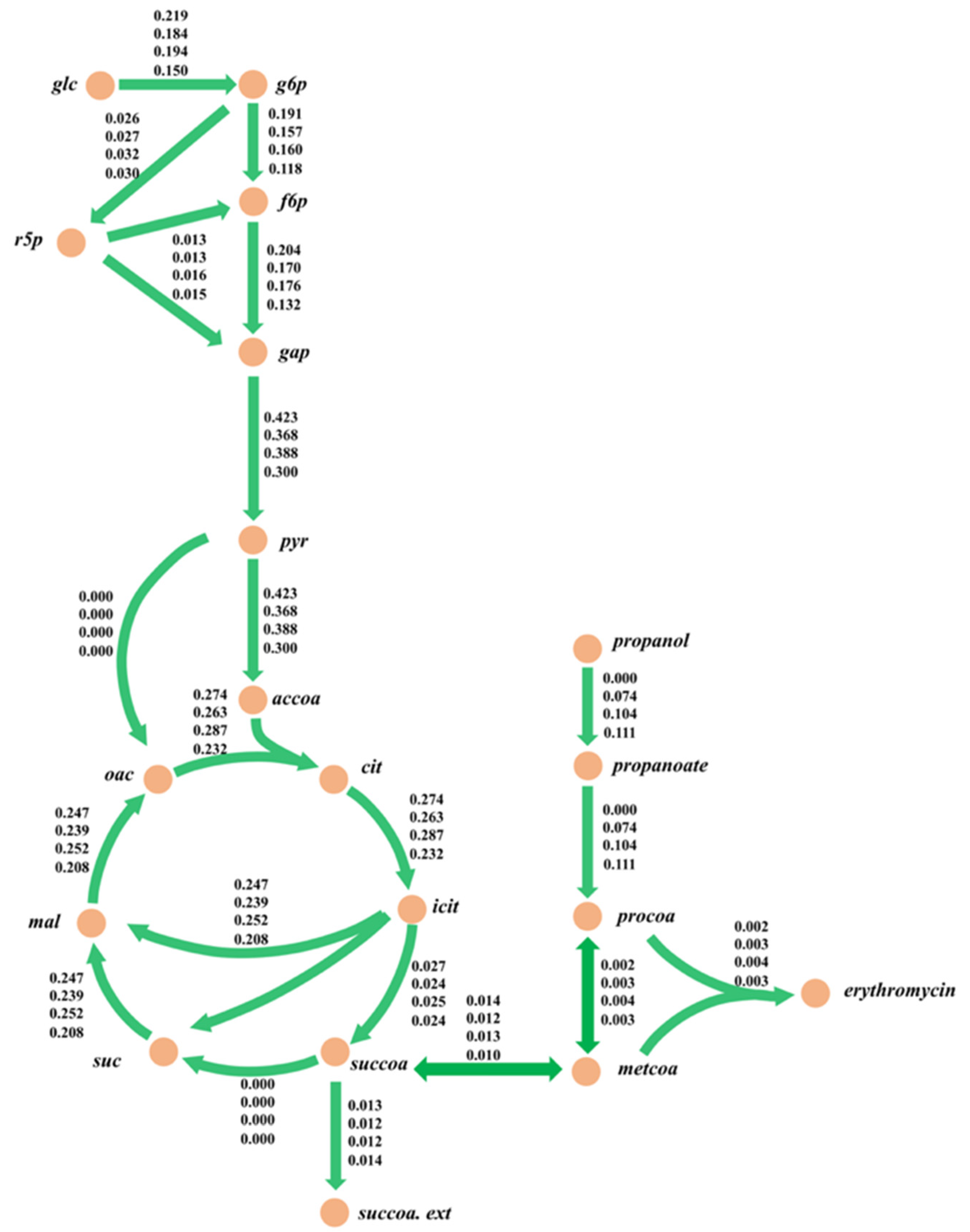
2.2.3. 13C metabolic Flux Analysis Validation
2.2.4. Validation of Knockout Phenotypes
2.3. Model Prediction of Essential Gene Targets In Silico for Strain Design
2.4. Model Application of Process Optimization for n-Propanol Supplementation
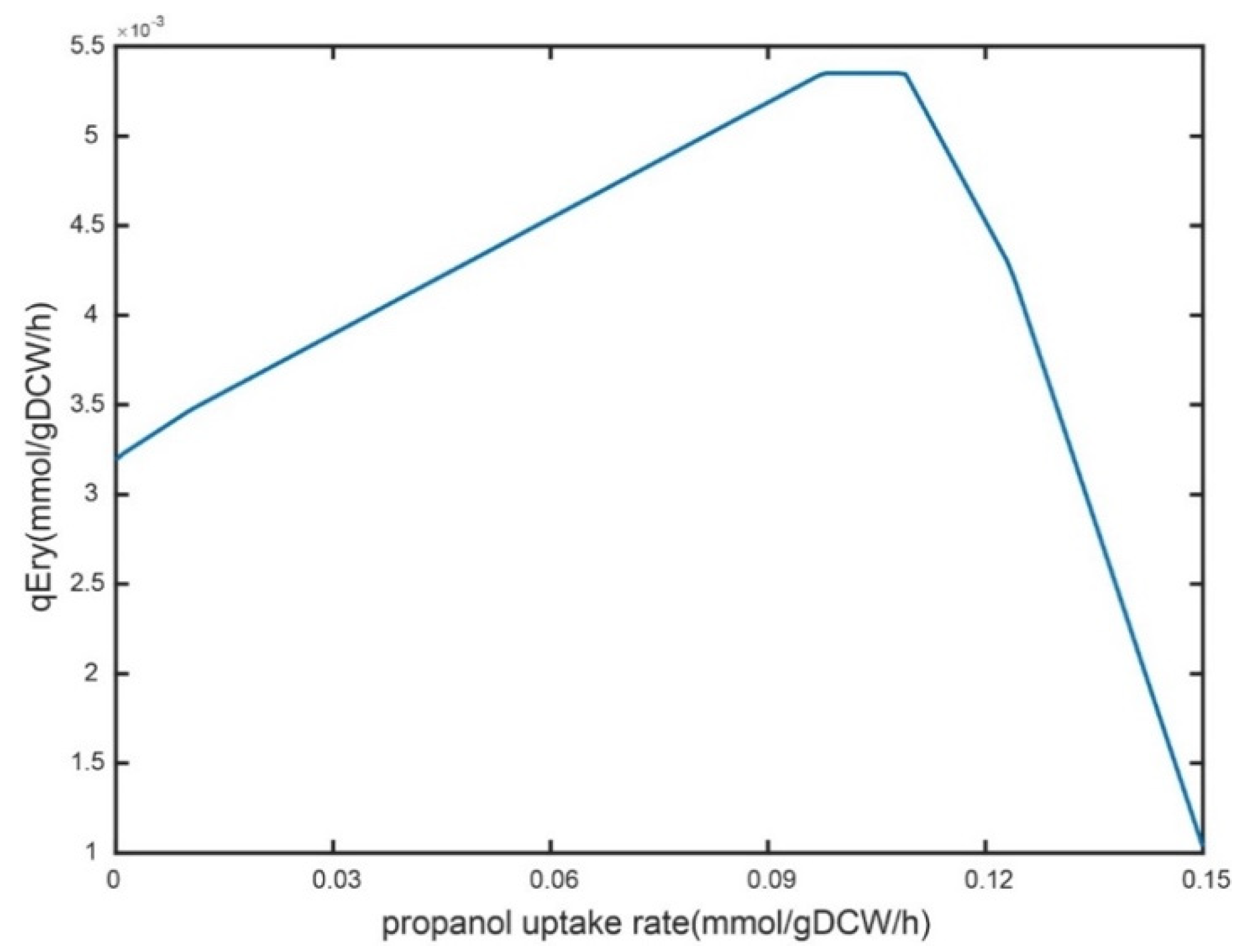
2.4.1. Analysis of n-Propanol Supplementation on Erythromycin Metabolism
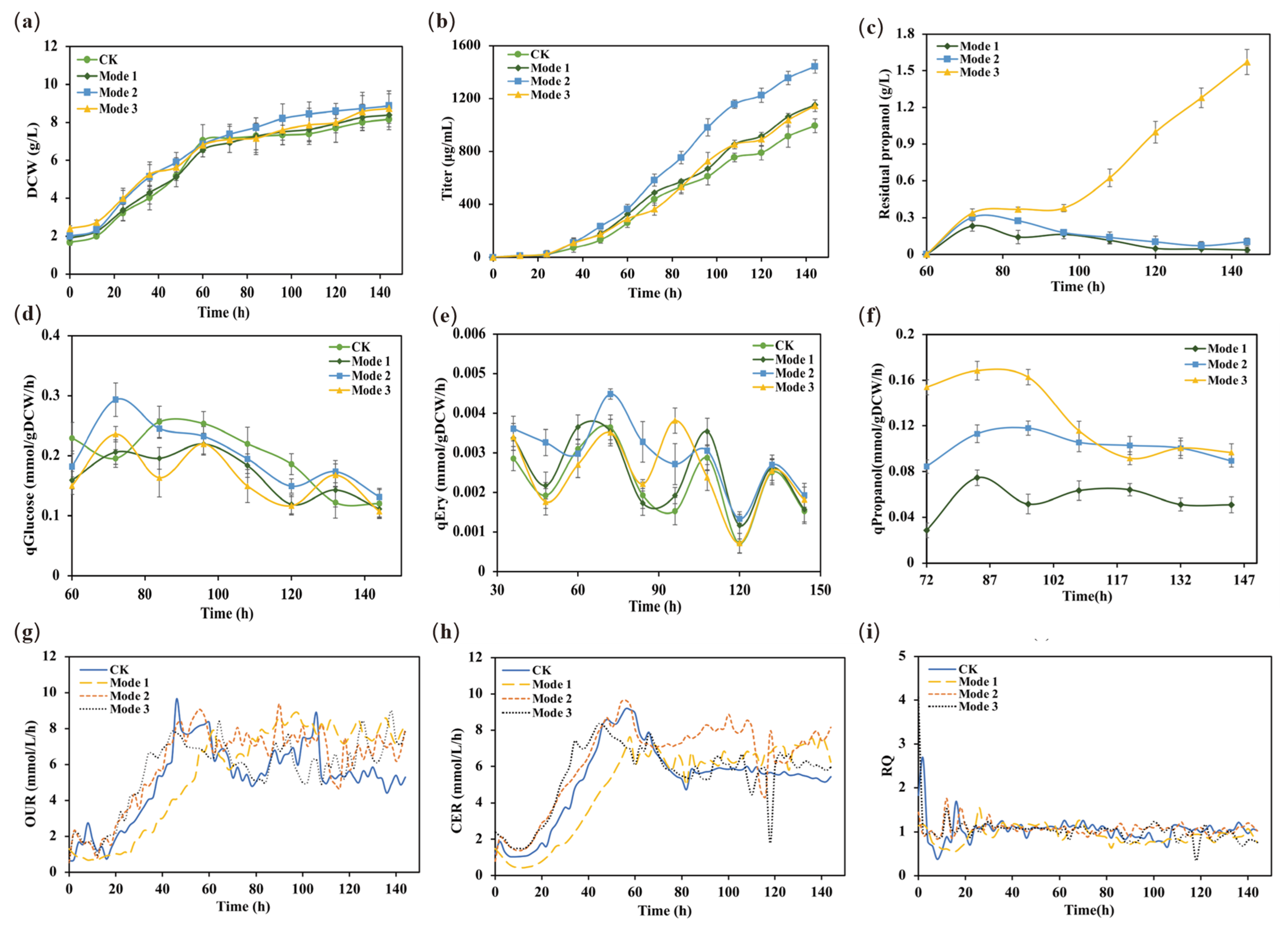
2.4.2. Cellular Physiological Parameters at Different Propanol Feeding Rates
2.4.3. Metabolic Flux Analysis
3. Discussion
4. Materials and Methods
4.1. Microorganism, Media, and Culture Conditions
4.2. Analytical Methods
4.3. iJL1426 Model Reconstruction
4.3.1. Draft Model Reconstruction
4.3.2. Gap-Filling
4.3.3. Curation of Directionality and Reversibility
4.3.4. Manual Refinement
4.3.5. Biomass Reactions
4.4. In Silico Computation Using Flux Balance Analysis
4.5. Model Validation
4.6. Model Simulation and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliynyk, M.; Samborskyy, M.; Lester, J.B.; Mironenko, T.; Scott, N.; Dickens, S.; Leadlay, P.F. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 2007, 25, 447–453. [Google Scholar] [CrossRef]
- Mironov, V.; Sergienko, O.; Nastasyak, I.; Danilenko, V. Biogenesis and regulation of biosynthesis of erythromycins in Saccharopolyspora erythraea. Appl. Biochem. Microbiol. 2004, 40, 531–541. [Google Scholar] [CrossRef]
- Zou, X.; Hang, H.F.; Chu, J.; Zhuang, Y.P.; Zhang, S.L. Enhancement of erythromycin A production with feeding available nitrogen sources in erythromycin biosynthesis phase. Bioresour. Technol. 2009, 100, 3358–3365. [Google Scholar] [CrossRef]
