Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Lipid Peroxidation in Liposomes Using Lipid-Soluble AMVN
2.2. Inhibition of Lipid Peroxidation in Liposomes Using Water-Soluble AIPH
2.3. Dynamic Light Scattering Analysis of Tocochromanols Containing Liposomes
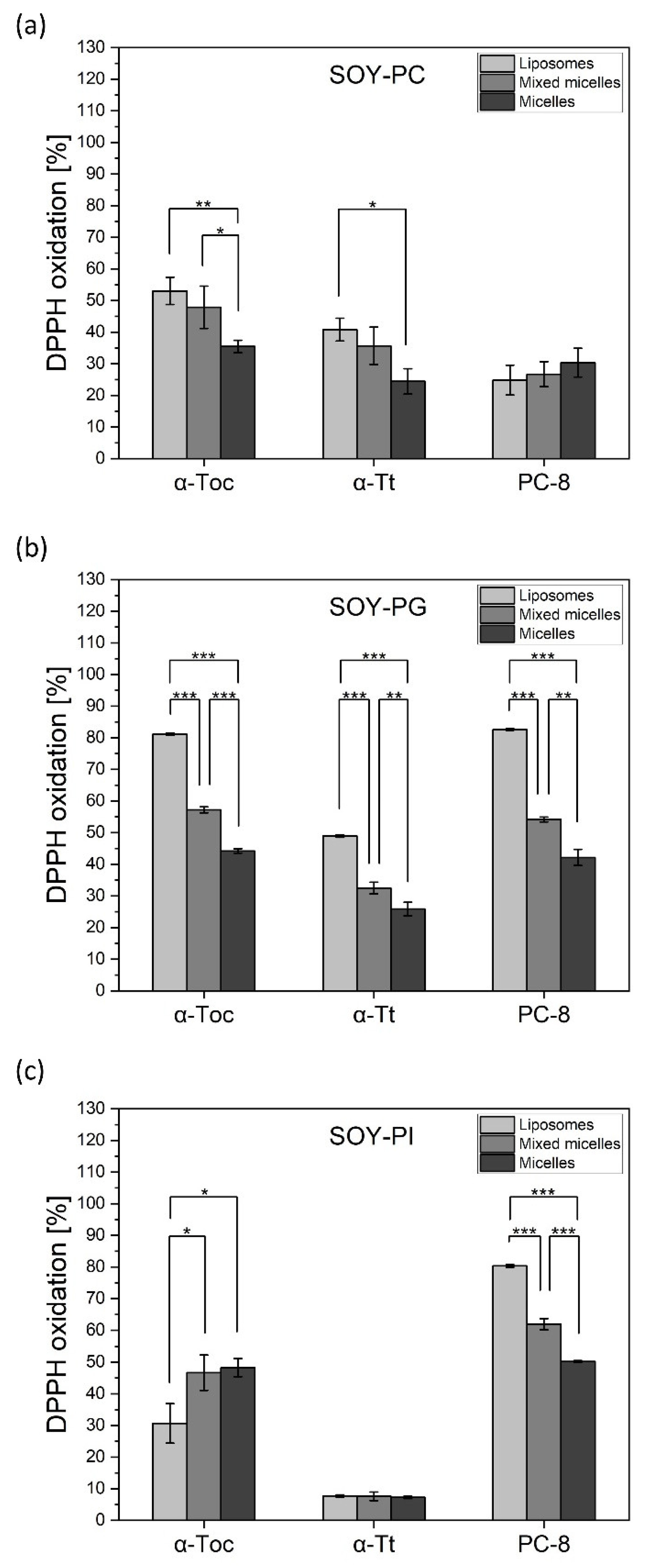
2.4. Antioxidant Activity of Tocochromanols in Different Nanocarriers—DPPH Assay
2.5. Effect of Tocochromanols against H2O2-Induced Damage in RA-SH-SY5Y Cells
3. Discussion
3.1. Free Radical-Initiated Lipid Peroxidation
3.2. Antioxidant Activity of Tocochromanols in Nanocarriers
3.3. Neuroprotective Activity of Tocochromanols
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Lipid Peroxidation of Tocochromanol-Containing Liposomes
4.2.2. HPLC Analysis
4.2.3. Dynamic Light Scattering (DLS) Measurements
4.2.4. Preparation of Tocochromanol-Containing Nanocarriers
4.2.5. DPPH Scavenging Assay
4.2.6. SH-SY5Y Cell Culture and Treatment
4.2.7. Cell Viability Assay
4.2.8. Cell Death Assessment
4.2.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fritsche, S.; Wang, X.; Jung, C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mene-Saffrane, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Szymanska, R.; Nowicka, B.; Kruk, J. Vitamin E—Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini Rev. Med. Chem. 2017, 17, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.M.; Schröder, R.; da Frota, M.L., Jr.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Kruk, J. Novel and rare prenyllipids—Occurrence and biological activity. Plant Physiol. Biochem. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Papas, A.M. CHAPTER Vitamin E: Tocopherols and Tocotrienols. In Antioxidant Status, Diet, Nutrition, and Health; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–23. [Google Scholar]
- Fiorentino, A.; Mastellone, C.; D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; Cefarelli, G.; Caputo, R.; Monaco, P. δ-Tocomonoenol: A new vitamin E from kiwi (Actinidia chinensis) fruits. Food Chem. 2009, 115, 187–192. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. CHAPTER 1 Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; The Royal Society of Chemistry: London, UK, 2019; pp. 1–11. [Google Scholar] [CrossRef]
- Kruk, J.; Pisarski, A.; Szymanska, R. Novel vitamin E forms in leaves of Kalanchoe daigremontiana and Phaseolus coccineus. J. Plant Physiol. 2011, 168, 2021–2027. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Wagner, T.; Sus, N.; Müller, M.; Kröpfl, A.; Vetter, W.; Frank, J. Cytotoxicity, cellular uptake, and metabolism to short-chain metabolites of 11′-α-tocomonoenol is similar to RRR-α-tocopherol in HepG2 cells. Free Radic. Biol. Med. 2021, 177, 24–30. [Google Scholar] [CrossRef]
