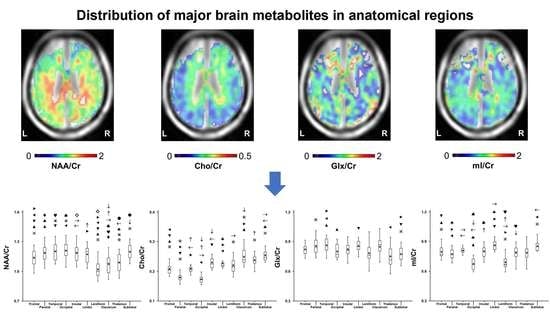
The Distribution of Major Brain Metabolites in Normal Adults: Short Echo Time Whole-Brain MR Spectroscopic Imaging Findings
Abstract
:1. Introduction
2. Results
2.1. Variation in Metabolite Ratios with Anatomical Location
2.2. Laterality
2.3. Sex-Related Variation
2.4. Correlation with Age
2.5. Relationship among Regional Metabolite Ratios
2.6. The Influence of Scan Conditions on Laterality in Major Metabolite Ratios
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. MR Imaging
4.2.1. For Evaluation of the Distribution of Major Metabolite Ratios
4.2.2. Test of the Influence of Scan Conditions on Laterality in Major Metabolite Ratios
4.3. Data Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Kwock, L.; Green, C. MELAS syndrome: Imaging and proton MR spectroscopic findings. AJNR Am. J. Neuroradiol. 1995, 16, 233–239. [Google Scholar]
- Takahashi, S.; Oki, J.; Miyamoto, A.; Okuno, A. Proton magnetic resonance spectroscopy to study the metabolic changes in the brain of a patient with Leigh syndrome. Brain Dev. 1999, 21, 200–204. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Andronesi, O.C.; Barker, P.B.; Bizzi, A.; Bogner, W.; Henning, A.; Nelson, S.J.; Posse, S.; Shungu, D.C.; Soher, B.J. Advanced magnetic resonance spectroscopic neuroimaging: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4309. [Google Scholar] [CrossRef]
- Bogner, W.; Otazo, R.; Henning, A. Accelerated MR spectroscopic imaging—A review of current and emerging techniques. NMR Biomed. 2021, 34, e4314. [Google Scholar] [CrossRef]
- Ebel, A.; Soher, B.J.; Maudsley, A.A. Assessment of 3D proton MR echo-planar spectroscopic imaging using automated spectral analysis. Magn. Reson. Med. 2001, 46, 1072–1078. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Darkazanli, A.; Alger, J.R.; Hall, L.O.; Schuff, N.; Studholme, C.; Yu, Y.; Ebel, A.; Frew, A.; Goldgof, D.; et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006, 19, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Taub, E.; Salibi, N.; Uswatte, G.; Maudsley, A.A.; Sheriff, S.; Womble, B.; Mark, V.W.; Knight, D.C. Comparison of reproducibility of single voxel spectroscopy and whole-brain magnetic resonance spectroscopy imaging at 3T. NMR Biomed. 2018, 31, e3898. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Govind, V.; Saigal, G.; Gold, S.G.; Harris, L.; Sheriff, S. Longitudinal MR Spectroscopy Shows Altered Metabolism in Traumatic Brain Injury. J. Neuroimaging 2017, 27, 562–569. [Google Scholar] [CrossRef]
