Vitamin and Mineral Supplementation and Rate of Weight Gain during the First Trimester of Gestation in Beef Heifers Alters the Fetal Liver Amino Acid, Carbohydrate, and Energy Profile at Day 83 of Gestation
Abstract
1. Introduction
2. Materials and Methods
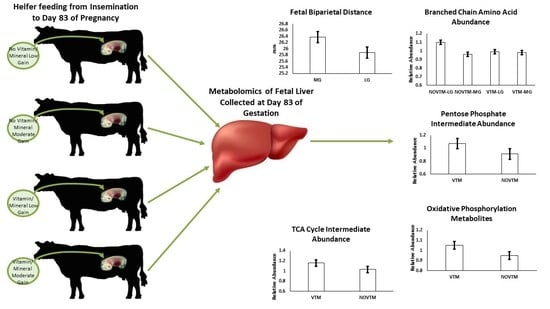
2.1. Animals, Experimental Design, and Dietary Treatments
2.2. Sample Collection
2.3. Sample Preparation, UPLC-MS/MS, and QC
2.4. Data Extraction and Compound Identification
2.5. Statistical Analysis
3. Results
3.1. Specific Interactive and Main Effects
3.1.1. Amino Acid Metabolism
3.1.2. Carbohydrate Metabolism
3.1.3. Energy Metabolism
3.2. Specific Interactive and Main Effects
3.2.1. Amino Acids
3.2.2. Carbohydrates and Energy
3.3. Total Pathway Analysis
3.3.1. Amino Acids
3.3.2. Carbohydrates and Energy
4. Discussion
- (1)
- Fetuses from LG and NOVTM heifers may be storing metabolites for use due to limited or reduced nutrient availability. This is supported by research from Crouse et al. [17] and Diniz et al. [30] that found fetuses from heifers on a restricted diet during the first 50 d of gestation had an increased number of genes upregulated in their liver, muscle, and cerebrum compared with fetuses from control dams, which suggests that when nutrients are restored, a compensatory mechanism is in place to recapture growth.
- (2)
- Vitamins and minerals are cofactors for multiple metabolic reactions. Menezes et al. [31] reported that multiple minerals were decreased in NOVTM fetal liver compared with VTM fetal liver including Se, Cu, Mn, and Co that are involved in multiple metabolic reactions including those involved in high energy phosphate bonds as well as being involved in the electron transport chain. Furthermore, cAMP levels in the same study (NOVTM-LG: 0.9585, VTM-LG: 0.9666, NOVTM-MG: 0.9781, and VTM-MG: 1.1080; gain: p = 0.03 and vitamin: p = 0.06) were decreased in LG compared with MG, and NOVTM compared with VTM. Cyclic AMP is involved in regulating protein kinases and is a marker of energy abundance. The decrease in cAMP demonstrates decreased energy availability in the LG and NOVTM compared with the MG and VTM fetal livers and suggests that fetuses do require their dams to be on an increased rate of weight gain to meet the requirements for proper fetal metabolism and growth. Crouse et al. [17] reported that fetuses from restricted vs. control dams had a greater transcript abundance of Serine/Threonine protein kinases, ATP-binding, and nucleotide-binding genes, which further supports the above hypothesis that fetuses from heifers fed on a lower rate of weight gain may be compensating for reduced energy and growth in anticipation of restored energy intake. Prezotto et al. [32] reported that when dams were feed restricted in early to mid-gestation and subsequently realimented during mid to late-gestation (Restricted-Restricted-Control or Restricted-Control-Control) the fetal liver weight was increased compared with fetal livers from dams on the control treatment (Control, Control, Control). These data demonstrate the compensation response to nutrient restriction in early gestation followed by realimentation. However, it must be noted that impacts of nutrient restriction such as that of intrauterine growth restriction on postnatal performance (metabolic, reproductive, etc.) should continue and understanding the impacts of the severity and duration of nutrient restriction and the timing of realimentation are warranted.
- (3)
- There is greater fetal hepatic metabolic activity in the MG and VTM fetuses which would explain the decreased metabolites and increased urea in fetal liver and is confirmed by the increase in markers of energy metabolism such as cAMP. The increased metabolic activity in MG compared with LG fetal livers is further evidenced by Baumgaertner et al. [33], who reported that heifers fed to gain 0.75 kg/day from breeding to d 84 of gestation were not only 41.5 kg heaver at parturition, but birthed calves were 2.1 kg heavier with a larger chest circumference compared with calves born to heifers fed to gain 0.20 kg/day, which are similar targeted gains to those in this study. Furthermore, when combining the ultrasonography measurements from the current study with data from Baumgaertner et al. [33] in which heifers were from the same genetic makeup and on similar rates of weight gains and diets, fetuses from the MG treatments had a greater biparietal distance at d 83 of gestation than those on the LG treatment (26.38 ± 0.18 mm vs. 25.88 ± 0.18 mm; p = 0.05).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Crouse, S.M.; Greseth, N.P.; McLean, K.J.; Crosswhite, M.R.; Pereira, N.N.; Ward, A.K.; Reynolds, L.P.; Dahlen, C.R.; Neville, B.W.; Borowicz, P.P.; et al. Maternal nutrition and stage of early pregnancy in beef heifers: Impacts on hexose and AA concentrations in maternal and fetal fluids. J. Anim. Sci. 2019, 97, 1296–1316. [Google Scholar] [CrossRef]
- Kegley, B.E.; Ball, J.J.; Beck, P.A.; Bill, E. Kunkle interdisciplinary beef symposium: Impact of mineral and vitamin status on beef cattle immune function and health. J. Anim. Sci. 2016, 94, 5401–5413. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Freetly, C.H.; Cundiff, L.V. Postweaning growth and reproduction characteristics of heifers sired by bulls of seven breeds and raised on different levels of nutrition. J. Anim. Sci. 1997, 75, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Freetly, C.H.; Cundiff, L.V. Reproductive performance, calf growth, and milk production of first-calf heifers sired by seven breeds and raised on different levels of nutrition. J. Anim. Sci. 1998, 76, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, D.Z.; Cope, E.R.; Hobbs, J.D.; Oakes, R.N.; Pohler, K.G.; Mulliniks, J.T. Winter grazing of stockpiled native forages during heifer development delays body weight gain without influencing final pregnancy rates. J. Anim. Sci. 2018, 96, 4633–4643. [Google Scholar] [CrossRef]
- Freetly, C.H.; Ferrell, C.L.; Jenkins, T.G. Production performance of beef cows raised on three different nutritionally controlled heifer development programs. J. Anim. Sci. 2001, 79, 819–826. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Alves, B.R.; Prezotto, L.D.; Thorson, J.F.; Tedeschi, L.O.; Keisler, D.H.; Park, C.S.; Amstalden, M.; Williams, G.L. Use of a stair-step compensatory gain nutritional regimen to program the onset of puberty in beef heifers. J. Anim. Sci. 2014, 92, 2942–2949. [Google Scholar] [CrossRef]
- Amundson, L.O.; Fountain, T.H.; Larimore, E.L.; Richardson, B.N.; McNeel, A.K.; Wright, E.C.; Keisler, D.H.; Cushman, R.A.; Perry, G.A.; Freetly, H.C. Postweaning nutritional programming of ovarian development in beef heifers. J. Anim. Sci. 2015, 93, 5232–5239. [Google Scholar] [CrossRef]
- Rosasco, L.S.; Melchior, E.A.; Cox, S.H.; Dunlap, R.L.; Gifford, J.A.H.; Scholljegerdes, E.J.; Cushman, R.A.; Summers, A.F. Effect of stair-step nutritional programming on ovarian development in replacement beef heifers. Transl. Anim. Sci. 2020, 4 (Suppl. S1), S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Freetly, C.H.; Cushman, R.A.; Bennett, G.L. Production performance of cows raised with different postweaning growth patterns. Transl. Anim. Sci. 2021, 5, txab031. [Google Scholar] [CrossRef]
- Pinney, O.D.; Stephens, D.F.; Pope, L.S. Lifetime effects of winter supplemental feed level and age at first parturition on range beef cows. J. Anim. Sci. 1972, 34, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Arias, R.; Gunn, P.; Lemanager, R.; Lake, S. Effects of post-AI nutrition on growth performance and fertility of yearling beef heifers. Proc. West. Sec. Amer. Soc. Anim. Sci. 2012, 63, 117–121. [Google Scholar]
- Perry, A.G.; Perry, B.L.; Walker, J.A.; Wright, C.L.; Salverson, R.R.; Patterson, H.H. Evaluation of prior grazing experience on reproductive performance in beef heifers. The Prof. Anim. Scientist 2013, 29, 595–600. [Google Scholar] [CrossRef]
- Crouse, S.M.; Caton, J.S.; Cushman, R.A.; McLean, K.J.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Ward, A.K. Moderate nutrient restriction of beef heifers alters expression of genes associated with tissue metabolism, accretion, and function in fetal liver, muscle, and cerebrum by day 50 of gestation. Transl. Anim. Sci. 2019, 3, 855–866. [Google Scholar] [CrossRef]
- USDA. Beef 2017, “Beef Cow-Calf Management Practices in the United States, 2017, Report 1.” USDA-APHIS-VS-CEAH-NAHMS. Fort Collins, CO. #.782.0520. 2020. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/nahms/NAHMS_Beef_CowCalf_Studies (accessed on 7 January 2022).