- Gee, P.T. Unleashing the untold and misunderstood observations on vitamin E. Genes Nutr. 2011, 6, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Maita, N.; Fujisawa, A.; Takashima, J.; Ishii, Y.; Dunlap, W.C. A new vitamin E (alpha-tocomonoenol) from eggs of the Pacific salmon Oncorhynchus Keta. J. Nat. Prod. 1999, 62, 1685–1687. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Kruk, J.; Szymanska, R.; Cela, J.; Munne-Bosch, S. Plastochromanol-8: Fifty years of research. Phytochemistry 2014, 108, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Kruk, J. Plastoquinol is the main prenyllipid synthesized during acclimation to high light conditions in Arabidopsis and is converted to plastochromanol by tocopherol cyclase. Plant Cell Physiol. 2010, 51, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Gianello, R.; Libinaki, R.; Azzi, A.; Gavin, P.D.; Negis, Y.; Zingg, J.M.; Holt, P.; Keah, H.H.; Griffey, A.; Smallridge, A.; et al. Alpha-tocopheryl phosphate: A novel, natural form of vitamin E. Free Radic. Biol. Med. 2005, 39, 970–976. [Google Scholar] [CrossRef]
- Trela, A.; Szymanska, R. Less widespread plant oils as a good source of vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, B.; Gruszka, J.; Kruk, J. Function of plastochromanol and other biological prenyllipids in the inhibition of lipid peroxidation-A comparative study in model systems. Biochim. Biophys. Acta 2013, 1828, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Myung, S.K.; Ju, W.; Cho, B.; Oh, S.W.; Park, S.M.; Koo, B.K.; Park, B.J.; Korean Meta-Analysis Study Group. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.Y.; Poudyal, H.; Ward, L.C.; Brown, L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients 2012, 4, 1527–1541. [Google Scholar] [CrossRef] [Green Version]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef] [Green Version]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369s–373s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef] [Green Version]
- Basambombo, L.L.; Carmichael, P.H.; Cote, S.; Laurin, D. Use of Vitamin E and C Supplements for the Prevention of Cognitive Decline. Ann. Pharm. 2017, 51, 118–124. [Google Scholar] [CrossRef]
- de Rijk, M.C.; Breteler, M.M.; den Breeijen, J.H.; Launer, L.J.; Grobbee, D.E.; van der Meche, F.G.; Hofman, A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch. Neurol. 1997, 54, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Cheng, M.L.; Chiang, M.C.; Chen, C.M. Lipophilic antioxidants in neurodegenerative diseases. Clin. Chim. Acta 2018, 485, 79–87. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Sarin, N.B. Antioxidant value addition in human diets: Genetic transformation of Brassica juncea with γ-TMT gene for increased α-tocopherol content. Transgenic Res. 2007, 16, 109–113. [Google Scholar] [CrossRef]
- Wolf, R.; Wolf, D.; Ruocco, V. Vitamin E: The radical protector. J. Eur. Acad. Derm. Venereol. 1998, 10, 103–117. [Google Scholar] [CrossRef]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630–636. [Google Scholar] [CrossRef]
- Takahashi, T.; Nakaso, K.; Horikoshi, Y.; Hanaki, T.; Yamakawa, M.; Nakasone, M.; Kitagawa, Y.; Koike, T.; Matsura, T. Rice bran dietary supplementation improves neurological symptoms and loss of Purkinje cells in vitamin E-deficient mice. Yonago Acta Med. 2016, 59, 188–195. [Google Scholar]