- Verma, G.; Woo, J.H.; Chawla, S.; Wang, S.; Sheriff, S.; Elman, L.B.; McCluskey, L.F.; Grossman, M.; Melhem, E.R.; Maudsley, A.A.; et al. Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology 2013, 267, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Maudsley, A.A.; Roy, B.; Gupta, R.K.; Sheriff, S.; Awasthi, R.; Gu, M.; Husain, N.; Mohakud, S.; Behari, S.; Spielman, D.M. Association of metabolite concentrations and water diffusivity in normal appearing brain tissue with glioma grade. J. Neuroimaging 2014, 24, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Maudsley, A.A.; Govind, V.; Arheart, K.L. Associations of age; gender and body mass with 1H MR-observed brain metabolites and tissue distributions. NMR Biomed. 2012, 25, 580–593. [Google Scholar] [CrossRef] [Green Version]
- Mascalchi, M.; Brugnoli, R.; Guerrini, L.; Belli, G.; Nistri, M.; Politi, L.S.; Gavazzi, C.; Lolli, F.; Argenti, G.; Villari, N. Single-voxel long TE 1H -MR spectroscopy of the normal brainstem and cerebellum. J. Magn. Reson. Imaging 2002, 16, 532–537. [Google Scholar] [CrossRef]
- Goryawala, M.Z.; Sheriff, S.; Maudsley, A.A. Regional distributions of brain glutamate and glutamine in normal subjects. NMR Biomed. 2016, 29, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.Q.; Maudsley, A.A.; Sabati, M.; Sheriff, S.; Schmitz, B.; Schütze, M.; Bronzlik, P.; Kahl, K.G.; Lanfermann, H. Physiological neuronal decline in healthy aging human brain—An in vivo study with MRI and short echo-time whole-brain 1H MR spectroscopic imaging. Neuroimage 2016, 137, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Kanowski, M.; Kaufmann, J.; Braun, J.; Bernarding, J.; Tempelmann, C. Quantitation of simulated short echo time 1H human brain spectra by LCModel and AMARES. Magn. Reson. Med. 2004, 51, 904–912. [Google Scholar] [CrossRef]
- Cheng, L.L.; Newell, K.; Mallory, A.E.; Hyman, B.T.; Gonzalez, R.G. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn. Reson. Imaging 2002, 20, 527–533. [Google Scholar] [CrossRef]
- Hammen, T.; Hildebrandt, M.; Stadlbauer, A.; Doelken, M.; Engelhorn, T.; Kerling, F.; Kasper, B.; Romstoeck, J.; Ganslandt, O.; Nimsky, C. Non-invasive detection of hippocampal sclerosis: Correlation between metabolite alterations detected by 1H-MRS and neuropathology. NMR Biomed. 2008, 21, 545–552. [Google Scholar] [CrossRef]