- Davy, S.J.; Forero, L.C.; Shapero, M.W.; Rao, D.R.; Becchetti, T.A.; Rivers, C.K.; Stackhouse, J.W.; DeAtley, K.L.; McNabb, B.R. Mineral status of California beef cattle. Transl. Anim. Sci. 2019, 3, 66–73. [Google Scholar] [CrossRef]
- Hidiroglou, M. Trace elements in the fetal and neonate ruminant: A review. Can. Vet. J. 1980, 21, 328. [Google Scholar] [PubMed]
- Hostetler, E.C.; Kincaid, R.L.; Mirando, M.A. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef]
- Menezes, B.A.C.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation affect concentrations of amino acids in maternal serum and allantoic fluid of beef heifers. J. Anim. Sci. 2021, 99, skab024. [Google Scholar] [CrossRef]
- Menezes, B.A.C.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers 1: Effects on dam hormonal and metabolic status, fetal tissue and organ mass, and concentration of glucose and fructose in fetal fluids at d 83 of gestation. Animals 2022, 12, 1757. [Google Scholar] [CrossRef]
- Lamb, C.G.; Dahlen, C.R.; Larson, J.E.; Marquezini, G.; Stevenson, J.S. Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: A review. J. Anim. Sci. 2010, 88 (Suppl. S13), E181–E192. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.K.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Crosswhite, M.R.; Neville, B.W.; Walden, S.D.; Caton, J.S. Technical note: A new surgical technique for ovariohysterectomy during early pregnancy in beef heifers. J. Anim. Sci. 2016, 94, 5089–5096. [Google Scholar] [CrossRef] [PubMed]
- Simintiras, A.C.; Sánchez, J.M.; McDonald, M.; Martins, T.; Binelli, M.; Lonergan, P. Biochemical characterization of progesterone-induced alterations in bovine uterine fluid amino acid and carbohydrate composition during the conceptus elongation window. Biol. Reprod. 2018, 100, 672–685. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS 9.4 Statements: Reference; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Ferrell, L.C.; Ford, S.P.; Prior, R.L.; Christenson, R.K. Blood flow, steroid secretion and nutrient uptake of the gravid bovine uterus and fetus. J. Anim. Sci. 1983, 56, 656–667. [Google Scholar] [CrossRef][Green Version]
- Wintour, E.M.; Laurence, B.M.; Lingwood, B.E. Anatomy, physiology and pathology of the amniotic and allantoic compartments in the sheep and cow. Aust. Vet. J. 1986, 63, 216–221. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Crouse, M.S.; Cushman, R.A.; McLean, K.J.; Caton, J.S.; Dahlen, C.R.; Reynolds, L.P.; Ward, A.K. Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy. Sci. Rep. 2021, 11, 2771. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; McCarthy, K.L.L.; Kassetas, C.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.T.; Neville, T.L.L.; Ward, A.K.K.; Borowicz, P.P.P.; Reynolds, L.P.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers: Effects on fetal trace mineral reserves at day 83 of gestation. J. Anim. Sci. 2021, 99 (Suppl. S3), 164–165. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Camacho, L.E.; Lemley, C.O.; Keomanivong, F.E.; Caton, J.S.; Vonnahme, K.A.; Swanson, K.C. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef]
- Baumgaertner, F.; Underdahl, S.R.; McCarthy, K.L.; Menezes, A.C.B.; Diniz, W.J.d.; Ward, A.K.; Sedivec, K.K.; Dorsam, S.T.; Kirsch, J.D.; Caton, J.; et al. Effects of energy supplementation during early gestation in beef heifers on body weight, concentrations of IGF-1, and calf characteristics. J. Anim. Sci. 2020, 98 (Suppl. S4), 163–164. [Google Scholar] [CrossRef]
- Micke, G.C.; Sullivan, T.M.; Magalhaes, R.J.S.; Rolls, P.J.; Norman, S.T.; Perry, V.E.A. Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim. Reprod. Sci. 2010, 117, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ford, S.P.; Zhu, M.-J. Optimizing livestock production efficiency through maternal nutritional management and fetal developmental programming. Anim. Front. 2017, 7, 5–11. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.; Zhu, M.; Ford, S.; Nathanielsz, P. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88 (Suppl. S13), E51–E60. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, A.L.; Weselowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the powerhouse: Mitochondrial dysfunction in the liver and skeletal muscle of intrauterine growth restricted fetuses. Front. Endocrinol. 2021, 12, 612888. [Google Scholar] [CrossRef]
- Jones, A.K.; Brown, L.D.; Rozance, P.J.; Serkova, N.J.; Hay, W.W., Jr.; Friedman, J.E.; Wesolowski, S.R. Differential effects of intrauterine growth restriction and a hyperinsulinemic-isoglycemic clamp on metabolic pathways and insulin action in the fetal liver. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R427–R440. [Google Scholar] [CrossRef]
- Molinaro, A.; Lassen, P.B.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.-O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Van Son, J.; Serlie, M.J.; Ståhlman, M.; Bäckhed, F.; Nieuwdorp, M.; Aron-Wisnewsky, J. Plasma imidazole propionate is positively correlated with blood pressure in overweight and obese humans. Nutrients 2021, 13, 2706. [Google Scholar] [CrossRef]
- Russell, J.B.; Forsberg, N. Production of tricarballylic acid by rumen microorganisms and its potential toxicity in ruminant tissue metabolism. Br. J. Nutr. 1986, 56, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]



| Superpathway | Subpathway | Pathway Enrichment Score 1 | ||
|---|---|---|---|---|
| Gain | Vitamin | Gain × Vitamin | ||
