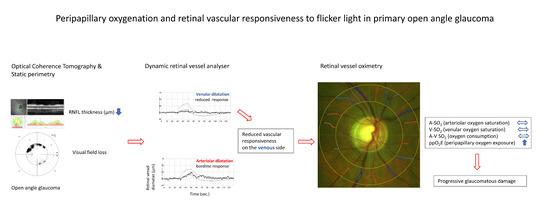
Peripapillary Oxygenation and Retinal Vascular Responsiveness to Flicker Light in Primary Open Angle Glaucoma
Abstract
1. Introduction
2. Results
2.1. Oxygen Saturation Parameters in Glaucoma vs. Controls
2.2. Oxygen Saturation by Vascular Responsiveness in Glaucoma Patients
2.3. Oxygen Saturation and Glaucomatous Damage
2.4. Peripapillary Oxygen Exposure and Glaucomatous Damage
2.5. Vascular Flicker Light Responsiveness vs. Glaucomatous Damage
2.6. Oxygen Saturation vs. Peripapillary Oxygen Exposure
3. Discussion
3.1. Oxygen Saturation and Glaucomatous Damage
3.2. Vascular Responsiveness to Flicker Light Exposure in Glaucoma
3.3. Vascular Flicker Light Responsiveness vs. Oxygen Saturation in Glaucoma
3.4. Peripapillary Oxygen Exposure in RNFL
3.5. Peripapillary Oxygen Exposure and Vascular Dysregulation in Glaucoma
3.6. Precautions
4. Materials and Methods
4.1. Visual Field Examination
4.2. Spectral Domain OCT
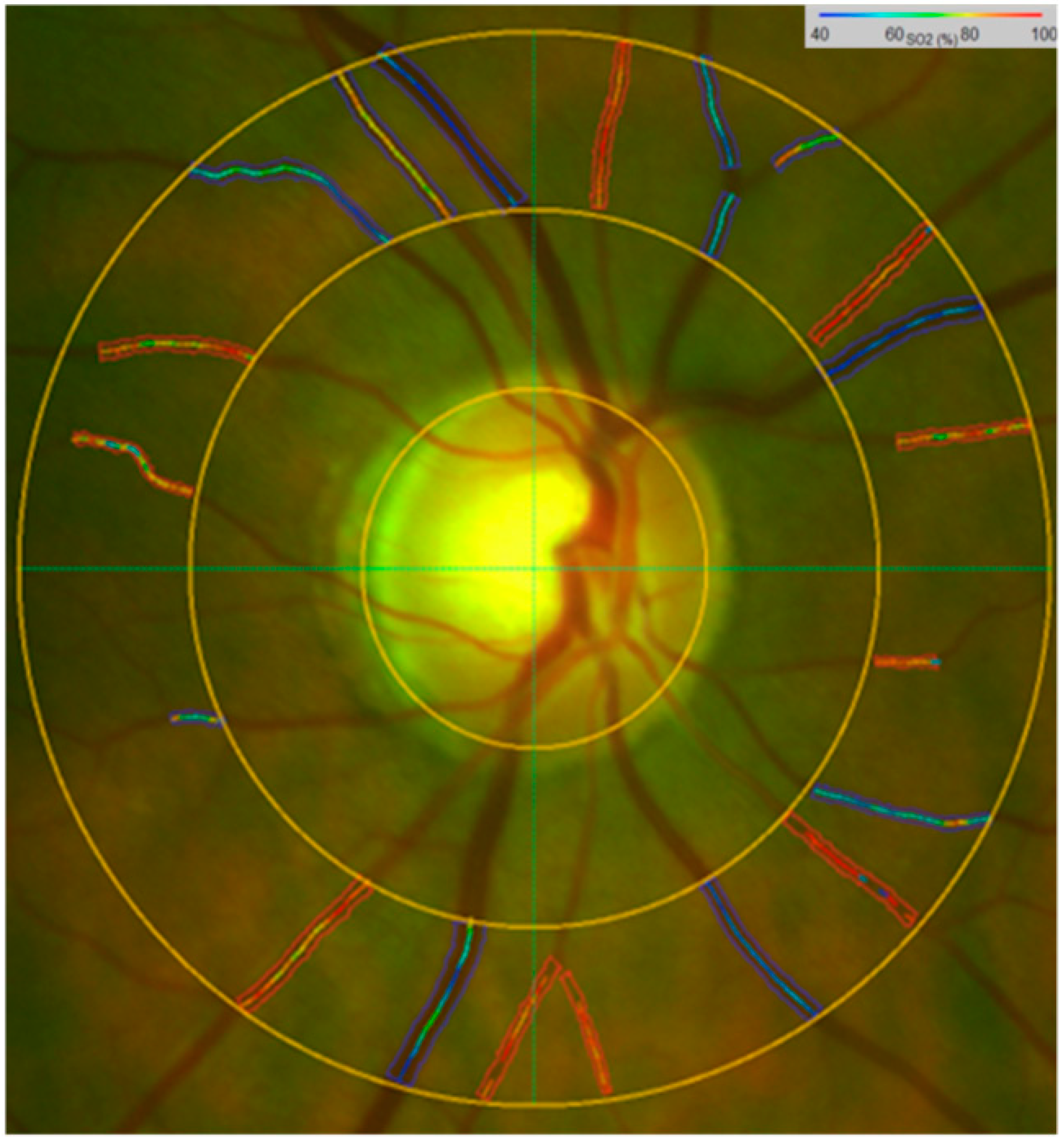
4.3. Retinal Vessel Oximetry and Retinal Vessel Analyser Imaging
4.4. Retinal Vessel Oximetry Imaging
4.5. Retinal Vessel Analyser
4.6. Statistical Analysis