- Pakkenberg, B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol. Psychiatry 1993, 34, 768–772. [Google Scholar] [CrossRef]
- Wegiel, J.; Flory, M.; Kuchna, I.; Nowicki, K.; Ma, S.Y.; Imaki, H.; Wegiel, J.; Cohen, I.L.; London, E.; Wisniewski, T. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum; amygdala and cerebellum. Acta. Neuropathol. Commun. 2014, 2, 141. [Google Scholar] [CrossRef] [Green Version]
- Nitsch, R.M.; Blusztajn, J.K.; Doyle, F.M.; Robitaille, Y.; Wurtman, R.J.; Growdon, J.H.; Kish, S.J. Phospholipid metabolite levels are altered in cerebral cortex of patients with dominantly inherited olivopontocerebellar atrophy. Neurosci. Lett. 1993, 161, 191–194. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Minati, L.; Aquino, D.; Bruzzone, M.G.; Erbetta, A. Quantitation of normal metabolite concentrations in six brain regions by in-vivo 1H-MR spectroscopy. J. Med. Phys. 2010, 35, 154–163. [Google Scholar] [CrossRef]
- Karlsen, A.S.; Pakkenberg, B. Total numbers of neurons and glial cells in cortex and basal ganglia of aged brains with Down syndrome—A stereological study. Cereb. Cortex 2011, 21, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H.; Capelle, L. Preferential brain locations of low-grade gliomas. Cancer 2004, 100, 2622–2626. [Google Scholar] [CrossRef]
- Parisot, S.; Darlix, A.; Baumann, C.; Zouaoui, S.; Yordanova, Y.; Blonski, M.; Rigau, V.; Chemouny, S.; Taillandier, L.; Bauchet, L.; et al. A Probabilistic Atlas of Diffuse WHO Grade II Glioma Locations in the Brain. PLoS ONE 2016, 11, e0144200. [Google Scholar] [CrossRef] [Green Version]
- Tartaglia, M.C.; Narayanan, S.; De Stefano, N.; Arnaoutelis, R.; Antel, S.B.; Francis, S.J.; Santos, A.C.; Lapierre, Y.; Arnold, D.L. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J. Neurol. 2002, 249, 1382–1390. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Geng, K.; Cheng, Y.; Li, Y.; Qiu, J.; Huang, H.; Wang, R.; Zhang, Y.; Wu, R. Glutamate Chemical Exchange Saturation Transfer (GluCEST) Magnetic Resonance Imaging in Pre-clinical and Clinical Applications for Encephalitis. Front. Neurosci. 2020, 14, 750. [Google Scholar] [CrossRef]
- Uylings, H.B.; Jacobsen, A.M.; Zilles, K.; Amunts, K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex 2006, 42, 652–658. [Google Scholar] [CrossRef]