- Karničar, K.; Drobnak, I.; Petek, M.; Magdevska, V.; Horvat, J.; Vidmar, R.; Baebler, Š.; Rotter, A.; Jamnik, P.; Fujs, Š.; et al. Integrated omics approaches provide strategies for rapid erythromycin yield increase in Saccharopolyspora erythraea. Microb. Cell Fact. 2016, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Hong, M.; Chu, J.; Huang, M.; Ouyang, L.; Tian, X.; Zhuang, Y. Blocking the flow of propionate into TCA cycle through a mutB knockout leads to a significant increase of erythromycin production by an industrial strain of Saccharopolyspora erythraea. Bioprocess Biosyst. Eng. 2017, 40, 201–209. [Google Scholar] [CrossRef]
- Hong, M.; Liao, J.; Chu, J. High-throughput optimization of the chemically defined synthetic medium for the production of erythromycin A. Bioprocess Biosyst. Eng. 2018, 41, 1529–1538. [Google Scholar] [CrossRef]
- Xu, F.; Ke, X.; Hong, M.; Huang, M.; Chen, C.; Tian, X.; Hang, H.; Chu, J. Exploring the metabolic fate of propanol in industrial erythromycin-producing strain via 13C labeling experiments and enhancement of erythromycin production by rational metabolic engineering of Saccharopolyspora erythraea. Biochem. Biophys. Res. Commun. 2021, 542, 73–79. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, M.Z.; Wang, Z.J.; Chu, J.; Zhuang, Y.P.; Zhang, S.L. Controlling the feed rate of glucose and propanol for the enhancement of erythromycin production and exploration of propanol metabolism fate by quantitative metabolic flux analysis. Bioprocess Biosyst. Eng. 2013, 36, 1445–1453. [Google Scholar] [CrossRef]
- Kumelj, T.; Sulheim, S.; Wentzel, A.; Almaas, E. Predicting Strain Engineering Strategies Using iKS1317: A Genome-Scale Metabolic Model of Streptomyces coelicolor. Biotechnol. J. 2019, 14, 1800180. [Google Scholar] [CrossRef] [Green Version]
- Thiele, I.; Palsson, B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93–121. [Google Scholar] [CrossRef] [Green Version]
- Licona-Cassani, C.; Marcellin, E.; Quek, L.E.; Jacob, S.; Nielsen, L.K. Reconstruction of the Saccharopolyspora erythraea genome-scale model and its use for enhancing erythromycin production. Antonie Van Leeuwenhoek 2012, 102, 493–502. [Google Scholar] [CrossRef]