- Li, Y.; Liu, L.; Barger, S.W. Vitamin E suppression of microglial activation is neuroprotective. J. Neurosci. Res. 2001, 66, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols as natural neuroprotective vitamins. In Tocotrienols: Vitamin E beyond Tocopherols; Watson, R.R., Preedy, V.R., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 361–398. [Google Scholar]
- Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.; Margina, D. Vitamin E beyond its antioxidant label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Ramdas, P.; Radhakrishnan, A.K.; Kutty, M.K.; Haleagrahara, N. Tocotrienols ameliorate neurodegeneration and motor deficits in the 6-OHDA-induced rat model of parkinsonism: Behavioural and immunohistochemistry analysis. Nutrients 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Maruyama, W.; Shaomoto-Nagai, M.; Kato, Y.; Hisaka, S.; Osawa, T.; Naoi, M. Role of lipid peroxide in the neurodegenerative disorders. Subcell. Biochem. 2014, 77, 127–136. [Google Scholar]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease 2019. Oxid Med. Cell Longev. 2019, 2019, 7147235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
- Suarna, C.; Hood, R.L.; Dean, R.T.; Stocker, R. Comparative antioxidant activity of tocotrienols and other natural lipid-soluble antioxidants in a homogeneous system, and in rat and human lipoproteins. Biochim. Biophys. Acta 1993, 1166, 163–170. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Yoshida, Y.; Itoh, N.; Saito, Y.; Hayakawa, M.; Niki, E. Application of water-soluble radical initiator, 2,2′-azobis [2-(2-imidazolin-2-yl)propane] dihydrochloride, to a study of oxidative stress. Free Radic. Res. 2004, 38, 375–384. [Google Scholar] [CrossRef]
- Jantas, D.; Pytel, M.; Mozrzymas, J.W.; Leskiewicz, M.; Regulska, M.; Antkiewicz-Michaluk, L.; Lason, W. The attenuating effect of memantine on staurosporine-, salsolinol- and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2008, 52, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Greda, A.; Golda, S.; Korostynski, M.; Grygier, B.; Roman, A.; Pilc, A.; Lason, W. Neuroprotective effects of metabotropic glutamate receptor group II and III activators against MPP(+)-induced cell death in human neuroblastoma SH-SY5Y cells: The impact of cell differentiation state. Neuropharmacology 2014, 83, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Szymanska, R.; Kruk, J. Identification of hydroxy-plastochromanol in Arabidopsis leaves. Acta Biochim. Pol. 2010, 57, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Szymanska, R.; Nowicka, B.; Kruk, J. Hydroxy-plastochromanol and plastoquinone-C as singlet oxygen products during photo-oxidative stress in Arabidopsis. Plant Cell Environ. 2014, 37, 1464–1473. [Google Scholar] [CrossRef]
- Doba, T.; Burton, G.W.; Ingold, K.U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim. Biophys. Acta 1985, 835, 298–303. [Google Scholar] [CrossRef]
- Huang, S.-W.; Frankel, E.N.; German, J.B. Antioxidant activity of alpha- and gamma-tocopherols in bulk oils and in oil-in-water emulsions. J. Agric. Food Chem. 1994, 42, 2108–2114. [Google Scholar] [CrossRef]