- Cullen, T.J.; Walker, M.A.; Eastwood, S.L.; Esiri, M.M.; Harrison, P.J.; Crow, T.J. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br. J. Psychiatry 2006, 188, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, P.J.; Frahm, J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn. Reson. Med. 1998, 39, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushton, W.A.H. A theory of the effects of fibre size in medullated nerve. J. Physiol. 1951, 115, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Southern, B.D.; Powers, R.E. Anatomic asymmetries of the posterior superior temporal lobes: A postmortem study. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999, 12, 247–254. [Google Scholar]
- Cercignani, M.; Giulietti, G.; Dowell, N.G.; Gabel, M.; Broad, R.; Leigh, P.N.; Harrison, N.A.; Bozzali, M. Characterizing axonal myelination within the healthy population: A tract-by-tract mapping of effects of age and gender on the fiber g-ratio. Neurobiol. Aging 2017, 49, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Ernst, T.; Strickland, T.; Mehringer, C.M. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am. J. Psychiatry 1999, 156, 716–722. [Google Scholar]
- Cichocka, M.; Kozub, J.; Karcz, P.; Urbanik, A. Sex differences in brain metabolite concentrations in healthy children—Proton magnetic resonance spectroscopy study (1HMRS). Pol. J. Radiol. 2018, 83, e24–e31. [Google Scholar] [CrossRef]
- Loy, R.; Sheldon, R.A. Sexually dimorphic development of cholinergic enzymes in the rat septohippocampal system. Brain Res. 1987, 431, 156–160. [Google Scholar] [CrossRef]
- Craig, M.C.; Daly, E.M.; O’Gorman, R.; Rymer, J.; Lythgoe, D.; Ng, G.; Simmons, A.; Maki, P.M.; Murphy, D.G. Effects of acute ovarian hormone suppression on the human brain: An in vivo 1H MRS study. Psychoneuroendocrinology 2007, 32, 1128–1132. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Bhagat, Y.A.; Beaulieu, C. Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF-suppression. J. Magn. Reson. Imaging 2004, 20, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ernst, T.; Poland, R.E.; Jenden, D.J. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996, 58, 2049–2056. [Google Scholar] [CrossRef]
- Hansen, L.A.; Armstrong, D.M.; Terry, R.D. An immunohistochemical quantification of fibrous astrocytes in the aging human cerebral cortex. Neurobiol. Aging 1987, 8, 1–6. [Google Scholar] [CrossRef]