- Zhuang, Z.; Huang, M.; Chu, J. In silico reconstruction and experimental validation of Saccharopolyspora erythraea genome-scale metabolic model iZZ1342 that accounts for 1685 ORFs. Bioresour. Bioprocess. 2018, 5, 26. [Google Scholar] [CrossRef]
- Ankrah, N.; Bernstein, D.B.; Biggs, M.; Carey, M.; Engevik, M.; García-Jiménez, B.; Lakshmanan, M.; Pacheco, A.R.; Sulheim, S.; Medlock, G.L. Enhancing Microbiome Research through Genome-Scale Metabolic Modeling. mSystems 2021, 6, e0059921. [Google Scholar] [CrossRef]
- Colarusso, A.; Goodchild-Michelman, I.; Rayle, M.; Zomorrodi, A.R. Computational modeling of metabolism in microbial communities on a genome-scale. Curr. Opin. Syst. Biol. 2021, 26, 46–57. [Google Scholar] [CrossRef]
- El-Enshasy, H.A.; Mohamed, N.A.; Farid, M.A.; El-Diwany, A.I. Improvement of erythromycin production by Saccharopolyspora erythraea in molasses based medium through cultivation medium optimization. Bioresour. Technol. 2008, 99, 4263–4268. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using Genome-Scale Models to Predict Biological Capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minas, W.; Brünker, P.; Kallio, P.T.; Bailey, J.E. Improved erythromycin production in a genetically engineered industrial strain of Saccharopolyspora erythraea. Biotechnol. Prog. 1998, 14, 561–566. [Google Scholar] [CrossRef]
- Weber, J.M.; Cernota, W.H.; Gonzalez, M.C.; Leach, B.I.; Reeves, A.R.; Wesley, R.K. An erythromycin process improvement using the diethyl methylmalonate-responsive (Dmr) phenotype of the Saccharopolyspora erythraea mutB strain. Appl. Microbiol. Biotechnol. 2012, 93, 1575–1583. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Mou, H.; Liu, X.; Huang, M.Z.; Chu, J. 13C-assisted metabolomics analysis reveals the positive correlation between specific erythromycin production rate and intracellular propionyl-CoA pool size in Saccharopolyspora erythraea. Bioprocess Biosyst. Eng. 2017, 40, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Kittikunapong, C.; Ye, S.; Magadán-Corpas, P.; Pérez-Valero, Á.; Villar, C.J.; Lombó, F.; Kerkhoven, E.J. Reconstruction of a Genome-Scale Metabolic Model of Streptomyces albus J1074: Improved Engineering Strategies in Natural Product Synthesis. Metabolites 2021, 11, 304. [Google Scholar] [CrossRef]
- Zhang, Q.; Hang, H.; Tian, X.; Zeng, W.; Yu, Z.H.; Wang, X.J.; Tang, Y.; Zhuang, Y.P.; Chu, J. Combined available nitrogen resources enhanced erythromycin production and preliminary exploration of metabolic flux analysis under nitrogen perturbations. Bioprocess Biosyst. Eng. 2019, 42, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.W. Biochemical Mechanisms in the Biosynthesis of the Erythromycins; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Reeves, A.R.; Brikun, I.A.; Cernota, W.H.; Leach, B.I.; Gonzalez, M.C.; Weber, J.M. Engineering of the methylmalonyl-CoA metabolite node for increased erythromycin production in oil-based fermentations of Saccharopolyspora erythraea. Metab. Eng. 2007, 9, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Y.; Chang, X.; Yu, W.B.; Li, H.; Ye, Z.Q.; Yu, H.; Liu, B.H.; Zhang, Y.; Zhang, S.L.; Ye, B.C. Systems perspectives on erythromycin biosynthesis by comparative genomic and transcriptomic analyses of S. erythraea E3 and NRRL23338 strains. BMC Genom. 2013, 14, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Chu, J.; Zhuang, Y.P.; Gao, Y. Controlling the feed rate of propanol to optimize erythromycin fermentation by on-line capacitance and oxygen uptake rate measurement. Bioprocess Biosyst. Eng. 2016, 39, 255–265. [Google Scholar] [CrossRef]
- Henry, C.S.; Dejongh, M.; Best, A.A.; Frybarger, P.M.; Linsay, B.; Stevens, R.L. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat. Biotechnol. 2010, 28, 977–982. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef] [Green Version]