- According to the median retinal vascular responsiveness to flicker light, glaucoma subjects were grouped as follows:
- By median arteriolar flicker responsiveness (1.80%; AFR) in:
- AFR ≤ 1.80% group: indicates ≤1.80% dilatation from the baseline diameter;
- AFR ≤ 1.80% group: indicates >1.80% dilatation from the baseline diameter.
- By median venular flicker responsiveness (2.90%; VFR) in:
- VFR ≤ 2.90% group: indicates ≤2.90% dilatation from the baseline diameter;
- VFR > 2.90% group: indicates >2.90% dilatation from the baseline diameter.
- Furthermore, we tested a hypothesis that the responsiveness to FL stimulation depends on the structural and functional alterations in glaucoma patients and sought to find out whether this relationship is different between the vascular responsiveness glaucoma groups (AFR, VFR).
- In addition, in order to obtain the mean peripapillary oxygen exposure per micron of nerve fibre layer thickness, ppO2E [%/µm], the mean A-V SO2 was divided by the mean RNFL thickness. Glaucoma patients were divided depending on the ppO2E values into two groups as follows:
- ppO2E ≤ 0.46 group: indicates ppO2E values ≤ 0.46%/µm;
- ppO2E > 0.46 group: indicates ppO2E values > 0.46%/µm.