- Schitine, C.; Nogaroli, L.; Costa, M.R.; Hedin-Pereira, C. Astrocyte heterogeneity in the brain: From development to disease. Front. Cell. Neurosci. 2015, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waragai, M.; Moriya, M.; Nojo, T. Decreased N-Acetyl Aspartate/Myo-Inositol Ratio in the Posterior Cingulate Cortex Shown by Magnetic Resonance Spectroscopy May Be One of the Risk Markers of Preclinical Alzheimer′s Disease: A 7-Year Follow-Up Study. J. Alzheimers Dis. 2017, 60, 1411–1427. [Google Scholar] [PubMed] [Green Version]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Waddell, K.W.; Zanjanipour, P.; Pradhan, S.; Xu, L.; Welch, E.B.; Joers, J.M.; Martin, P.R.; Avison, M.J.; Gore, J.C. Anterior cingulate and cerebellar GABA and Glu correlations measured by ¹H J-difference spectroscopy. Magn. Reson. Imaging 2011, 29, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Jiru, F.; Skoch, A.; Klose, U.; Grodd, W.; Hajek, M. Error images for spectroscopic imaging by LCModel using Cramer–Rao bounds. Magn. Reson. Mater. Phy. 2006, 19, 1–14. [Google Scholar] [CrossRef]
- Guerrini, L.; Belli, G.; Mazzoni, L.; Foresti, S.; Ginestroni, A.; Della Nave, R.; Diciotti, S.; Mascalchi, M. Impact of cerebrospinal fluid contamination on brain metabolites evaluation with 1H-MR spectroscopy: A single voxel study of the cerebellar vermis in patients with degenerative ataxias. J. Magn. Reson. Imaging 2009, 30, 11–17. [Google Scholar]
- Wahlund, L.O.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjögren, M.; Wallin, A.; Ader, H.; Leys, D.; Pantoni, L.; et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ebel, A.; Maudsley, A.A. Detection and correction of frequency instabilities for volumetric 1H echo-planar spectroscopic imaging. Magn. Reson. Med. 2005, 53, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Maudsley, A.A.; Gupta, R.K.; Stoyanova, R.; Parra, N.A.; Roy, B.; Sheriff, S.; Hussain, N.; Behari, S. Mapping of glycine distributions in gliomas. AJNR Am. J. Neuroradiol. 2014, 35, S31–S36. [Google Scholar] [CrossRef] [Green Version]
- Soher, B.J.; Young, K.; Govindaraju, V.; Maudsley, A.A. Automated spectral analysis III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn. Reson. Med. 1998, 40, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.; Peet, A.C.; Dehghani, H.; Wilson, M. Influence of macromolecule baseline on 1H MR spectroscopic imaging reproducibility. Magn. Reson. Med. 2017, 77, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Maghsudi, H.; Schmitz, B.; Maudsley, A.A.; Sheriff, S.; Bronzlik, P.; Schütze, M.; Lanfermann, H.; Ding, X. Regional Metabolite Concentrations in Aging Human Brain: Comparison of Short-TE Whole Brain MR Spectroscopic Imaging and Single Voxel Spectroscopy at 3T. Clin. Neuroradiol. 2020, 30, 251–261. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Rorden, C.; Karnath, H.O.; Bonilha, L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007, 19, 1081–1088. [Google Scholar] [CrossRef]