- Flamholz, A.; Noor, E.; Bar-Even, A.; Milo, R. eQuilibrator—The biochemical thermodynamics calculator. Nucleic Acids Res. 2012, 40, D770–D775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Thermodynamic constraints shape the structure of carbon fixation pathways. BBA-Bioenerg. 2012, 1817, 1646–1659. [Google Scholar] [CrossRef] [Green Version]
- Noor, E.; Haraldsdóttir, H.S.; Milo, R.; Fleming, R.M.T. Consistent estimation of Gibbs energy using component contribution. PLoS Comput. Biol. 2014, 9, e1003098. [Google Scholar] [CrossRef] [Green Version]
- Anne, K.; Sven, P.; Matthias, H. Systematic assignment of thermodynamic constraints in metabolic network models. BMC Bioinform. 2006, 7, 512. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.W.; Zeng, A.P. Reconstruction of metabolic networks from genome data and analysis of their global structure for various organisms. Bioinformatics 2003, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Donachie, W.; Begg, K. Growth of the Bacterial Cell. Nature 1970, 227, 1220–1224. [Google Scholar] [CrossRef]
- Borodina, I.; Krabben, P.; Nielsen, J. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res. 2005, 15, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Bordbar, A.; Monk, J.; King, Z.A.; Palsson, B.O. Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 2014, 15, 107–120. [Google Scholar] [CrossRef]
- Orth, J.; Thiele, I.; Palsson, B.O. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]

| Characteristics | iJL1426 | iZZ1342 [12] | NRRL23338-GEMR [11] |
|---|---|---|---|
| Genome size | 8.2Mb | 8.2 Mb | 8.2 Mb |
| Total genes | 7714 | 7233 | 7233 |
| Genes assigned | 1426 | 1342 | 1272 |
| effective genes | 1426 | 1291 | 1272 |
| Annotation coverage (%) | 18.5% | 17.9% | 17.5% |
| Total reactions | 1858 | 1684 | 3985 |