| Amino Acid Metabolism | Glycine, Serine, and Threonine | 0.35 | 1.56 | 5.62 |
| Alanine and Aspartate | 1.06 | 0.00 | 0.00 | |
| Glutamate | 0.65 | 4.53 | 3.81 | |
| Histidine | 1.01 | 0.88 | 0.00 | |
| Lysine | 0.46 | 3.25 | 0.00 | |
| Phenylalanine | 0.00 | 0.00 | 0.00 | |
| Tyrosine | 0.35 | 0.00 | 1.32 | |
| Tryptophan | 0.35 | 1.56 | 0.00 | |
| Leucine, Isoleucine, and Valine | 0.00 | 0.00 | 1.8 | |
| Methionine, Cysteine, SAM, and Taurine | 0.31 | 0.68 | 0.58 | |
| Urea Cycle; Arginine and Proline | 0.44 | 0.00 | 2.59 | |
| Creatine | 0.00 | 0.00 | 0.00 | |
| Polyamine | 0.00 | 0.00 | 0.00 | |
| Guanidino and Acetamido | 0.00 | 0.00 | 0.00 | |
| Glutathione | 0.56 | 0.00 | 0.00 | |
| Carbohydrate Metabolism | Glycolysis, Gluconeogenesis, and Pyruvate | 0.00 | 1.34 | 0.00 |
| Pentose Phosphate Pathway | 0.00 | 0.00 | 0.00 | |
| Pentose | 0.00 | 0.00 | 0.00 | |
| Glycogen | 0.00 | 0.00 | 0.00 | |
| Fructose, Mannose, and Galactose | 0.00 | 0.00 | 2.28 | |
| Nucleotide Sugar | 0.00 | 0.00 | 0.00 | |
| Aminosugar | 0.00 | 2.09 | 0.00 | |
| Advanced Glycation End-product | 0.00 | 0.00 | 0.00 | |
| Energy Metabolism | TCA Cycle | 0.47 | 2.09 | 0.00 |
| Oxidative Phosphorylation | 0.00 | 0.00 | 0.00 | |
| Metabolite | Two-Way ANOVA Main Effects | Two-Way ANOVA Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Vitamin | Gain:Vitamin | NOVTM-MG | VTM-LG | NOVTM-MG | VTM-MG | VTM-MG | VTM-MG | ||
| NOVTM-LG | NOVTM-LG | VTM-LG | NOVTM-LG | VTM-LG | NOVTM-MG | |||||
| Glycine, Serine and Threonine Metabolism | glycine | 0.6901 | 0.1548 | 0.1680 | 0.94 | 0.91 | 1.03 | 0.94 | 1.03 | 1.00 |
| N-acetylglycine | 0.6664 | 0.1463 | 0.9750 | 1.04 | 0.92 | 1.13 | 0.94 | 1.03 | 0.91 | |
| sarcosine | 0.5676 | 0.4727 | 0.0497 | 0.71 | 0.85 | 0.84 | 1.01 | 1.19 | 1.42 | |
| dimethylglycine | 0.0588 | 0.0611 | 0.0132 | 0.96 | 0.66 | 1.45 | 0.99 | 1.50 | 1.03 | |
| betaine | 0.0708 | 0.0197 | 0.0195 | 0.97 | 0.67 | 1.45 | 0.95 | 1.42 | 0.98 | |
| betaine aldehyde | 0.8557 | 0.5481 | 0.8424 | 0.98 | 0.97 | 1.00 | 0.93 | 0.95 | 0.95 | |
| serine | 0.0475 | 0.0730 | 0.0825 | 0.86 | 0.87 | 0.99 | 0.86 | 0.99 | 1.00 | |
| N-acetylserine | 0.6045 | 0.3075 | 0.7645 | 0.99 | 0.97 | 1.02 | 0.94 | 0.97 | 0.95 | |
| threonine | 0.9211 | 0.8148 | 0.2988 | 0.94 | 0.97 | 0.97 | 1.01 | 1.04 | 1.07 | |
| N-acetylthreonine | 0.6312 | 0.7174 | 0.2792 | 0.94 | 0.95 | 0.99 | 0.97 | 1.02 | 1.03 | |
| homoserine | 0.8946 | 0.9145 | 0.0467 | 0.89 | 0.88 | 1.01 | 1.03 | 1.16 | 1.15 | |
| homoserine lactone | 0.3595 | 0.9494 | 0.2773 | 0.85 | 0.94 | 0.90 | 0.92 | 0.98 | 1.09 | |
| Alanine and Aspartate Metabolism | alanine | 0.7514 | 0.2222 | 0.9764 | 0.99 | 1.05 | 0.94 | 1.03 | 0.99 | 1.05 |
| N-acetylalanine | 0.8720 | 0.3951 | 0.7095 | 1.02 | 0.98 | 1.04 | 0.98 | 0.99 | 0.96 | |
| N,N-dimethylalanine | 0.0746 | 0.2257 | 0.5782 | 1.11 | 0.86 | 1.29 | 1.04 | 1.21 | 0.94 | |
| aspartate | 0.0183 | 0.0779 | 0.4362 | 0.87 | 0.90 | 0.97 | 0.84 | 0.93 | 0.96 | |
| N-acetylaspartate (NAA) | 0.7745 | 0.5859 | 0.6148 | 0.95 | 0.76 | 1.26 | 0.80 | 1.06 | 0.84 | |
| asparagine | 0.1065 | 0.4365 | 0.6115 | 0.93 | 0.96 | 0.97 | 0.92 | 0.96 | 0.99 | |
| N-acetylasparagine | 0.0217 | 0.8178 | 0.9221 | 0.82 | 0.97 | 0.84 | 0.82 | 0.85 | 1.00 | |
| hydroxyasparagine ** | 0.9401 | 0.8651 | 0.2157 | 0.96 | 0.96 | 1.00 | 0.99 | 1.03 | 1.03 | |
| Glutamate Metabolism | glutamate | 0.0007 | 0.3580 | 0.9168 | 0.91 | 0.98 | 0.93 | 0.89 | 0.91 | 0.98 |
| glutamine | 0.5533 | 0.6350 | 0.3371 | 1.04 | 1.20 | 0.86 | 1.03 | 0.86 | 0.99 | |
| alpha-ketoglutaramate * | 0.4965 | 0.9267 | 0.1820 | 0.83 | 0.87 | 0.96 | 0.93 | 1.08 | 1.12 | |
| N-acetylglutamate | 0.1907 | 0.7624 | 0.0165 | 0.77 | 0.86 | 0.90 | 0.94 | 1.10 | 1.22 | |
| N-acetylglutamine | 0.6920 | 0.2855 | 0.7559 | 0.99 | 0.97 | 1.02 | 0.93 | 0.95 | 0.94 | |
| 4-hydroxyglutamate | 0.0185 | 0.0414 | 0.6237 | 0.82 | 0.85 | 0.96 | 0.73 | 0.86 | 0.89 | |
| gamma-carboxyglutamate | 0.8963 | 0.0472 | 0.1480 | 0.95 | 0.84 | 1.13 | 0.91 | 1.08 | 0.96 | |
| glutamate, gamma-methyl ester | 0.0925 | 0.5781 | 0.2678 | 0.92 | 0.99 | 0.93 | 0.97 | 0.98 | 1.05 | |
| N-acetyl-aspartyl-glutamate (NAAG) | 0.9706 | 0.3730 | 0.0069 | 1.23 | 1.15 | 1.07 | 0.94 | 0.82 | 0.77 | |
| beta-citrylglutamate | 0.1796 | 0.2694 | 0.1725 | 0.99 | 1.11 | 0.90 | 0.98 | 0.88 | 0.99 | |
| carboxyethyl-GABA | 0.4769 | 0.2633 | 0.0227 | 0.75 | 0.72 | 1.05 | 0.85 | 1.18 | 1.13 | |
| N-methyl-GABA | 0.1183 | 0.0161 | 0.2525 | 1.04 | 0.63 | 1.64 | 0.89 | 1.40 | 0.85 | |
| S-1-pyrroline-5-carboxylate | 0.7169 | 0.2078 | 0.4386 | 1.04 | 0.96 | 1.08 | 0.89 | 0.92 | 0.85 | |
| Histidine Metabolism | histidine | 0.3143 | 0.9044 | 0.3428 | 0.95 | 0.97 | 0.98 | 0.97 | 1.00 | 1.02 |
| 1-methylhistidine | 0.0428 | 0.2295 | 0.9430 | 0.84 | 0.90 | 0.93 | 0.75 | 0.84 | 0.90 | |
| 3-methylhistidine | 0.0945 | 0.1445 | 0.6573 | 0.92 | 0.93 | 0.99 | 0.80 | 0.86 | 0.87 | |
| N-acetylhistidine | 0.9703 | 0.1184 | 0.6513 | 1.05 | 1.16 | 0.90 | 1.13 | 0.97 | 1.08 | |
| 1-carboxyethylhistidine | 0.4200 | 0.9911 | 0.1420 | 0.82 | 0.91 | 0.90 | 0.93 | 1.01 | 1.13 | |
| hydantoin-5-propionate | 0.0193 | 0.5519 | 0.4896 | 1.60 | 1.23 | 1.29 | 1.45 | 1.18 | 0.91 | |
| imidazole propionate | 0.0115 | 0.2605 | 0.6547 | 1.91 | 0.79 | 2.43 | 1.25 | 1.59 | 0.65 | |
| formiminoglutamate | 0.0292 | 0.7594 | 0.3609 | 0.21 | 0.50 | 0.42 | 0.41 | 0.83 | 1.96 | |
| imidazole lactate | 0.4196 | 0.6874 | 0.2216 | 1.85 | 1.52 | 1.22 | 1.28 | 0.84 | 0.69 | |