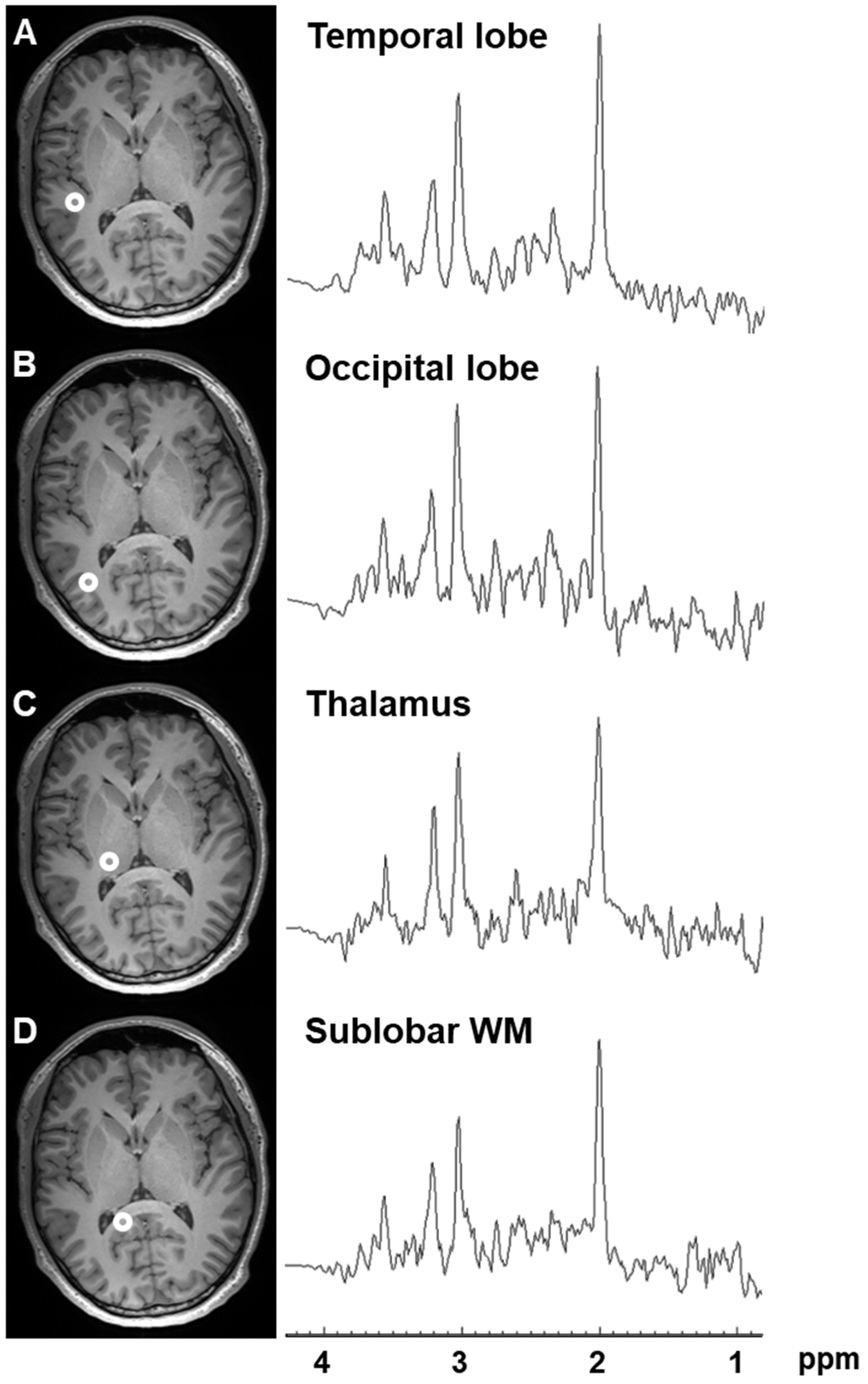
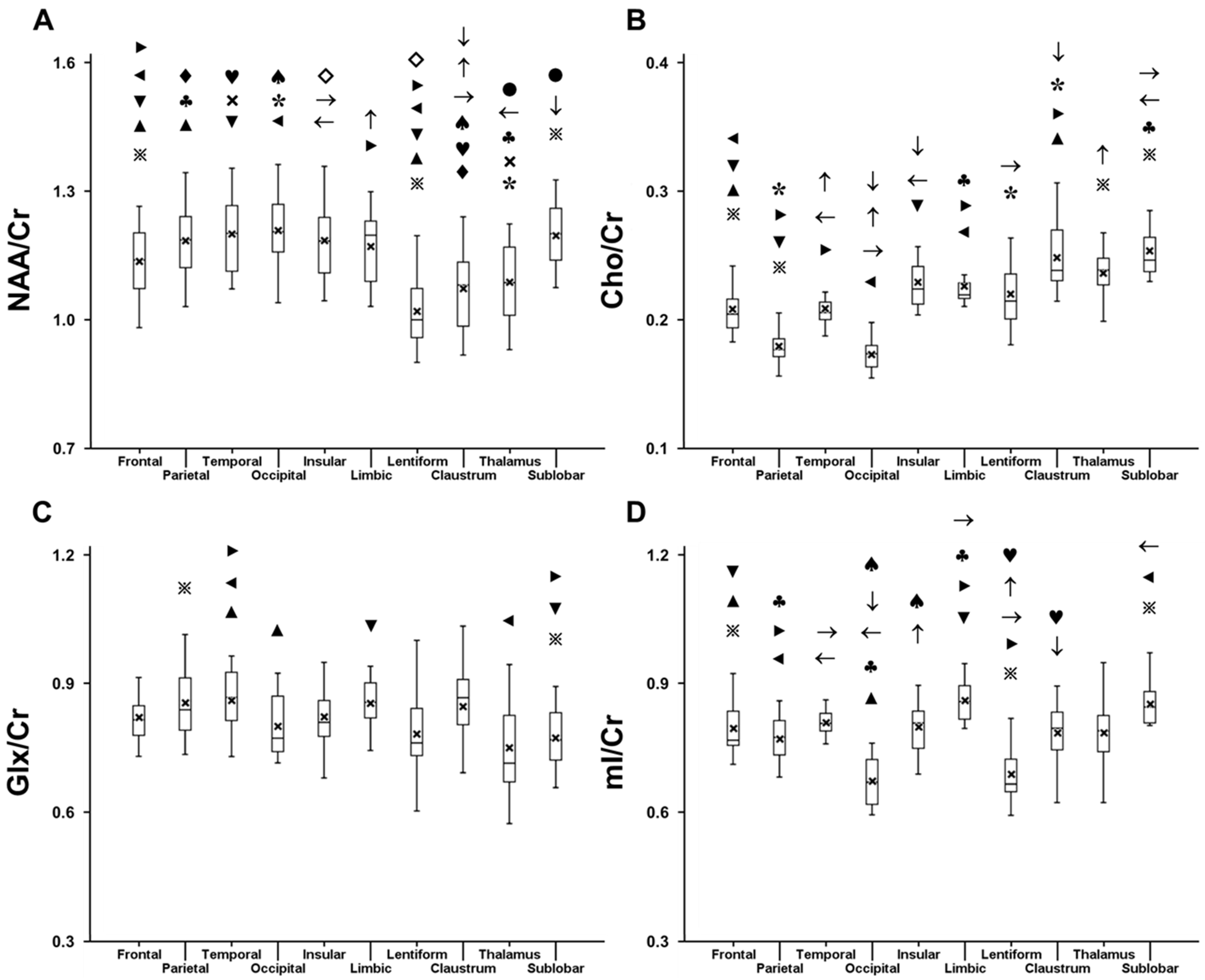


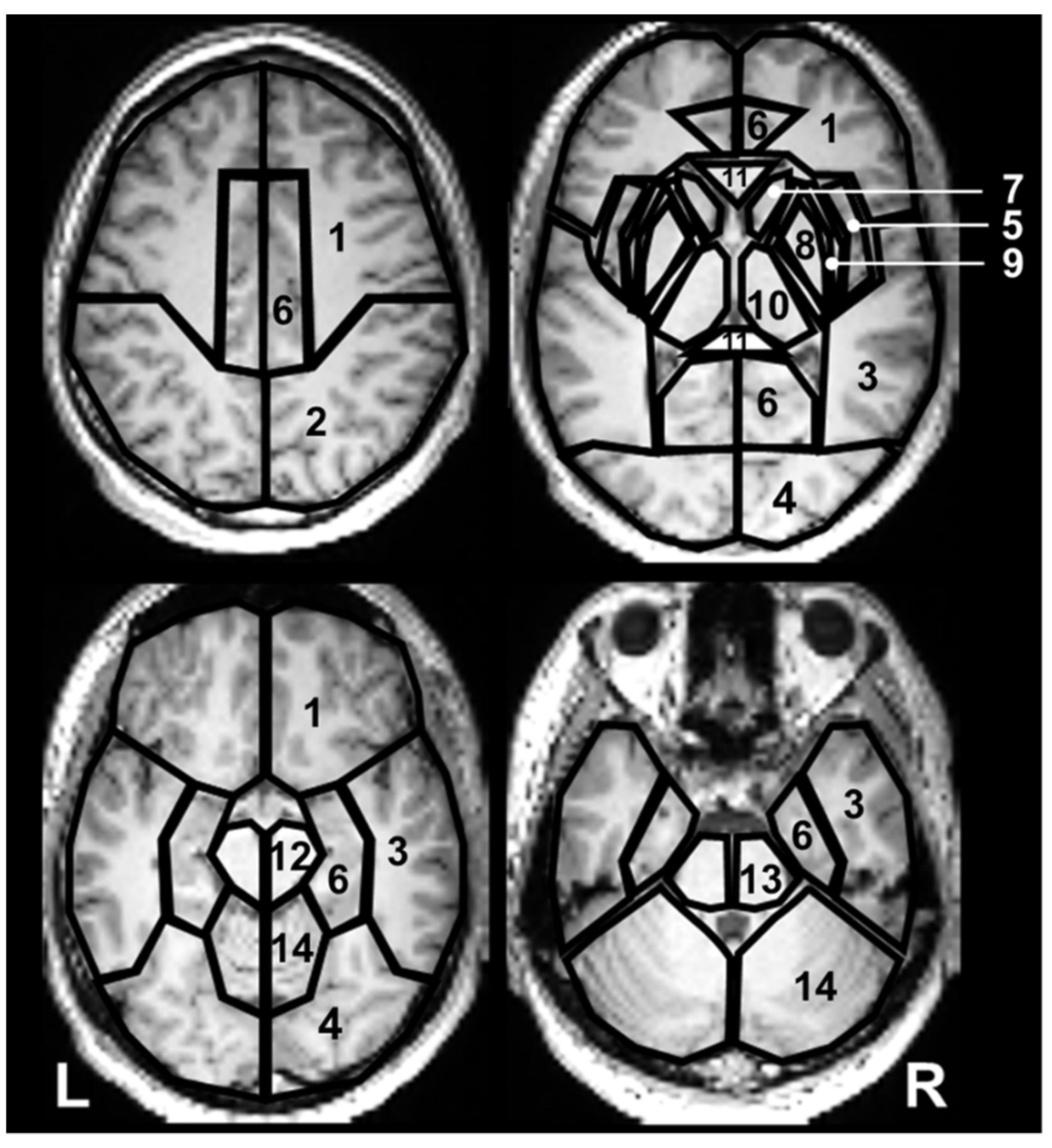
| Metabolite Ratio | Frontal Lobe | Parietal Lobe | Temporal Lobe | Occipital Lobe | Insula | Limbic Lobe | Claustrum | Lentiform Nucleus | Thalamus | Sublobar White Matter | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr | Left | 1.140 ± 0.086 * | 1.188 ± 0.093 * | 1.221 ± 0.085 * | 1.240 ± 0.061 | 1.165 ± 0.095 | 1.173 ± 0.092 | 1.040 ± 0.093 | 1.021 ± 0.076 | 1.096 ± 0.08 | 1.212 ± 0.071 |
| Right | 1.123 ± 0.077 * | 1.163 ± 0.079 * | 1.166 ± 0.086 * | 1.232 ± 0.070 | 1.158 ± 0.099 | 1.175 ± 0.085 | 1.034 ± 0.110 | 1.043 ± 0.071 | 1.068 ± 0.087 | 1.204 ± 0.083 | |
| Women | 1.122 ± 0.069 | 1.173 ± 0.064 | 1.198 ± 0.071 | 1.226 ± 0.080 | 1.158 ± 0.095 | 1.164 ± 0.069 | 1.019 ± 0.111 | 1.015 ± 0.096 | 1.063 ± 0.105 | 1.208 ± 0.087 | |
| Men | 1.147 ± 0.069 | 1.181 ± 0.062 | 1.198 ± 0.072 | 1.214 ± 0.076 | 1.157 ± 0.093 | 1.182 ± 0.066 | 1.052 ± 0.111 | 1.037 ± 0.102 | 1.082 ± 0.104 | 1.194 ± 0.088 | |
| Cho/Cr | Left | 0.218 ± 0.022 | 0.184 ± 0.020 * | 0.207 ± 0.017 * | 0.166 ± 0.015 * | 0.230 ± 0.022 * | 0.234 ± 0.026 * | 0.246 ± 0.022 | 0.217 ± 0.020 | 0.239 ± 0.022 | 0.253 ± 0.020 * |
| Right | 0.217 ± 0.021 | 0.193 ± 0.020 * | 0.219 ± 0.018 * | 0.178 ± 0.016 * | 0.234 ± 0.021 * | 0.239 ± 0.025 * | 0.248 ± 0.024 | 0.216 ± 0.019 | 0.245 ± 0.023 | 0.257 ± 0.020 * | |
| Women | 0.207 ± 0.025 ^ | 0.185 ± 0.021 | 0.202 ± 0.020 ^ | 0.167 ± 0.018 | 0.223 ± 0.026 ^ | 0.229 ± 0.029 | 0.236 ± 0.033 | 0.214 ± 0.030 | 0.237 ± 0.028 | 0.250 ± 0.025 | |
| Men | 0.227 ± 0.025 ^ | 0.193 ± 0.021 | 0.220 ± 0.020 ^ | 0.176 ± 0.017 | 0.242 ± 0.026 ^ | 0.244 ± 0.027 | 0.255 ± 0.033 | 0.227 ± 0.032 | 0.249 ± 0.028 | 0.262 ± 0.025 | |
| Glx/Cr | Left | 0.832 ± 0.075 * | 0.834 ± 0.096 | 0.875 ± 0.061 * | 0.783 ± 0.071 | 0.845 ± 0.086 | 0.843 ± 0.067 * | 0.865 ± 0.100 * | 0.770 ± 0.103 | 0.752 ± 0.112 | 0.785 ± 0.054 * |
| Right | 0.783 ± 0.070 * | 0.807 ± 0.068 | 0.810 ± 0.054 * | 0.778 ± 0.066 | 0.813 ± 0.083 | 0.808 ± 0.085 * | 0.806 ± 0.114 * | 0.717 ± 0.123 | 0.738 ± 0.086 | 0.747 ± 0.055 * | |