| Unique reactions | 1858 | 1611 | 1482 |
| Metabolic reactions | 1632 | 1525 | 3872 |
| Transport and exchange reactions | 225 | 133 | 113 |
| Metabolites | 1687 | 1614 | 1546 |
| GPR associations | 1492 | 1441 | - |
| Reactions with genes assigned | 1492 | 1441 | 1223 |
| Reactions without genes assigned | 366 | 243 | 2762 |
| Carbon Source | Observed in Experiment | Predicted in Model | Reference |
|---|---|---|---|
| D-Glucose | + | + | [15] |
| sucrose | + | + | [15] |
| D-Xylose | + | + | [15] |
| Mannose | + | + | [12] |
| Mannitol | + | + | [12] |
| L-Rhamnose | + | + | [12] |
| L-Arabinose | + | + | [15] |
| D-Mannose | + | + | [12] |
| D-Fructose | + | + | [15] |
| Raffinose | + | + | [12] |
| D-Galactose | + | + | [15] |
| inost | + | + | [12] |
| Melibiose | + | + | [12] |
| D-Ribose | + | + | [15] |
| alpha,alpha-Trehalose | + | + | [15] |
| Maltose | + | + | [15] |
| β-Lactose | + | + | [15] |
| α-Lactose | + | + | [15] |
| Pyruvate | + | − | [12] |
| 2-Oxoglutarate | − | − | [12] |
| Succinate | − | − | [12] |
| Fumarate | − | − | [12] |
| Acetate | + | − | [12] |
| Propanoate | + | + | [12] |
| Citrate | + | + | [12] |
| (S)-Malate | + | + | [12] |
| (S)-Lactate | − | − | [12] |
| Nitrogen Source | Observed in Experiment | Predicted in Model | Reference |
|---|---|---|---|
| L-Valine | + | + | [12] |
| L-Threonine | + | + | [12] |
| L-Isoleucine | + | + | [12] |
| L-Leucine | + | + | [12] |
| L-Methionine | + | + | [12] |
| L-Aspartate | + | + | [12] |
| L-Glutamine | + | + | [12] |
| L-Phenylalanine | + | + | [12] |
| L-Glutamate | + | + | [12] |
| L-Serine | + | + | [12] |
| L-Proline | + | + | [12] |
| Glycine | + | + | [12] |
| L-Lysine | − | − | [12] |
| L-Histidine | + | + | [12] |
| L-Cysteine | + | + | [12] |
| L-Asparagine | + | + | [12] |
| L-Alanine | + | + | [12] |
| L-Arginine | + | + | [12] |
| L-Tyrosine | − | − | [12] |