| carnosine | 0.3240 | 0.5726 | 0.1250 | 0.84 | 0.92 | 0.91 | 0.96 | 1.04 | 1.15 | |
| homocarnosine | 0.8970 | 0.6877 | 0.1204 | 0.91 | 0.94 | 0.97 | 1.04 | 1.11 | 1.14 | |
| N-acetylcarnosine | 0.9427 | 0.9365 | 0.1132 | 0.92 | 0.90 | 1.02 | 0.99 | 1.10 | 1.08 | |
| anserine | 0.2994 | 0.2889 | 0.6144 | 0.92 | 0.92 | 1.01 | 0.90 | 0.98 | 0.97 | |
| histamine | 0.6434 | 0.4986 | 0.2738 | 0.81 | 0.91 | 0.90 | 1.05 | 1.16 | 1.29 | |
| 1-methylhistamine | 0.6493 | 0.4623 | 0.9973 | 1.00 | 0.88 | 1.14 | 0.88 | 1.01 | 0.89 | |
| 1-methyl-4-imidazoleacetate | 0.0684 | 0.6038 | 0.4095 | 0.74 | 0.87 | 0.85 | 0.78 | 0.90 | 1.06 | |
| 1-methyl-5-imidazoleacetate | 0.0128 | 0.2055 | 0.9617 | 0.77 | 0.88 | 0.87 | 0.70 | 0.79 | 0.91 | |
| 1-methyl-5-imidazolelactate | 0.0576 | 0.2149 | 0.5774 | 0.78 | 0.83 | 0.94 | 0.74 | 0.89 | 0.95 | |
| 1-ribosyl-imidazoleacetate * | 0.8999 | 0.7201 | 0.7769 | 1.09 | 1.05 | 1.04 | 1.09 | 1.04 | 1.00 | |
| 4-imidazoleacetate | 0.6833 | 0.7021 | 0.3190 | 1.25 | 1.08 | 1.16 | 1.01 | 0.94 | 0.81 | |
| histidine methyl ester | 0.7766 | 0.0492 | 0.7283 | 0.96 | 0.87 | 1.11 | 0.86 | 0.99 | 0.89 | |
| Lysine Metabolism | lysine | 0.2567 | 0.6274 | 0.4615 | 0.95 | 0.97 | 0.98 | 0.96 | 0.99 | 1.01 |
| N2-acetyllysine | 0.0627 | 0.3400 | 0.7550 | 1.11 | 1.06 | 1.04 | 1.25 | 1.18 | 1.13 | |
| N6-acetyllysine | 0.8815 | 0.1030 | 0.4186 | 0.97 | 1.04 | 0.93 | 1.06 | 1.02 | 1.10 | |
| N6-methyllysine | 0.2053 | 0.0056 | 0.1704 | 1.46 | 0.87 | 1.68 | 0.82 | 0.94 | 0.56 | |
| N6,N6-dimethyllysine | 0.8597 | 0.0295 | 0.9459 | 1.00 | 0.90 | 1.11 | 0.89 | 0.99 | 0.89 | |
| N6,N6,N6-trimethyllysine | 0.8456 | 0.0704 | 0.7623 | 0.99 | 0.95 | 1.05 | 0.95 | 1.01 | 0.96 | |
| hydroxy-N6,N6,N6-trimethyllysine * | 0.2745 | 0.5473 | 0.3013 | 0.99 | 0.87 | 1.14 | 1.04 | 1.20 | 1.05 | |
| 5-hydroxylysine | 0.1056 | 0.2497 | 0.6162 | 1.08 | 1.07 | 1.02 | 1.11 | 1.04 | 1.03 | |
| 5-(galactosylhydroxy)-l-lysine | 0.6524 | 0.3259 | 0.9437 | 0.98 | 0.95 | 1.03 | 0.93 | 0.98 | 0.95 | |
| fructosyllysine | 0.2990 | 0.6047 | 0.3281 | 0.98 | 0.89 | 1.10 | 1.01 | 1.13 | 1.03 | |
| saccharopine | 0.0063 | 0.2847 | 0.1755 | 0.75 | 1.28 | 0.58 | 0.72 | 0.56 | 0.97 | |
| 2-aminoadipate | 0.0893 | 0.3553 | 0.2686 | 0.45 | 0.51 | 0.88 | 0.47 | 0.93 | 1.05 | |
| 2-oxoadipate | 0.0690 | 0.6160 | 0.2133 | 0.61 | 0.70 | 0.86 | 0.62 | 0.88 | 1.02 | |
| glutarylcarnitine (C5-DC) | 0.0868 | 0.5505 | 0.8451 | 0.74 | 1.15 | 0.64 | 0.91 | 0.79 | 1.24 | |
| pipecolate | 0.0271 | 0.7271 | 0.5053 | 0.79 | 0.90 | 0.88 | 0.81 | 0.91 | 1.03 | |
| 6-oxopiperidine-2-carboxylate | 0.9827 | 0.0855 | 0.0779 | 0.81 | 0.97 | 0.84 | 1.32 | 1.37 | 1.62 | |
| 5-aminovalerate | 0.1118 | 0.6292 | 0.5313 | 1.10 | 1.03 | 1.07 | 1.22 | 1.19 | 1.12 | |
| N,N,N-trimethyl-5-aminovalerate | 0.1651 | 0.0204 | 0.5350 | 1.22 | 0.64 | 1.90 | 0.85 | 1.32 | 0.69 | |
| Pathway | Metabolite | Two-Way ANOVA Main Effects | Two-Way ANOVA Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Vitamin | Gain:Vitamin | NOVTM-MG | VTM-LG | NOVTM-MG | VTM-MG | VTM-MG | VTM-MG | ||
| NOVTM-LG | NOVTM-LG | VTM-LG | NOVTM-LG | VTM-LG | NOVTM-MG | |||||
| Phenylalanine Metabolism | phenylalanine | 0.5262 | 0.1995 | 0.3534 | 0.97 | 1.03 | 0.95 | 1.13 | 1.09 | 1.16 |
| N-acetylphenylalanine | 0.1710 | 0.8251 | 0.9814 | 0.93 | 1.01 | 0.92 | 0.95 | 0.94 | 1.02 | |
| 1-carboxyethylphenylalanine | 0.9206 | 0.5831 | 0.2268 | 0.90 | 0.99 | 0.91 | 1.06 | 1.07 | 1.18 | |
| phenyllactate (PLA) | 0.1825 | 0.7811 | 0.3047 | 1.00 | 0.93 | 1.08 | 1.12 | 1.20 | 1.12 | |
| Tyrosine Metabolism | tyrosine | 0.2839 | 0.1136 | 0.5203 | 0.90 | 0.87 | 1.04 | 0.85 | 0.98 | 0.94 |
| N-acetyltyrosine | 0.1829 | 0.3086 | 0.7077 | 0.95 | 1.10 | 0.86 | 0.99 | 0.90 | 1.04 | |
| 1-carboxyethyltyrosine | 0.3515 | 0.6551 | 0.2005 | 0.82 | 0.87 | 0.94 | 0.88 | 1.02 | 1.08 | |
| 4-hydroxyphenylpyruvate | 0.6571 | 0.7175 | 0.4672 | 0.91 | 0.95 | 0.96 | 0.93 | 0.99 | 1.03 | |
| 3-(4-hydroxyphenyl)lactate | 0.1194 | 0.7794 | 0.1299 | 1.01 | 0.94 | 1.08 | 1.14 | 1.21 | 1.12 | |
| phenol sulfate | 0.7156 | 0.6369 | 0.9161 | 1.01 | 1.16 | 0.87 | 1.09 | 0.94 | 1.07 | |
| 4-methoxyphenol sulfate | 0.4551 | 0.6799 | 0.2942 | 1.15 | 0.95 | 1.21 | 0.35 | 0.37 | 0.31 | |
| vanillactate | 0.2774 | 0.5352 | 0.5575 | 0.92 | 1.15 | 0.80 | 0.95 | 0.82 | 1.02 | |
| 3-methoxytyrosine | 0.0112 | 0.7596 | 0.2593 | 0.85 | 1.17 | 0.73 | 0.80 | 0.69 | 0.94 | |
| O-methyltyrosine | 0.6826 | 0.6289 | 0.0352 | 0.93 | 0.90 | 1.03 | 0.99 | 1.10 | 1.07 | |
| dopamine 4-sulfate | 0.7152 | 0.2172 | 0.7477 | 0.98 | 0.94 | 1.05 | 0.90 | 0.96 | 0.92 | |
| N-formylphenylalanine | 0.2733 | 0.7616 | 0.8693 | 0.77 | 1.03 | 0.75 | 0.92 | 0.89 | 1.20 | |
| Tryptophan Metabolism | tryptophan | 0.3681 | 0.9275 | 0.1313 | 0.97 | 0.95 | 1.02 | 1.05 | 1.11 | 1.08 |
| N-acetyltryptophan | 0.1276 | 0.7993 | 0.8266 | 0.89 | 1.06 | 0.85 | 0.91 | 0.86 | 1.02 | |
| C-glycosyltryptophan | 0.4762 | 0.4647 | 0.7849 | 1.02 | 1.02 | 1.00 | 1.06 | 1.04 | 1.04 | |
| oxindolylalanine | 0.9601 | 0.2572 | 0.2679 | 0.94 | 0.89 | 1.07 | 0.96 | 1.09 | 1.02 | |
| kynurenine | 0.6684 | 0.6015 | 0.4260 | 0.88 | 0.79 | 1.12 | 0.89 | 1.13 | 1.01 | |
| kynurenate | 0.6685 | 0.3135 | 0.8373 | 0.96 | 0.95 | 1.01 | 0.83 | 0.87 | 0.86 | |
| N-formylanthranilic acid | 0.7313 | 0.4260 | 0.7723 | 1.00 | 0.96 | 1.04 | 0.88 | 0.91 | 0.87 | |
| picolinate | 0.3397 | 0.1535 | 0.8080 | 0.88 | 0.78 | 1.13 | 0.61 | 0.78 | 0.69 | |