| Women | 0.816 ± 0.086 | 0.825 ± 0.082 | 0.859 ± 0.071 | 0.759 ± 0.085 | 0.828 ± 0.111 | 0.842 ± 0.087 | 0.829 ± 0.145 | 0.714 ± 0.151 | 0.726 ± 0.122 | 0.771 ± 0.071 | |
| Men | 0.804 ± 0.086 | 0.813 ± 0.079 | 0.822 ± 0.072 | 0.783 ± 0.080 | 0.813 ± 0.108 | 0.810 ± 0.084 | 0.827 ± 0.146 | 0.753 ± 0.153 | 0.759 ± 0.120 | 0.751 ± 0.071 | |
| mI/Cr | Left | 0.790 ± 0.082 | 0.723 ± 0.070 | 0.775 ± 0.055 | 0.661 ± 0.089 | 0.781 ± 0.088 | 0.834 ± 0.086 | 0.785 ± 0.102 | 0.678 ± 0.084 | 0.733 ± 0.150 | 0.848 ± 0.082 |
| Right | 0.781 ± 0.074 | 0.736 ± 0.066 | 0.785 ± 0.051 | 0.680 ± 0.098 | 0.791 ± 0.066 | 0.820 ± 0.088 | 0.799 ± 0.092 | 0.673 ± 0.071 | 0.770 ± 0.138 | 0.842 ± 0.086 | |
| Women | 0.785 ± 0.085 | 0.700 ± 0.078 ^ | 0.770 ± 0.063 | 0.605 ± 0.110 ^ | 0.784 ± 0.091 | 0.817 ± 0.096 | 0.769 ± 0.131 | 0.629 ± 0.112 ^ | 0.772 ± 0.179 | 0.822 ± 0.101 | |
| Men | 0.782 ± 0.085 | 0.750 ± 0.076 ^ | 0.785 ± 0.062 | 0.709 ± 0.106 ^ | 0.802 ± 0.090 | 0.838 ± 0.092 | 0.826 ± 0.132 | 0.757 ± 0.115 ^ | 0.754 ± 0.178 | 0.865 ± 0.102 | |
| Metabolite Ratio | Frontal Lobe | Parietal Lobe | Temporal Lobe | Occipital Lobe | Insula | Limbic Lobe | Claustrum | Lentiform Nucleus | Thalamus | Sublobar White Matter | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr | All | −0.741 | −0.791 | −0.819 | −0.668 | −0.605 | −0.780 | −0.512 | −0.359 | −0.471 | −0.568 |
| Left | −0.757 | −0.821 | −0.820 | −0.672 | −0.574 | −0.783 | −0.502 | −0.421 | −0.111 | −0.429 | |
| Right | −0.719 | −0.766 | −0.888 | −0.620 | −0.628 | −0.779 | −0.528 | −0.326 | −0.644 | −0.639 | |
| Women | −0.794 | −0.783 | −0.832 | −0.636 | −0.437 | −0.834 | −0.329 | −0.266 | −0.342 | −0.484 | |
| Men | −0.757 | −0.817 | −0.839 | −0.696 | −0.686 | −0.776 | −0.637 | −0.450 | −0.587 | −0.677 | |
| Cho/Cr | All | 0.051 | 0.214 | 0.234 | 0.195 | 0.218 | 0.245 | 0.052 | 0.122 | 0.116 | 0.154 |
| Left | −0.008 | 0.123 | 0.348 | 0.241 | 0.176 | 0.181 | 0.052 | 0.252 | 0.108 | 0.128 | |
| Right | 0.106 | 0.307 | 0.062 | 0.225 | 0.229 | 0.293 | 0.049 | 0.033 | 0.099 | 0.156 | |
| Women | −0.542 | −0.313 | −0.152 | 0.318 | −0.249 | −0.402 | −0.313 | −0.287 | −0.054 | −0.148 | |
| Men | 0.453 | 0.494 | 0.527 | 0.276 | 0.476 | 0.510 | 0.273 | 0.662 | 0.331 | 0.501 | |
| Glx/Cr | All | −0.262 | −0.532 | −0.262 | −0.259 | −0.143 | −0.422 | −0.023 | 0.004 | −0.092 | −0.248 |