- We studied then the relationships between oxygen saturation, flicker responsiveness and ppO2E within the glaucoma group.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.P.; Stefansson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef]
- Harris, A.; Ciulla, T.A.; Chung, H.S.; Martin, B. Regulation of retinal and optic nerve blood flow. Arch. Ophthalmol. 1998, 116, 1491–1495. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Vaegan; Millar, T.J.; Beaumont, P.; Sarks, S. Widespread choroidal insufficiency in primary open-angle glaucoma. J. Glaucoma 1997, 6, 23–32. [Google Scholar] [CrossRef]
- Galassi, F.; Sodi, A.; Ucci, F.; Harris, A.; Chung, H.S. Ocular haemodynamics in glaucoma associated with high myopia. Int. Ophthalmol. 1998, 22, 299–305. [Google Scholar] [CrossRef]
- Galassi, F.; Sodei, A.; Ucci, F.; Renieri, G.; Pieri, B.; Baccini, M. Ocular hemodynamics and glaucoma prognosis: A color Doppler imaging study. Arch. Ophthalmol. 2003, 121, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Rojanapongpun, P.; Drance, S.M.; Morrison, B.J. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br. J. Ophthalmol. 1993, 77, 25–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butt, Z.; McKillop, G.; O’Brien, C.; Allan, P.; Aspinall, P. Measurement of ocular blood flow velocity using colour Doppler imaging in low tension glaucoma. Eye 1995, 9, 29–33. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S. Optic nerve blood-flow abnormalities in glaucoma. Prog. Retin. Eye Res. 1998, 17, 267–289. [Google Scholar] [CrossRef]
- Chung, H.S.; Harris, A.; Kagemann, L.; Martin, B. Peripapillary retinal blood flow in normal tension glaucoma. Br. J. Ophthalmol. 1999, 83, 466–469. [Google Scholar] [CrossRef]
- Marangoni, D.; Falsini, B.; Colotto, A.; Salgarello, T.; Anselmi, G.; Fadda, A.; Di Renzo, A.; Campos, E.C.; Riva, C.E. Subfoveal choroidal blood flow and central retinal function in early glaucoma. Acta Ophthalmol. 2012, 90, e288–e294. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Haefliger, I.O.; Orgül, S.; Resink, T. Vascular dysregulation: A principal risk factor for glaucomatous damage? J. Glaucoma 1999, 8, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Orgül, S.; Gugleta, K.; Flammer, J. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br. J. Ophthalmol. 2004, 88, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, M.C.; Flammer, J. Blood flow in glaucoma. Curr. Opin. Ophthalmol. 2005, 16, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Orgül, S.; Gugleta, K.; Gekkieva, M.; Flammer, J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am. J. Ophthalmol. 2000, 130, 597–605. [Google Scholar] [CrossRef]
- Breil, P.; Krummenauer, F.; Schmitz, S.; Pfeiffer, N. The relationship between retrobulbar blood flow velocity and glaucoma damage. An intraindividual comparison. Ophthalmologe 2002, 99, 613–616. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Grieshaber, M.; Flammer, J. Oxygen and blood flow: Players in the pathogenesis of glaucoma. Mol. Vis. 2008, 14, 224–233. [Google Scholar]
- Anderson, B., Jr.; Saltzman, H. Retinal Oxygen utilisation measured by hyperbaric blackout. Arch. Ophthalmol. 1964, 72, 792–795. [Google Scholar] [CrossRef]
- Wangsa-Wirawan, N.D.; Linsenmeier, R. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef]