| serotonin | 0.3438 | 0.4071 | 0.5512 | 1.01 | 1.00 | 1.00 | 1.21 | 1.21 | 1.21 | |
| indolelactate | 0.0007 | 0.8106 | 0.1900 | 1.20 | 0.93 | 1.29 | 1.33 | 1.43 | 1.11 | |
| indoleacetylglycine | 0.4239 | 0.0513 | 0.8167 | 0.95 | 0.83 | 1.15 | 0.74 | 0.90 | 0.78 | |
| 3-hydroxy-2-ethylpropionate | 0.5943 | 0.0291 | 0.3183 | 0.88 | 0.74 | 1.19 | 0.77 | 1.04 | 0.87 | |
| Leucine, Isoleucine and Valine Metabolism | leucine | 0.0750 | 0.9078 | 0.1090 | 0.90 | 0.95 | 0.95 | 0.94 | 0.99 | 1.05 |
| N-acetylleucine | 0.6266 | 0.1516 | 0.5715 | 0.87 | 0.82 | 1.06 | 0.81 | 0.99 | 0.93 | |
| 1-carboxyethylleucine | 0.0289 | 0.8616 | 0.3462 | 0.79 | 0.94 | 0.84 | 0.84 | 0.89 | 1.06 | |
| alpha-hydroxyisocaproate | 0.6909 | 0.8436 | 0.3239 | 0.85 | 0.91 | 0.94 | 0.96 | 1.06 | 1.12 | |
| isovalerylglycine | 0.6364 | 0.1351 | 0.5646 | 0.94 | 0.79 | 1.18 | 0.82 | 1.04 | 0.87 | |
| beta-hydroxyisovalerate | 0.1620 | 0.6902 | 0.9776 | 1.22 | 1.06 | 1.16 | 1.27 | 1.20 | 1.04 | |
| beta-hydroxyisovaleroylcarnitine | 0.5844 | 0.8602 | 0.2612 | 0.96 | 0.92 | 1.05 | 1.03 | 1.12 | 1.07 | |
| 3-methylglutaconate | 0.0897 | 0.6683 | 0.2842 | 0.65 | 0.90 | 0.73 | 0.78 | 0.87 | 1.19 | |
| isoleucine | 0.1041 | 0.8273 | 0.2485 | 0.93 | 0.97 | 0.96 | 0.95 | 0.99 | 1.03 | |
| N-acetylisoleucine | 0.0883 | 0.2759 | 0.0122 | 1.10 | 1.15 | 0.96 | 0.72 | 0.62 | 0.65 | |
| 1-carboxyethylisoleucine | 0.3750 | 0.9650 | 0.1246 | 0.82 | 0.91 | 0.90 | 0.92 | 1.01 | 1.12 | |
| 2-hydroxy-3-methylvalerate | 0.5237 | 0.8014 | 0.4875 | 0.76 | 0.80 | 0.95 | 0.81 | 1.01 | 1.06 | |
| 2-methylbutyrylcarnitine (C5) | 0.3794 | 0.1415 | 0.0255 | 0.77 | 0.74 | 1.04 | 0.85 | 1.16 | 1.11 | |
| 2-methylbutyrylglycine | 0.5173 | 0.4655 | 0.2779 | 0.97 | 0.84 | 1.15 | 0.99 | 1.18 | 1.03 | |
| tiglylcarnitine (C5:1-DC) | 0.7393 | 0.3314 | 0.0011 | 0.77 | 0.70 | 1.10 | 0.99 | 1.40 | 1.28 | |
| 3-hydroxy-2-ethylpropionate | 0.7844 | 0.1849 | 0.1361 | 0.92 | 0.84 | 1.10 | 0.93 | 1.11 | 1.01 | |
| butyryl/isobutyryl CoA | 0.9978 | 0.8187 | 0.5227 | 0.87 | 0.86 | 1.00 | 0.96 | 1.11 | 1.11 | |
| ethylmalonate | 0.0175 | 0.6535 | 0.7402 | 0.88 | 1.07 | 0.82 | 0.88 | 0.82 | 1.01 | |
| methylsuccinate | 0.6889 | 0.0841 | 0.6010 | 0.91 | 0.79 | 1.15 | 0.78 | 0.98 | 0.86 | |
| valine | 0.0089 | 0.3786 | 0.2571 | 0.88 | 0.99 | 0.89 | 0.94 | 0.95 | 1.07 | |
| N-acetylvaline | 0.8181 | 0.7265 | 0.1477 | 0.90 | 0.87 | 1.04 | 0.96 | 1.11 | 1.07 | |
| 1-carboxyethylvaline | 0.0769 | 0.8592 | 0.2042 | 0.81 | 0.92 | 0.88 | 0.87 | 0.95 | 1.08 | |
| 3-methyl-2-oxobutyrate | 0.1586 | 0.9675 | 0.8355 | 0.83 | 0.98 | 0.84 | 0.84 | 0.86 | 1.02 | |
| alpha-hydroxyisovalerate | 0.0277 | 0.3426 | 0.8951 | 0.86 | 0.94 | 0.91 | 0.81 | 0.87 | 0.95 | |
| isobutyrylcarnitine (C4) | 0.5091 | 0.5229 | 0.9301 | 0.89 | 0.90 | 0.99 | 0.87 | 0.96 | 0.97 | |
| isobutyrylglycine | 0.0777 | 0.1447 | 0.3581 | 0.91 | 0.94 | 0.98 | 0.72 | 0.77 | 0.78 | |
| 3-hydroxyisobutyrate | 0.3841 | 0.8606 | 0.2047 | 0.86 | 0.91 | 0.95 | 0.93 | 1.02 | 1.08 | |
| Methionine, Cysteine, SAM and Taurine Metabolism | methionine | 0.8320 | 0.9846 | 0.7597 | 0.97 | 0.98 | 0.99 | 0.99 | 1.01 | 1.02 |
| N-acetylmethionine | 0.7988 | 0.6990 | 0.3953 | 0.92 | 0.91 | 1.02 | 0.94 | 1.03 | 1.01 | |
| N-formylmethionine | 0.8162 | 0.6270 | 0.7966 | 1.02 | 1.00 | 1.02 | 0.94 | 0.94 | 0.91 | |
| S-methylmethionine | 0.2451 | 0.0804 | 0.2018 | 0.99 | 0.94 | 1.05 | 0.79 | 0.83 | 0.80 | |
| methionine sulfone | 0.0048 | 0.0341 | 0.2338 | 0.85 | 0.89 | 0.96 | 0.62 | 0.69 | 0.72 | |
| methionine sulfoxide | 0.9801 | 0.5705 | 0.9165 | 1.01 | 1.05 | 0.96 | 1.05 | 1.00 | 1.04 | |
| N-acetylmethionine sulfoxide | 0.8310 | 0.9902 | 0.7681 | 1.07 | 1.07 | 1.01 | 1.05 | 0.98 | 0.97 | |
| S-adenosylmethionine (SAM) | 0.5308 | 0.3777 | 0.4083 | 0.91 | 1.02 | 0.89 | 1.00 | 0.99 | 1.11 | |
| S-adenosylhomocysteine (SAH) | 0.3151 | 0.2339 | 0.2600 | 0.99 | 1.01 | 0.98 | 1.11 | 1.09 | 1.12 | |
| 5-methylthioribose ** | 0.4375 | 0.5182 | 0.8871 | 1.04 | 0.98 | 1.06 | 1.01 | 1.03 | 0.97 | |
| 2,3-dihydroxy-5-methylthio-4-pentenoate | 0.9072 | 0.0509 | 0.3284 | 0.97 | 1.03 | 0.94 | 1.07 | 1.04 | 1.11 | |
| 2-hydroxy-4-(methylthio)butanoic | 0.2529 | 0.6619 | 0.6947 | 1.44 | 1.21 | 1.19 | 1.50 | 1.24 | 1.04 | |
| homocysteine | 0.2852 | 0.2269 | 0.9529 | 0.92 | 1.13 | 0.81 | 0.99 | 0.88 | 1.08 | |
| cystathionine | 0.3153 | 0.4010 | 0.9092 | 0.94 | 1.05 | 0.89 | 0.99 | 0.94 | 1.05 | |
| cysteine | 0.8144 | 0.5822 | 0.5550 | 1.05 | 1.13 | 0.93 | 1.04 | 0.92 | 0.99 | |
| N-acetylcysteine | 0.3834 | 0.3336 | 0.3481 | 0.98 | 1.17 | 0.83 | 0.98 | 0.83 | 1.00 | |
| S-methylcysteine | 0.3039 | 0.6297 | 0.3937 | 1.05 | 0.97 | 1.09 | 1.49 | 1.54 | 1.41 | |
| S-methylcysteine sulfoxide | 0.0657 | 0.7767 | 0.0704 | 1.01 | 0.84 | 1.19 | 1.12 | 1.33 | 1.11 | |
| cysteine s-sulfate | 0.6742 | 0.9318 | 0.9945 | 1.05 | 1.02 | 1.03 | 1.10 | 1.08 | 1.04 | |
| cystine | 0.9628 | 0.5386 | 0.8525 | 0.83 | 0.80 | 1.04 | 0.88 | 1.11 | 1.06 | |
| cysteine sulfinic acid | 0.9516 | 0.4598 | 0.7822 | 0.98 | 1.14 | 0.86 | 1.05 | 0.92 | 1.07 | |
| hypotaurine | 0.0125 | 0.0937 | 0.3937 | 1.19 | 1.09 | 1.09 | 1.53 | 1.41 | 1.29 | |
| taurine | 0.0925 | 0.5849 | 0.0396 | 0.98 | 0.85 | 1.16 | 1.08 | 1.27 | 1.10 | |
| N-acetyltaurine | 0.8753 | 0.3345 | 0.0888 | 0.88 | 0.78 | 1.12 | 0.93 | 1.19 | 1.06 | |
| succinoyltaurine | 0.0619 | 0.6499 | 0.3868 | 1.09 | 0.84 | 1.29 | 1.19 | 1.41 | 1.09 | |
| taurocyamine | 0.2638 | 0.1720 | 0.2067 | 1.66 | 1.01 | 1.64 | 1.08 | 1.07 | 0.65 | |