- Palozza, P.; Verdecchia, S.; Avanzi, L.; Vertuani, S.; Serini, S.; Iannone, A.; Manfredini, S. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol. Cell Biochem. 2006, 287, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.B.; Pownall, H.J. Interaction of alpha-tocopherol with model human high-density lipoproteins. Biophys. J. 1998, 75, 2923–2931. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Amiri, S.; Ghanbarzadeh, B.; Hamishehkar, H.; Hosein, M.; Babazadeh, A.; Adun, P. Vitamin E loaded nanoliposomes: Effects of gammaoryzanol, polyethylene glycol and lauric acid on physicochemical properties. Colloid Interface Sci. Commun. 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Sakdiset, P.; Okada, A.; Todo, H.; Sugibayashi, K. Selection of phospholipids to design liposome preparations with high skin penetration-enhancing effects. J. Drug Deliv. Sci. Technol. 2018, 44, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal delivery of natural product: A promising approach in health research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Ye, A.; Liu, W.; Tian, M.; Lu, Y.; Wu, K.; Liu, J.; Lou, M.P. Influence of phospholipids structure on the physicochemical properties and in vitro digestibility of lactoferrin-loaded liposomes. Food Biophys. 2019, 14, 287–299. [Google Scholar] [CrossRef]
- Gruszka, J.; Pawlak, A.; Kruk, J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers-determination of singlet oxygen quenching rate constants and oxidation products. Free Radic. Biol. Med. 2008, 45, 920–928. [Google Scholar] [CrossRef]
- Hincha, D.K. Effects of alpha-tocopherol (vitamin E) on the stability and lipid dynamics of model membranes mimicking the lipid composition of plant chloroplast membranes. FEBS Lett. 2008, 582, 3687–3692. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.; Kinnun, J.J.; Marquardt, D.; Ghefli, M.; Kučerka, N.; Katsaras, J.; Atkinson, J.; Harroun, T.A.; Feller, S.E.; Wassall, S.R. α-Tocopherol Is Well Designed to Protect Polyunsaturated Phospholipids: MD Simulations. Biophys. J. 2015, 109, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Kagan, V.E. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann. N. Y. Acad Sci. 1989, 570, 121–135. [Google Scholar] [CrossRef]
- Urano, S.; Shichita, N.; Matsuo, M. Interaction of vitamin E and its model compounds with unsaturated fatty acids in homogeneous solution. J. Nutr. Sci. Vitam. 1988, 34, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Erin, A.N.; Skrypin, V.V.; Kagan, V.E. Formation of alpha-tocopherol complexes with fatty acids. Nature of complexes. Biochim. Biophys. Acta 1985, 815, 209–214. [Google Scholar] [CrossRef]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of alpha-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, A.; Pisal, D.S.; Doty, A.; Balu-Iyer, S.V. Phosphatidylinositol induces fluid phase formation and packing defects in phosphatidylcholine model membranes. Chem. Phys. Lipids 2012, 165, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, J.P.; Bushby, R.J.; Giles, C.C.; Saunders, M.R. Orientation of the headgroup of phosphatidylinositol in a model biomembrane as determined by neutron diffraction. Biochemistry 1999, 38, 8393–8401. [Google Scholar] [CrossRef]