- Beach, J.M.; Schwenzer, K.J.; Srinivas, S.; Kim, D.; Tiedeman, J.S. Oximetry of retinal vessels by dual-wavelength imaging: Calibration and influence of pigmentation. J. Appl. Physiol. 1999, 86, 748–758. [Google Scholar] [CrossRef]
- Michelson, G.; Scibor, M. Intravascular oxygen saturation in retinal vessels in normal subjects and open-angle glaucoma subjects. Acta Ophthalmol. Scand. 2006, 84, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, O.B.; Hardarson, S.; Gottfredsdottir, M.S.; Harris, A.; Stefánsson, E. Retinal oximetry in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, E.; Abegão Pinto, L.; Olafsdottir, O.B.; De Clerck, E.; Stalmans, P.; Van Calster, J.; Zeyen, T.; Stefánsson, E.; Stalmans, I. Oximetry in glaucoma: Correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014, 92, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, O.B.; Vandewalle, E.; Abegão Pinto, L.; Geirsdottir, A.; De Clerck, E.; Stalmans, P.; Gottfredsdottir, M.S.; Kristjansdottir, J.V.; Van Calster, J.; Zeyen, T.; et al. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br. J. Ophthalmol. 2014, 98, 329–333. [Google Scholar] [CrossRef]
- Ito, M.; Murayama, K.; Deguchi, T.; Takasu, M.; Gil, T.; Araie, M.; Peyman, G.; Yoneya, S. Oxygen saturation levels in the juxta-papillary retina in eyes with glaucoma. Exp. Eye Res. 2008, 86, 512–518. [Google Scholar] [CrossRef]
- Na, J.H.; Sung, K.; Baek, S.; Lee, J.Y.; Kim, S. Progression of retinal nerve fiber layer thinning in glaucoma assessed by cirrus optical coherence tomography-guided progression analysis. Curr. Eye Res. 2013, 38, 386–395. [Google Scholar] [CrossRef]
- Ramm, L.; Jentsch, S.; Peters, S.; Augsten, R.; Hammer, M. Investigation of blood flow regulation and oxygen saturation of the retinal vessels in primary open-angle glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2014, 252, 1803–1810. [Google Scholar] [CrossRef]
- Graham, N.; Hood, D.C. Modeling the dynamics of light adaptation: The merging of two traditions. Vis. Res. 1992, 32, 1373–1393. [Google Scholar] [CrossRef]
- Luo, X.; Shen, Y.; Jiang, M.N.; Lou, X.F.; Shen, Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. J. Ophthalmol. 2015, 864871. [Google Scholar] [CrossRef]
- Garhöfer, G.; Zawinka, C.; Resch, H.; Huemer, K.H.; Schmetterer, L.; Dorner, G.T. Response of retinal vessel diameters to flicker stimulation in patients with early open angle glaucoma. J. Glaucoma 2004, 13, 340–344. [Google Scholar] [CrossRef]
- Gugleta, K.; Waldmann, N.; Polunina, A.; Kochkorov, A.; Katamay, R.; Flammer, J.; Orgul, S. Retinal neurovascular coupling in patients with glaucoma and ocular hypertension and its association with the level of glaucomatous damage. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Gugleta, K.; Zawinka, C.; Rickenbacher, I.; Kochkorov, A.; Katamay, R.; Flammer, J.; Orgul, S. Analysis of retinal vasodilation after flicker light stimulation in relation to vasospastic propensity. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4034–4041. [Google Scholar] [CrossRef]
- Seifert, B.U.; Vilser, W. Retinal Vessel Analyzer (RVA)-design and function. Biomed. Tech. 2002, 47, 678–681. [Google Scholar] [CrossRef]