| L-Tryptophan | + | + | [12] |
| Urea | + | + | [12] |
| 4-Aminobutanoate | + | + | [12] |
| Xanthine | − | − | [12] |
| Hypoxanthine | − | − | [12] |
| Ammonium chloride | + | + | [12] |
| Ammonium nitrate | + | + | [15] |
| Ammonium acetate | + | + | [15] |
| Ammonium oxalate | + | + | [15] |
| Ammonium carbonate | + | + | [15] |
| Ammonium sulfate | + | + | [12] |
| Ammonium dihydrogen phosphate | + | + | [15] |
| CK | Mode 1 | Mode 2 | Mode 3 | |
|---|---|---|---|---|
| qGlucose (mmol/gDCW/h) | 0.219 ± 0.003 | 0.184 ± 0.002 | 0.194 ± 0.002 | 0.150 ± 0.001 |
| qpropanol (mmol/gDCW/h) | 0 | 0.073 ± 0.003 | 0.105 ± 0.005 | 0.115 ± 0.005 |
| qCO2 (mmol/gDCW/h) | 0.826 ± 0.004 | 0.837 ± 0.003 | 0.968 ± 0.005 | 0.701 ± 0.005 |
| qEry (mmol/gDCW/h) | 0.002 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| qsuc-coA (mmol/gDCW/h) | 0.013 ± 0.001 | 0.012 ± 0.001 | 0.012 ± 0.002 | 0.014 ± 0.001 |
| μ(h-1) | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.002 ± 0.001 | 0.001 ± 0.001 |
| Carbon recoveries (%) * | 95.5 | 96.6 | 97.4 | 95.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Lu, J.; Ke, X.; Shao, M.; Huang, M.; Chu, J. Reconstruction of the Genome-Scale Metabolic Model of Saccharopolyspora erythraea and Its Application in the Overproduction of Erythromycin. Metabolites 2022, 12, 509. https://doi.org/10.3390/metabo12060509
Xu F, Lu J, Ke X, Shao M, Huang M, Chu J. Reconstruction of the Genome-Scale Metabolic Model of Saccharopolyspora erythraea and Its Application in the Overproduction of Erythromycin. Metabolites. 2022; 12(6):509. https://doi.org/10.3390/metabo12060509
Chicago/Turabian StyleXu, Feng, Ju Lu, Xiang Ke, Minghao Shao, Mingzhi Huang, and Ju Chu. 2022. "Reconstruction of the Genome-Scale Metabolic Model of Saccharopolyspora erythraea and Its Application in the Overproduction of Erythromycin" Metabolites 12, no. 6: 509. https://doi.org/10.3390/metabo12060509