| 3-sulfo-l-alanine | 0.7564 | 0.4612 | 0.2764 | 1.16 | 1.17 | 0.99 | 1.07 | 0.91 | 0.92 | |
| Pathway | Metabolite | Two-Way ANOVA Main Effects | Two-Way ANOVA Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Vitamin | Gain:Vitamin | NOVTM-MG | VTM-LG | NOVTM-MG | VTM-MG | VTM-MG | VTM-MG | ||
| NOVTM-LG | NOVTM-LG | VTM-LG | NOVTM-LG | VTM-LG | NOVTM-MG | |||||
| Urea cycle; Arginine and Proline Metabolism | arginine | 0.8478 | 0.1751 | 0.4076 | 1.05 | 0.97 | 1.08 | 0.95 | 0.98 | 0.91 |
| argininosuccinate | 0.3443 | 0.1471 | 0.6434 | 0.98 | 0.93 | 1.06 | 0.84 | 0.90 | 0.85 | |
| urea | 0.0540 | 0.8952 | 0.2783 | 1.17 | 1.06 | 1.10 | 1.11 | 1.05 | 0.95 | |
| ornithine | 0.9752 | 0.0671 | 0.9283 | 1.01 | 0.93 | 1.08 | 0.93 | 1.00 | 0.92 | |
| 3-amino-2-piperidone | 0.7296 | 0.3557 | 0.5588 | 1.01 | 0.99 | 1.02 | 0.95 | 0.96 | 0.94 | |
| 2-oxoarginine * | 0.6161 | 0.7755 | 0.2100 | 0.83 | 0.84 | 0.98 | 1.00 | 1.18 | 1.21 | |
| citrulline | 0.1861 | 0.3133 | 0.0377 | 0.74 | 0.77 | 0.97 | 0.84 | 1.10 | 1.13 | |
| homoarginine | 0.3467 | 0.9645 | 0.0129 | 1.15 | 1.25 | 0.92 | 0.94 | 0.75 | 0.82 | |
| homocitrulline | 0.0038 | 0.9535 | 0.6506 | 1.29 | 0.94 | 1.38 | 1.39 | 1.49 | 1.08 | |
| proline | 0.4551 | 0.9809 | 0.2805 | 0.99 | 0.97 | 1.02 | 1.03 | 1.06 | 1.04 | |
| dimethylarginine (SDMA + ADMA) | 0.8330 | 0.6314 | 0.4786 | 1.05 | 1.01 | 1.03 | 0.98 | 0.97 | 0.94 | |
| N-acetylarginine | 0.2266 | 0.1772 | 0.5058 | 0.92 | 1.03 | 0.89 | 1.01 | 0.98 | 1.09 | |
| N-delta-acetylornithine | 0.1555 | 0.6141 | 0.6692 | 1.04 | 0.80 | 1.30 | 1.03 | 1.28 | 0.98 | |
| trans-4-hydroxyproline | 0.5460 | 0.3362 | 0.0697 | 1.04 | 1.08 | 0.96 | 1.01 | 0.94 | 0.98 | |
| pro-hydroxy-pro | 0.9930 | 0.8139 | 0.0483 | 0.91 | 0.92 | 0.98 | 1.01 | 1.10 | 1.11 | |
| N-methylproline | 0.0091 | 0.8882 | 0.2628 | 2.39 | 1.20 | 2.00 | 1.54 | 1.28 | 0.64 | |
| N,N,N-trimethyl-alanylproline betaine (TMAP) | 0.1842 | 0.3672 | 0.0642 | 0.79 | 0.93 | 0.85 | 0.97 | 1.04 | 1.23 | |
| argininate * | 0.6041 | 0.2495 | 0.0575 | 0.73 | 0.66 | 1.10 | 0.84 | 1.27 | 1.15 | |
| dimethylguanidino valeric acid (DMGV) * | 0.9002 | 0.9542 | 0.6950 | 0.91 | 0.96 | 0.95 | 0.99 | 1.03 | 1.08 | |
| Creatine Metabolism | guanidinoacetate | 0.2718 | 0.1371 | 0.9793 | 1.09 | 0.91 | 1.20 | 0.97 | 1.06 | 0.88 |
| creatine | 0.3056 | 0.3923 | 0.3726 | 0.92 | 0.93 | 0.99 | 0.92 | 1.00 | 1.00 | |
| creatinine | 0.0263 | 0.3155 | 0.1463 | 0.81 | 0.87 | 0.93 | 0.84 | 0.96 | 1.04 | |
| creatine phosphate | 0.4778 | 0.3963 | 0.1362 | 0.82 | 0.97 | 0.85 | 1.03 | 1.07 | 1.26 | |
| Polyamine Metabolism | putrescine | 0.5608 | 0.8046 | 0.7262 | 1.00 | 1.00 | 1.00 | 1.04 | 1.04 | 1.04 |
| N-acetylputrescine | 0.4842 | 0.0708 | 0.4958 | 1.00 | 1.07 | 0.93 | 1.14 | 1.07 | 1.15 | |
| N-acetyl-isoputreanine | 0.6131 | 0.6805 | 0.4274 | 1.16 | 1.04 | 1.12 | 1.05 | 1.01 | 0.90 | |
| spermidine | 0.8145 | 0.2942 | 0.8581 | 0.97 | 0.89 | 1.08 | 0.88 | 0.99 | 0.91 | |
| N(1′)-acetylspermidine | 0.4290 | 0.4354 | 0.8203 | 1.24 | 0.95 | 1.30 | 1.00 | 1.05 | 0.81 | |
| spermine | 0.1310 | 0.7210 | 0.9401 | 0.89 | 0.97 | 0.92 | 0.85 | 0.88 | 0.96 | |
| N1,N12-diacetylspermine | 0.2559 | 0.1314 | 0.6602 | 1.23 | 0.52 | 2.38 | 0.72 | 1.39 | 0.58 | |
| 5-methylthioadenosine (MTA) | 0.8384 | 0.7439 | 0.4964 | 0.95 | 1.00 | 0.95 | 1.00 | 1.00 | 1.05 | |
| 4-acetamidobutanoate | 0.4564 | 0.4522 | 0.8593 | 0.95 | 0.97 | 0.98 | 0.90 | 0.93 | 0.94 | |
| Guanidino and Acetamido Metabolism | 4-guanidinobutanoate | 0.6857 | 0.4207 | 0.0671 | 1.86 | 1.05 | 1.78 | 0.82 | 0.79 | 0.44 |
| guanidinosuccinate | 0.2206 | 0.1726 | 0.8548 | 0.65 | 0.74 | 0.89 | 0.53 | 0.72 | 0.82 | |
| Glutathione Metabolism | glutathione, reduced (GSH) | 0.9470 | 0.5327 | 0.8642 | 0.98 | 1.08 | 0.91 | 1.07 | 0.99 | 1.09 |
| glutathione, oxidized (GSSG) | 0.6325 | 0.1749 | 0.7609 | 1.02 | 1.07 | 0.95 | 1.13 | 1.06 | 1.11 | |
| cysteine-glutathione disulfide | 0.9078 | 0.8347 | 0.5081 | 0.93 | 0.91 | 1.03 | 0.99 | 1.09 | 1.06 | |
| S-methylglutathione | 0.9103 | 0.4645 | 0.5765 | 0.97 | 1.02 | 0.95 | 1.04 | 1.02 | 1.07 | |
| S-lactoylglutathione | 0.3135 | 0.5594 | 0.8245 | 0.82 | 0.87 | 0.94 | 0.74 | 0.86 | 0.91 | |
| cysteinylglycine | 0.7261 | 0.9655 | 0.7495 | 0.96 | 0.99 | 0.96 | 0.87 | 0.87 | 0.91 | |
| cysteinylglycine disulfide * | 0.8032 | 0.2323 | 0.5772 | 0.85 | 0.64 | 1.32 | 0.87 | 1.36 | 1.03 | |
| cys-gly, oxidized | 0.9903 | 0.5298 | 0.4338 | 0.89 | 0.80 | 1.11 | 0.95 | 1.19 | 1.07 | |
| 5-oxoproline | 0.0403 | 0.3065 | 0.2612 | 0.89 | 1.01 | 0.88 | 0.73 | 0.73 | 0.82 | |
| 2-hydroxybutyrate/2-hydroxyisobutyrate | 0.2133 | 0.4544 | 0.2458 | 0.88 | 0.90 | 0.98 | 0.90 | 1.01 | 1.03 | |
| ophthalmate | 0.0062 | 0.9343 | 0.8049 | 0.75 | 1.07 | 0.70 | 0.77 | 0.72 | 1.02 | |
| S-(1,2-dicarboxyethyl)glutathione | 0.5503 | 0.5480 | 0.9660 | 0.96 | 0.98 | 0.98 | 0.92 | 0.95 | 0.96 | |
| 4-hydroxy-nonenal-glutathione | 0.2902 | 0.5776 | 0.2652 | 1.02 | 1.27 | 0.81 | 0.93 | 0.73 | 0.91 | |
| 3′-dephospho-CoA-glutathione * | 0.4941 | 0.9283 | 0.6141 | 1.00 | 0.93 | 1.07 | 1.02 | 1.09 | 1.02 | |
| CoA-glutathione * | 0.1656 | 0.5005 | 0.3299 | 1.07 | 0.99 | 1.08 | 1.21 | 1.22 | 1.13 | |
| Pathway | Metabolite | Two-Way ANOVA Main Effects | Two-Way ANOVA Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Vitamin | Gain:Vitamin | NOVTM-MG | VTM-LG | NOVTM-MG | VTM-MG | VTM-MG | VTM-MG | ||
| NOVTM-LG | NOVTM-LG | VTM-LG | NOVTM-LG | VTM-LG | NOVTM-MG | |||||
| Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | 1,5-anhydroglucitol (1,5-AG) | 0.0585 | 0.6067 | 0.3522 | 1.21 | 0.96 | 1.27 | 1.40 | 1.46 | 1.15 |
| glucose | 0.3648 | 0.9280 | 0.6851 | 0.97 | 0.89 | 1.08 | 1.02 | 1.14 | 1.05 | |
| glucose 6-phosphate | 0.5025 | 0.7956 | 0.0634 | 1.11 | 1.26 | 0.88 | 0.98 | 0.77 | 0.88 | |
| glucose 1-phosphate | 0.9300 | 0.0131 | 0.5007 | 1.06 | 1.49 | 0.71 | 1.40 | 0.94 | 1.32 | |
| fructose-6-phosphate | 0.1441 | 0.5077 | 0.1772 | 0.91 | 1.13 | 0.81 | 0.71 | 0.63 | 0.78 | |
| fructose 1,6-diphosphate/glucose 1,6-diphosphate/myo-inositol diphosphates | 0.0776 | 0.1107 | 0.7328 | 0.75 | 0.82 | 0.91 | 0.58 | 0.70 | 0.77 | |
| 2,3-diphosphoglycerate | 0.0880 | 0.2964 | 0.3735 | 0.78 | 0.86 | 0.91 | 0.78 | 0.91 | 1.00 | |
| dihydroxyacetone phosphate (DHAP) | 0.0805 | 0.1028 | 0.9521 | 0.78 | 0.81 | 0.97 | 0.61 | 0.75 | 0.77 | |
| 2-phosphoglycerate | 0.9482 | 0.6197 | 0.3701 | 1.03 | 1.02 | 1.01 | 0.98 | 0.96 | 0.95 | |
| 3-phosphoglycerate | 0.7457 | 0.3718 | 0.6649 | 1.00 | 1.00 | 1.01 | 0.92 | 0.93 | 0.92 | |
| phosphoenolpyruvate (PEP) | 0.7712 | 0.3166 | 0.7888 | 0.99 | 1.00 | 0.99 | 0.89 | 0.90 | 0.90 | |
| pyruvate | 0.4192 | 0.4839 | 0.5195 | 0.74 | 0.84 | 0.89 | 0.79 | 0.94 | 1.06 | |
| lactate | 0.7617 | 0.6477 | 0.2892 | 0.95 | 0.98 | 0.97 | 1.01 | 1.03 | 1.05 | |
| glycerate | 0.1138 | 0.6427 | 0.6373 | 0.95 | 1.04 | 0.91 | 0.95 | 0.91 | 1.00 | |
| Pentose Phosphate Pathway | 6-phosphogluconate | 0.3169 | 0.3609 | 0.5766 | 1.15 | 1.16 | 0.99 | 1.22 | 1.05 | 1.06 |
| ribulose/xylulose 5-phosphate | 0.8198 | 0.1524 | 0.4411 | 1.55 | 2.55 | 0.61 | 1.69 | 0.66 | 1.09 | |
| ribose 5-phosphate | 0.9493 | 0.9017 | 0.1523 | 1.12 | 1.17 | 0.96 | 0.99 | 0.84 | 0.88 | |
| ribose 1-phosphate | 0.8756 | 0.8145 | 0.7914 | 1.02 | 1.03 | 0.99 | 0.97 | 0.94 | 0.95 | |
| sedoheptulose-7-phosphate | 0.9215 | 0.0933 | 0.3919 | 1.12 | 1.41 | 0.80 | 1.30 | 0.93 | 1.16 | |
| Pentose Metabolism | ribose | 0.3843 | 0.1546 | 0.7655 | 0.97 | 0.86 | 1.12 | 0.70 | 0.81 | 0.72 |
| ribitol | 0.5039 | 0.5481 | 0.6527 | 0.95 | 0.95 | 1.00 | 0.94 | 0.99 | 0.99 | |
| ribonate | 0.3689 | 0.4237 | 0.3819 | 0.99 | 1.00 | 0.99 | 0.91 | 0.91 | 0.92 | |
| ribulose/xylulose | 0.2077 | 0.4605 | 0.7281 | 0.95 | 0.97 | 0.98 | 0.81 | 0.84 | 0.86 | |
| arabitol/xylitol | 0.3537 | 0.3204 | 0.8100 | 1.07 | 0.93 | 1.15 | 0.99 | 1.07 | 0.93 | |
| arabonate/xylonate | 0.2243 | 0.1567 | 0.8196 | 0.93 | 0.92 | 1.02 | 0.84 | 0.91 | 0.89 | |
| sedoheptulose | 0.0620 | 0.1501 | 0.3486 | 0.94 | 1.25 | 0.75 | 0.97 | 0.78 | 1.04 | |
| lyxonate | 0.8156 | 0.6675 | 0.7056 | 0.98 | 0.92 | 1.07 | 0.98 | 1.06 | 0.99 | |
| Glycogen Metabolism | maltotetraose | 0.8424 | 0.8885 | 0.2153 | 1.35 | 1.54 | 0.88 | 1.05 | 0.68 | 0.78 |
| maltotriose | 0.9137 | 0.7785 | 0.2539 | 1.32 | 1.47 | 0.90 | 1.07 | 0.73 | 0.81 | |
| maltose | 0.5077 | 0.9582 | 0.6315 | 1.27 | 1.08 | 1.17 | 1.13 | 1.05 | 0.90 | |
| Fructose, Mannose and Galactose Metabolism | fructose | 0.3265 | 0.1126 | 0.3292 | 1.00 | 1.03 | 0.97 | 1.14 | 1.11 | 1.14 |
| mannitol/sorbitol | 0.1781 | 0.6695 | 0.0241 | 0.96 | 0.90 | 1.07 | 1.04 | 1.15 | 1.08 | |
| mannose | 0.4865 | 0.7626 | 0.8222 | 0.97 | 1.05 | 0.92 | 0.93 | 0.89 | 0.96 | |
| mannose-6-phosphate | 0.3729 | 0.6587 | 0.1689 | 1.00 | 1.18 | 0.85 | 0.96 | 0.81 | 0.95 | |
| galactose 1-phosphate | 0.6926 | 0.2442 | 0.2481 | 1.14 | 1.23 | 0.92 | 1.15 | 0.93 | 1.01 | |
| 2-ketogluconate | 0.5514 | 0.0969 | 0.3804 | 1.01 | 0.96 | 1.05 | 0.82 | 0.85 | 0.82 | |
| galactonate | 0.0934 | 0.0538 | 0.2374 | 0.65 | 0.64 | 1.02 | 0.62 | 0.98 | 0.96 | |
| Nucleotide Sugar | UDP-glucose | 0.5041 | 0.7344 | 0.7394 | 1.20 | 1.07 | 1.12 | 1.11 | 1.04 | 0.93 |
| UDP-galactose | 0.7730 | 0.2830 | 0.6958 | 1.13 | 0.94 | 1.20 | 0.85 | 0.90 | 0.75 | |
| UDP-glucuronate | 0.9565 | 0.3460 | 0.3111 | 0.91 | 0.99 | 0.92 | 1.08 | 1.09 | 1.19 | |
| UDP-N-acetylglucosamine/galactosamine | 0.9726 | 0.2023 | 0.9219 | 0.94 | 1.13 | 0.83 | 1.15 | 1.02 | 1.23 | |
| cytidine 5′-monophospho-N-acetylneuraminic acid | 0.6326 | 0.5254 | 0.5279 | 1.02 | 1.26 | 0.81 | 1.00 | 0.79 | 0.98 | |
| Aminosugar Metabolism | glucosamine-6-phosphate | 0.4187 | 0.7581 | 0.1837 | 1.00 | 1.17 | 0.86 | 0.94 | 0.80 | 0.94 |
| glucuronate | 0.3349 | 0.7981 | 0.8994 | 1.22 | 0.99 | 1.23 | 1.10 | 1.11 | 0.90 | |
| N-acetylglucosamine 6-phosphate | 0.4180 | 0.3776 | 0.9366 | 0.89 | 0.92 | 0.97 | 0.83 | 0.90 | 0.93 | |
| N-acetyl-glucosamine 1-phosphate | 0.6283 | 0.7579 | 0.6548 | 1.08 | 1.11 | 0.97 | 0.95 | 0.86 | 0.88 | |
| N-acetylneuraminate | 0.4911 | 0.8673 | 0.4253 | 1.02 | 1.04 | 0.98 | 0.94 | 0.90 | 0.92 | |
| N-acetylglucosaminylasparagine | 0.5502 | 0.4829 | 0.5675 | 1.03 | 1.00 | 1.03 | 1.00 | 1.00 | 0.97 | |
| erythronate * | 0.4003 | 0.9382 | 0.2993 | 0.91 | 0.95 | 0.97 | 0.95 | 1.01 | 1.04 | |
| N-acetylglucosamine/N-acetylgalactosamine | 0.2608 | 0.0385 | 0.4075 | 0.95 | 0.83 | 1.15 | 0.64 | 0.77 | 0.67 | |
| N-glycolylneuraminate | 0.2983 | 0.9645 | 0.8015 | 0.93 | 0.99 | 0.94 | 0.92 | 0.94 | 0.99 | |
| Advanced Glycation End-product | N6-carboxymethyllysine | 0.0866 | 0.7937 | 0.6084 | 0.91 | 1.02 | 0.89 | 0.89 | 0.87 | 0.98 |
| Pathway | Metabolite | Two-Way ANOVA Main Effects | Two-Way ANOVA Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Vitamin | Gain:Vitamin | NOVTM-MG | VTM-LG | NOVTM-MG | VTM-MG | VTM-MG | VTM-MG | ||
| NOVTM-LG | NOVTM-LG | VTM-LG | NOVTM-LG | VTM-LG | NOVTM-MG | |||||