- Gugleta, K.; Türksever, C.; Polunina, A.; Orgül, S. Effect of ageing on the retinal vascular responsiveness to flicker light in glaucoma patients and in ocular hypertension. Br. J. Ophthalmol. 2013, 97, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.E.; Logean, E.; Falsini, B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog. Retin. Eye Res. 2005, 24, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, O.; Galambos, P.; Wagenfeld, L.; Wiermann, A.; Wlodarsch, P.; Praga, R.; Matthiessen, E.T.; Richard, G.; Klemm, M. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br. J. Ophthalmol. 2006, 90, 1245–1248. [Google Scholar] [CrossRef]
- Moore, N.A.; Harris, A.; Wentz, S.; Verticchio Vercellin, A.C.; Parekh, P.; Gross, J.; Hussain, R.M.; Thieme, C.; Siesky, B. Baseline retrobulbar blood flow is associated with both functional and structural glaucomatous progression after 4 years. Br. J. Ophthalmol. 2017, 101, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Gugleta, K.; Kochkorov, A.; Waldmann, N.; Polunina, A.; Katamay, R.; Flammer, J.; Orgul, S. Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 589–594. [Google Scholar] [CrossRef]
- Waldmann, N.P.; Kochkorov, A.; Polunina, A.; Orgül, S.; Gugleta, K. The prognostic value of retinal vessel analysis in primary open-angle glaucoma. Acta Ophthalmol. 2016, 94, e474–e480. [Google Scholar] [CrossRef]
- Kim, K.E.; Kim, D.; Flammer, J.; Kim, K.N. Central retinal venous pressure in eyes of normal-tension glaucoma patients with optic disc hemorrhage. PLoS ONE 2015, 10, e0127920. [Google Scholar] [CrossRef]
- Fang, L.; Baertschi, M.; Mozaffarieh, M. The effect of flammer-syndrome on retinal venous pressure. BMC Ophthalmol. 2014, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Turtschi, S.; Mozaffarieh, M. The effect of nifedipine on retinal venous pressure of glaucoma patients with the Flammer-Syndrome. Graefes. Arch. Clin. Exp. Ophthalmol. 2015, 253, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; Morbio, R.; Varotto, A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology 2000, 107, 1287–1293. [Google Scholar] [CrossRef]
- Aleksandra, M.; Jens, D.; Marcus, B.; Nicolle, M.; Christoph, K.; Gunter, W.; Walthard, V.; Heike, H.; Ulrich, A.M. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care 2007, 30, 3048–3052. [Google Scholar]
- Stefánsson, E.; Olafsdottir, O.B.; Eliasdottir, T.S.; Vehmeijer, W.; Einarsdottir, A.B.; Bek, T.; Torp, T.L.; Grauslund, J.; Eysteinsson, T.; Karlsson, R.A.; et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog. Retin. Eye Res. 2019, 70, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Heller, T.; Jentsch, S.; Dawczynski, J.; Schweitzer, D.; Peters, S.; Schmidtke, K.U.; Müller, U.A. Retinal vessel oxygen saturation under flicker light stimulation in patients with nonproliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4063–4068. [Google Scholar] [CrossRef][Green Version]
- Bojinova, R.I.; Türksever, C.; Schötzau, A.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G. Reduced metabolic function and structural alterations in inherited retinal dystrophies: Investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2017, 95, 252–261. [Google Scholar] [CrossRef]
- Palkovits, S.; Lasta, M.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; Schmetterer, L. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4707–4713. [Google Scholar] [CrossRef]