| Left | −0.196 | −0.577 | −0.293 | −0.427 | 0.094 | −0.480 | 0.144 | −0.068 | −0.257 | 0.017 | |
| Right | −0.316 | −0.503 | −0.243 | 0.139 | −0.291 | −0.364 | −0.124 | 0.084 | 0.122 | −0.360 | |
| Women | 0.001 | −0.319 | −0.051 | 0.035 | −0.102 | −0.114 | 0.083 | 0.296 | 0.125 | 0.102 | |
| Men | −0.461 | −0.634 | −0.510 | −0.360 | −0.186 | −0.588 | −0.091 | −0.207 | −0.195 | −0.646 | |
| mI/Cr | All | 0.417 | 0.140 | 0.380 | −0.269 | 0.452 | 0.385 | 0.316 | −0.158 | 0.298 | 0.145 |
| Left | 0.428 | 0.055 | 0.414 | −0.185 | 0.310 | 0.431 | 0.426 | −0.394 | 0.314 | 0.164 | |
| Right | 0.417 | 0.232 | 0.357 | −0.354 | 0.575 | 0.355 | 0.236 | 0.177 | 0.270 | 0.136 | |
| Women | 0.496 | 0.306 | 0.105 | −0.030 | 0.401 | 0.461 | 0.356 | 0.305 | 0.246 | 0.392 | |
| Men | 0.503 | 0.083 | 0.566 | −0.175 | 0.543 | 0.412 | 0.450 | 0.054 | 0.443 | 0.188 | |
| Sex | Total Number (Right-Handers) | Mean Age ± SD (Range) | Mean Years of Education ± SD (Range) | Mean BMI ± SD | |
|---|---|---|---|---|---|
| All | 38 (30) | 35.11 ± 11.97 (22–68) years | 17.88 ± 2.12 (16–22) years | 22.76 ± 2.92 | |
| Women | 17 (14) | 34.29 ± 9.76 (22–52) years | 18.00 ± 2.35 (16–22) years | 20.90 ± 2.42 | |
| Men | 21 (16) | 35.76 ± 13.71 (22–68) years | 17.75 ± 1.98 (16–22) years | 24.27 ± 2.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Abiko, K.; Sheriff, S.; Maudsley, A.A.; Urushibata, Y.; Ahn, S.; Tha, K.K. The Distribution of Major Brain Metabolites in Normal Adults: Short Echo Time Whole-Brain MR Spectroscopic Imaging Findings. Metabolites 2022, 12, 543. https://doi.org/10.3390/metabo12060543
Li X, Abiko K, Sheriff S, Maudsley AA, Urushibata Y, Ahn S, Tha KK. The Distribution of Major Brain Metabolites in Normal Adults: Short Echo Time Whole-Brain MR Spectroscopic Imaging Findings. Metabolites. 2022; 12(6):543. https://doi.org/10.3390/metabo12060543
Chicago/Turabian StyleLi, Xinnan, Kagari Abiko, Sulaiman Sheriff, Andrew A. Maudsley, Yuta Urushibata, Sinyeob Ahn, and Khin Khin Tha. 2022. "The Distribution of Major Brain Metabolites in Normal Adults: Short Echo Time Whole-Brain MR Spectroscopic Imaging Findings" Metabolites 12, no. 6: 543. https://doi.org/10.3390/metabo12060543
APA StyleLi, X., Abiko, K., Sheriff, S., Maudsley, A. A., Urushibata, Y., Ahn, S., & Tha, K. K. (2022). The Distribution of Major Brain Metabolites in Normal Adults: Short Echo Time Whole-Brain MR Spectroscopic Imaging Findings. Metabolites, 12(6), 543. https://doi.org/10.3390/metabo12060543