| TCA Cycle | citrate | 0.3331 | 0.8607 | 0.3612 | 0.96 | 1.17 | 0.82 | 0.81 | 0.69 | 0.85 |
| aconitate [cis or trans] | 0.1831 | 0.4470 | 0.7333 | 0.87 | 1.30 | 0.67 | 0.80 | 0.61 | 0.92 | |
| alpha-ketoglutarate | 0.4885 | 0.7102 | 0.8549 | 0.91 | 0.99 | 0.92 | 0.94 | 0.95 | 1.04 | |
| succinylcarnitine (C4-DC) | 0.6616 | 0.2368 | 0.1037 | 0.90 | 0.81 | 1.12 | 0.92 | 1.15 | 1.03 | |
| succinate | 0.3246 | 0.9539 | 0.1196 | 0.90 | 0.88 | 1.02 | 1.24 | 1.41 | 1.38 | |
| fumarate | 0.1093 | 0.0759 | 0.6135 | 0.96 | 0.96 | 1.00 | 0.89 | 0.93 | 0.93 | |
| malate | 0.7981 | 0.4002 | 0.4979 | 1.07 | 1.00 | 1.07 | 0.95 | 0.94 | 0.88 | |
| tricarballylate | 0.0218 | 0.0007 | 0.5281 | 1.71 | 0.49 | 3.49 | 0.68 | 1.38 | 0.39 | |
| 2-methylcitrate/homocitrate | 0.6635 | 0.1431 | 0.2733 | 0.94 | 0.90 | 1.05 | 0.92 | 1.03 | 0.98 | |
| Oxidative Phosphorylation | acetylphosphate | 0.4156 | 0.0617 | 0.0840 | 1.10 | 1.43 | 0.77 | 1.13 | 0.79 | 1.02 |
| phosphate | 0.4261 | 0.6431 | 0.1278 | 0.98 | 0.95 | 1.03 | 1.01 | 1.06 | 1.03 | |
| Superpathway | Subpathway | Treatment 1 | SEM 3 | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| NOVTM-LG | NOVTM-MG | VTM-LG | VTM-MG | Gain | Vitamin | Gain × Vitamin | |||
| Amino Acid Metabolism | Total 4 | 1.13 | 1.08 | 1.04 | 1.03 | 0.037 | 0.43 | 0.07 | 0.63 |
| Glycine, Serine, and Threonine | 1.09 a | 1.00 bc | 0.96 c | 1.05 ab | 0.028 | 0.98 | 0.11 | <0.01 | |
| Alanine and Aspartate | 1.19 | 1.14 | 1.08 | 1.08 | 0.091 | 0.78 | 0.38 | 0.75 | |
| Glutamate | 1.08 | 1.02 | 1.00 | 0.98 | 0.035 | 0.24 | 0.12 | 0.58 | |
| Histidine | 1.25 | 1.12 | 1.11 | 1.09 | 0.101 | 0.46 | 0.41 | 0.59 | |
| Lysine | 1.18 | 1.07 | 1.06 | 1.06 | 0.055 | 0.31 | 0.26 | 0.32 | |
| Phenylalanine | 1.02 | 0.97 | 1.00 | 1.08 | 0.058 | 0.83 | 0.38 | 0.29 | |
| Tyrosine | 1.07 | 1.00 | 1.06 | 0.95 | 0.060 | 0.14 | 0.67 | 0.70 | |
| Tryptophan | 1.08 | 1.04 | 0.97 | 1.00 | 0.051 | 0.85 | 0.17 | 0.51 | |
| Leucine, Isoleucine, and Valine | 1.10 a | 0.96 b | 0.99 b | 0.98 b | 0.026 | 0.01 | 0.08 | 0.02 | |
| Methionine, Cysteine, SAM, and Taurine | 1.04 | 1.08 | 1.04 | 1.10 | 0.028 | 0.11 | 0.75 | 0.71 | |
| Urea Cycle; Arginine and Proline | 1.04 | 1.07 | 0.99 | 1.05 | 0.040 | 0.29 | 0.35 | 0.66 | |
| Creatine | 1.07 | 0.97 | 0.99 | 1.01 | 0.041 | 0.35 | 0.52 | 0.15 | |
| Polyamine | 1.07 | 1.11 | 0.99 | 1.01 | 0.071 | 0.66 | 0.22 | 0.87 | |
| Gaunidino and Acetamido | 1.54 | 1.64 | 1.30 | 0.97 | 0.259 | 0.67 | 0.09 | 0.41 | |
| Glutathione | 1.10 | 1.01 | 1.05 | 1.01 | 0.035 | 0.09 | 0.48 | 0.48 | |
| Carbohydrate Metabolism | Total | 1.05 | 1.09 | 1.15 | 1.03 | 0.057 | 0.54 | 0.80 | 0.15 |
| Glycolysis, Gluconeogenesis, and Pyruvate | 1.08 | 1.01 | 1.07 | 0.98 | 0.053 | 0.11 | 0.66 | 0.87 | |
| Pentose Phosphate Pathway | 0.83 | 0.95 | 1.11 | 0.97 | 0.079 | 0.96 | 0.07 | 0.11 | |
| Pentose | 1.10 | 1.07 | 1.06 | 0.97 | 0.056 | 0.28 | 0.25 | 0.59 | |
| Glycogen | 1.18 | 1.54 | 1.62 | 1.27 | 0.308 | 0.97 | 0.79 | 0.25 | |
| Fructose, Mannose, and Galactose | 1.10 | 1.03 | 1.06 | 1.01 | 0.044 | 0.17 | 0.54 | 0.79 | |
| Nucleotide Sugar | 0.96 | 1.00 | 1.03 | 1.00 | 0.101 | 0.99 | 0.72 | 0.74 | |
| Aminosugar | 1.07 | 1.07 | 1.07 | 0.98 | 0.046 | 0.33 | 0.29 | 0.34 | |
| Advanced Glycation End-product | 1.03 | 0.94 | 1.05 | 0.92 | 0.075 | 0.09 | 0.79 | 0.61 | |
| Energy Metabolism | Total | 1.07 | 1.06 | 1.07 | 1.02 | 0.027 | 0.19 | 0.48 | 0.43 |
| TCA Cycle | 1.13 | 1.19 | 1.06 | 1.01 | 0.063 | 0.09 | 0.04 | 0.39 | |
| Oxidative Phosphorylation | 0.94 | 0.97 | 1.10 | 1.00 | 0.038 | 0.38 | 0.02 | 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crouse, M.S.; McCarthy, K.L.; Menezes, A.C.B.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; et al. Vitamin and Mineral Supplementation and Rate of Weight Gain during the First Trimester of Gestation in Beef Heifers Alters the Fetal Liver Amino Acid, Carbohydrate, and Energy Profile at Day 83 of Gestation. Metabolites 2022, 12, 696. https://doi.org/10.3390/metabo12080696
Crouse MS, McCarthy KL, Menezes ACB, Kassetas CJ, Baumgaertner F, Kirsch JD, Dorsam S, Neville TL, Ward AK, Borowicz PP, et al. Vitamin and Mineral Supplementation and Rate of Weight Gain during the First Trimester of Gestation in Beef Heifers Alters the Fetal Liver Amino Acid, Carbohydrate, and Energy Profile at Day 83 of Gestation. Metabolites. 2022; 12(8):696. https://doi.org/10.3390/metabo12080696
Chicago/Turabian StyleCrouse, Matthew S., Kacie L. McCarthy, Ana Clara B. Menezes, Cierrah J. Kassetas, Friederike Baumgaertner, James D. Kirsch, Sheri Dorsam, Tammi L. Neville, Alison K. Ward, Pawel P. Borowicz, and et al. 2022. "Vitamin and Mineral Supplementation and Rate of Weight Gain during the First Trimester of Gestation in Beef Heifers Alters the Fetal Liver Amino Acid, Carbohydrate, and Energy Profile at Day 83 of Gestation" Metabolites 12, no. 8: 696. https://doi.org/10.3390/metabo12080696
APA StyleCrouse, M. S., McCarthy, K. L., Menezes, A. C. B., Kassetas, C. J., Baumgaertner, F., Kirsch, J. D., Dorsam, S., Neville, T. L., Ward, A. K., Borowicz, P. P., Reynolds, L. P., Sedivec, K. K., Forcherio, J. C., Scott, R., Caton, J. S., & Dahlen, C. R. (2022). Vitamin and Mineral Supplementation and Rate of Weight Gain during the First Trimester of Gestation in Beef Heifers Alters the Fetal Liver Amino Acid, Carbohydrate, and Energy Profile at Day 83 of Gestation. Metabolites, 12(8), 696. https://doi.org/10.3390/metabo12080696