- Einarsdottir, A.B.; Hardarson, S.; Kristjansdottir, J.V.; Bragason, D.T.; Snaedal, J.; Stefánsson, E. Retinal oximetry imaging in Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 49, 79–83. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; The Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.A.; Jackson, K.; Schlappal, A.E.; Spangenburg, E.E.; Ward, C.W.; Park, J.H.; Dugger, N.; Shi, G.L.; Fishman, P.S. Mitochondrial oxygen consumption deficits in skeletal muscle isolated from an Alzheimer’s disease-relevant murine model. BMC Neurosci. 2014, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Kass, M.A.; Kolker, A.E.; Wax, M.B. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology 1996, 103, 2105–2113. [Google Scholar] [CrossRef]
- Osborne, N.N.; Álvarez, C.N.; del Olmo Aguado, S. Targeting mitochondrial dysfunction as in aging and glaucoma. Drug Discov. Today 2014, 19, 1613–1622. [Google Scholar] [CrossRef]
- Prada, D.; Harris, A.; Guidoboni, G.; Siesky, B.; Huang, A.M.; Arciero, J. Autoregulation and neurovascular coupling in the optic nerve head. Surv. Ophthalmol. 2016, 61, 164–186. [Google Scholar] [CrossRef]
- Gaspar, A.Z.; Gasser, P.; Flammer, J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica 1995, 209, 11–13. [Google Scholar] [CrossRef]
- Konieczka, K.; Todorova, M.G.; Bojinova, R.I.; Binggeli, T.; Chackathayil, T.N.; Flammer, J. Unexpected Effect of Calcium Channel Blockers on the Optic Nerve Compartment Syndrome. Klin. Mon. Augenheilkd. 2016, 233, 387–390. [Google Scholar] [CrossRef]
- Cellini, M.; Rossi, A.; Moretti, M. The use of polyunsaturated fatty acids in ocular hypertension.: A study with blue-on-yellow perimetry. Acta Ophthalmol. Scand. 1999, 77, 54–55. [Google Scholar] [CrossRef]
- Quaranta, L.; Bettelli, S.; Uva, M.G.; Semeraro, F.; Turano, R.; Gandolfo, E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 2003, 110, 359–362. [Google Scholar] [CrossRef]
- Resch, H.; Weigert, G.; Karl, K.; Pemp, B.; Garhofer, G.; Schmetterer, L. Effect of systemic moxaverine on ocular blood flow in humans. Acta Ophthalmol. 2009, 87, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Felder, A.E.; Wanek, J.; Blair, N.P.; Shahidi, M. Inner Retinal Oxygen Extraction Fraction in Response to Light Flicker Stimulation in Humans. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6633–6637. [Google Scholar] [CrossRef] [PubMed]
- Türksever, C.; Orgül, S.; Todorova, M.G. Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol. 2015, 93, e439–e445. [Google Scholar] [CrossRef] [PubMed]
- Terai, N.; Spoerl, E.; Pillunat, L.E.; Stodtmeister, R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol. 2012, 90, e524–e528. [Google Scholar] [CrossRef]

| Parameters | Groups | Nr. Eyes | Mean | SD | 95% CI | p-Values between Control and Glaucoma Patients | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| A–SO2 (%) | Controls Glaucoma | 21 | 93.44 | 4.97 | 91.58 | 95.31 | 0.016 |
| 46 | 96.18 | 3.64 | 94.98 | 97.38 | |||
| V–SO2 (%) | Controls Glaucoma | 21 | 54.21 | 9.10 | 52.97 | 57.44 | <0.001 |
| 46 | 61.66 | 6.04 | 59.58 | 63.73 | |||
| A–V SO2 (%) | Controls Glaucoma | 21 | 39.25 | 7.96 | 36.45 | 42.06 | 0.011 |
| 46 | 34.86 | 6.04 | 33.06 | 36.67 | |||
| ppO2E (%/µm) | Controls Glaucoma | 21 | 0.40 | 0.09 | 0.34 | 0.44 | 0.024 |
| 46 | 0.46 | 0.10 | 0.43 | 0.49 | |||
| Parameters | Control vs. Glaucoma Groups by Median VFR | Nr. Eyes | Mean | SD | 95% CI | p-Values between Glaucoma Groups | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| A–SO2 (%) | Controls | 46 | 93.44 | 4.97 | 91.58 | 95.31 | |