- Peng, A.; Straubinger, R.M.; Balu-Iyer, S.V. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. AAPS J. 2010, 12, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Kono, N.; Ohto, U.; Hiramatsu, T.; Urabe, M.; Uchida, Y.; Satow, Y.; Arai, H. Impaired alpha-TTP-PIPs interaction underlies familial vitamin E deficiency. Science 2013, 340, 1106–1110. [Google Scholar] [CrossRef]
- Chung, S.; Ghelfi, M.; Atkinson, J.; Parker, R.; Qian, J.; Carlin, C.; Manor, D. Vtamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic alpha-Tocopherol Transfer Protein. J. Biol. Chem. 2016, 291, 17028–17039. [Google Scholar] [CrossRef] [Green Version]
- Shichiri, M.; Takanezawa, Y.; Uchida, K.; Tamai, H.; Arai, H. Protection of cerebellar granule cells by tocopherols and to-cotrienols against methylmercury toxicity. Brain Res. 2007, 1182, 106–115. [Google Scholar] [CrossRef]
- Yang, L.; Shen, K.; Ji, D. Natural compounds attenuate heavy metal-induced PC12 cell damage. J. Int. Med. Res. 2020, 48, 300060520930847. [Google Scholar] [CrossRef]
- Selvaraju, T.R.; Khaza’ai, H.; Vidyadaran, S.; Abd Mutalib, M.S.; Vasudevan, R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn. J. Basic. Med. Sci. 2014, 14, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Torre, M.E.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Valenzano, A.; Pisanelli, D.; Panaro, M.A.; Tartaglia, N.; Am-brosi, A.; et al. Role of Vitamin E and the Orexin System in Neuroprotection. Brain Sci. 2021, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X. Effect of Vesicle-to-Micelle Transition on the Interactions of Phospholipid/Sodium Cholate Mixed Systems with Curcumin in Aqueous Solution. J. Phys. Chem. B 2016, 120, 7392–7400. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Jantas, D.; Piotrowski, M.; Staroń, J.; Leśkiewicz, M.; Regulska, M.; Lasoń, W.; Warszyński, P. Encapsulation of curcumin in polyelectrolyte nanocapsules and their neuroprotective activity. Nanotechnology 2016, 27, 355101. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trela-Makowej, A.; Leśkiewicz, M.; Kruk, J.; Żądło, A.; Basta-Kaim, A.; Szymańska, R. Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study. Metabolites 2022, 12, 608. https://doi.org/10.3390/metabo12070608
Trela-Makowej A, Leśkiewicz M, Kruk J, Żądło A, Basta-Kaim A, Szymańska R. Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study. Metabolites. 2022; 12(7):608. https://doi.org/10.3390/metabo12070608
Chicago/Turabian StyleTrela-Makowej, Agnieszka, Monika Leśkiewicz, Jerzy Kruk, Andrzej Żądło, Agnieszka Basta-Kaim, and Renata Szymańska. 2022. "Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study" Metabolites 12, no. 7: 608. https://doi.org/10.3390/metabo12070608
APA StyleTrela-Makowej, A., Leśkiewicz, M., Kruk, J., Żądło, A., Basta-Kaim, A., & Szymańska, R. (2022). Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study. Metabolites, 12(7), 608. https://doi.org/10.3390/metabo12070608