| VFR ≤ 2.9% | 27 | 96.50 | 4.39 | 93.85 | 99.16 | 0.517 | |
| VFR > 2.9% | 19 | 97.38 | 3.03 | 95.63 | 99.13 | ||
| V–SO2 (%) | Controls | 46 | 5.21 | 9.10 | 52.97 | 57.44 | |
| VFR ≤ 2.9% | 27 | 62.90 | 6.23 | 59.13 | 66.66 | 0.391 | |
| VFR > 2.9% | 19 | 60.72 | 6.28 | 57.09 | 64.34 | ||
| A–V SO2 (%) | Controls | 46 | 39.25 | 7.96 | 36.45 | 42.06 | |
| VFR ≤ 2.9% | 27 | 34.64 | 4.51 | 31.92 | 37.37 | 0.294 | |
| VFR > 2.9% | 19 | 36.68 | 5.28 | 33.64 | 39.72 | ||
| ppO2E (%/µm) | Controls | 21 | 0.40 | 0.09 | 0.34 | 0.44 | |
| VFR ≤ 2.9% | 27 | 0.49 | 0.08 | 0.44 | 0.54 | 0.027 | |
| VFR > 2.9% | 19 | 0.43 | 0.06 | 0.40 | 0.47 | ||
| Retinal Vessel Oxygen Saturation and Peripapillary Exposure | Nr. Eyes | Neuroretinal Rim Area (mm2) p-Values | RNFL Thickness (µm) p-Values | Visual Field (MD, dB) p-Values |
|---|---|---|---|---|
| A–SO2 (%) | 46 | 0.236 | 0.297 | 0.392 |
| V–SO2 (%) | 46 | 0.012 | 0.001 | 0.002 |
| A-V SO2 (%) | 46 | 0.007 | <0.001 | 0.001 |
| ppO2E (%/µm) | 46 | <0.001 | 0.017 | 0.012 |
| Glaucomatous Damage | Groups by Median VFR | Nr. Eyes | Mean | SD | 95% CI | p-Values between Glaucoma VFR Groups | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| Neuroretinal rim area (mm2) | VFR ≤ 2.9% | 27 | 0.78 | 0.32 | 0.98 | 0.29 | 0.098 |
| VFR > 2.9% | 19 | 0.95 | 0.21 | 1.08 | 0.60 | ||
| RNFL thickness (µm) | VFR ≤ 2.9% | 27 | 72.23 | 12.09 | 64.92 | 79.54 | 0.014 |
| VFR > 2.9% | 19 | 85.31 | 13.67 | 77.42 | 93.21 | ||
| Visual field (MD, dB) | VFR ≤ 2.9% | 27 | 4.73 | 3.66 | 2.52 | 6.94 | 0.270 |
| VFR > 2.9% | 19 | 3.49 | 5.29 | 0.44 | 6.55 | ||
| Parameters | Glaucoma Groups by Median ppO2E | Nr. Eyes | Mean | SD | 95% CI | p-Values between Subgroups: Controls: ≤0.46 Controls: >0.46 ≤0.46: >0.46 | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| A–SO2 (%) | Controls | 21 | 93.44 | 4.97 | 91.58 | 95.31 | 0.184 |
| ppO2E ≤ 0.46 | 25 | 95.43 | 3.73 | 93.80 | 97.07 | 0.065 | |
| ppO2E > 0.46 | 21 | 96.26 | 3.76 | 98.08 | 98.08 | 0.503 | |
| V–SO2 (%) | Controls | 21 | 54.21 | 9.10 | 52.97 | 57.44 | 0.015 |
| ppO2E ≤ 0.46 | 25 | 62.29 | 5.67 | 59.33 | 65.25 | 0.526 | |
| ppO2E > 0.46 | 21 | 57.92 | 8.86 | 54.63 | 61.21 | 0.053 | |
| A–V SO2 (%) | Controls | 21 | 39.25 | 7.96 | 36.45 | 42.06 | 0.060 |
| ppO2E ≤ 0.46 | 25 | 33.59 | 5.24 | 31.16 | 36.01 | 0.565 | |
| ppO2E > 0.46 | 21 | 38.54 | 6.48 | 35.84 | 41.24 | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türksever, C.; Todorova, M.G. Peripapillary Oxygenation and Retinal Vascular Responsiveness to Flicker Light in Primary Open Angle Glaucoma. Metabolites 2022, 12, 597. https://doi.org/10.3390/metabo12070597
Türksever C, Todorova MG. Peripapillary Oxygenation and Retinal Vascular Responsiveness to Flicker Light in Primary Open Angle Glaucoma. Metabolites. 2022; 12(7):597. https://doi.org/10.3390/metabo12070597
Chicago/Turabian StyleTürksever, Cengiz, and Margarita G. Todorova. 2022. "Peripapillary Oxygenation and Retinal Vascular Responsiveness to Flicker Light in Primary Open Angle Glaucoma" Metabolites 12, no. 7: 597. https://doi.org/10.3390/metabo12070597
APA StyleTürksever, C., & Todorova, M. G. (2022). Peripapillary Oxygenation and Retinal Vascular Responsiveness to Flicker Light in Primary Open Angle Glaucoma. Metabolites, 12(7), 597. https://doi.org/10.3390/metabo12070597