Enzymatic Chicken Pulp Promotes Appetite, Digestive Enzyme Activity, and Growth in Litopenaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diet
2.2. Experimental Procedure
2.3. Sample Collection
2.3.1. Proximate Composition and Analysis
2.3.2. Serum Biochemical Activity
2.3.3. Digestive Enzyme Activity in the Intestine
2.3.4. Intestinal Microbiota
2.3.5. Real-Time Quantitative PCR Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth Performance and Survival
3.2. Whole Body Composition
3.3. Serum Biochemical Indexes
3.4. Digestive Enzyme Activity in Intestine
3.5. Intestinal Microbiota
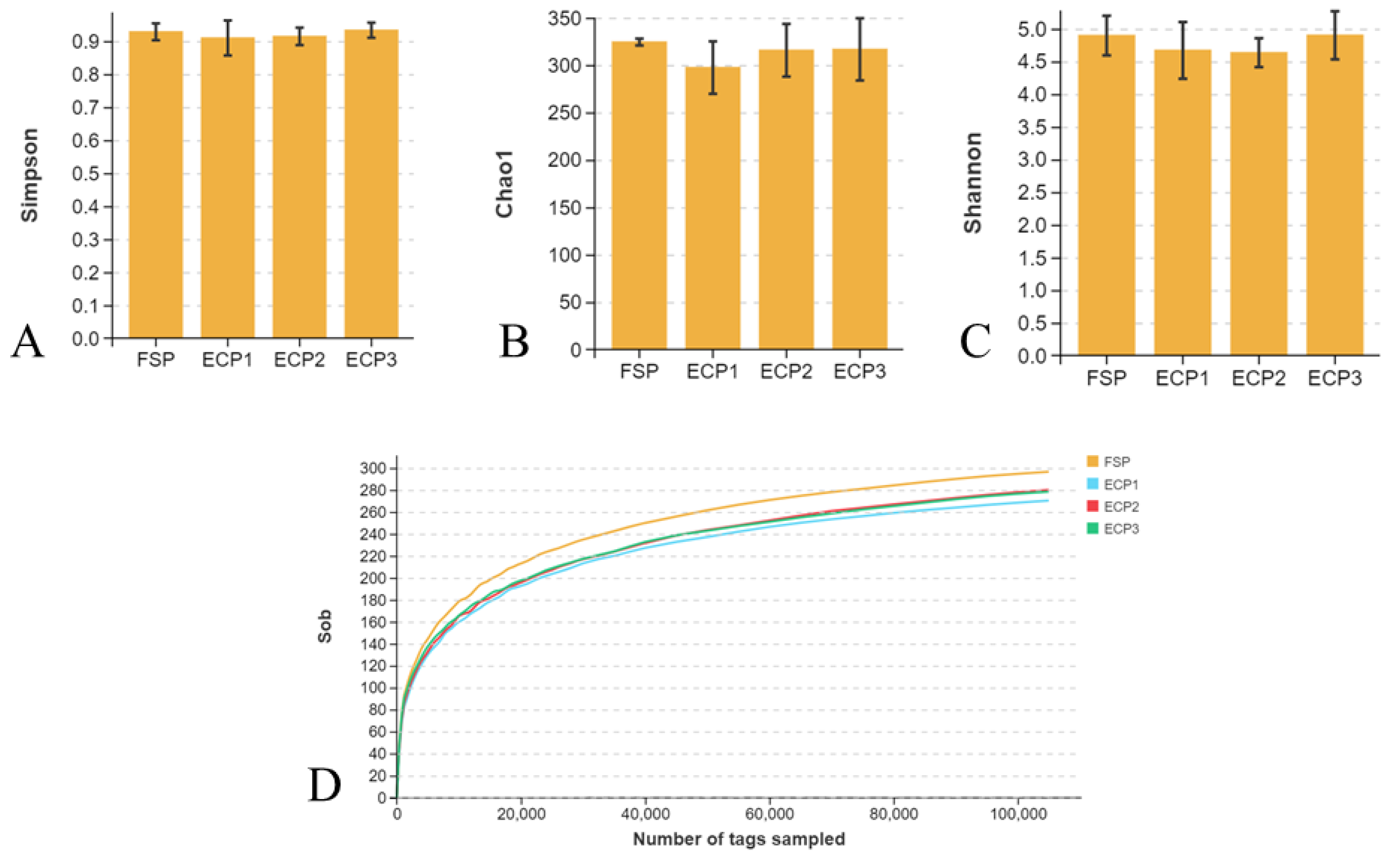
3.5.1. Species Composition
3.5.2. Alpha Diversity
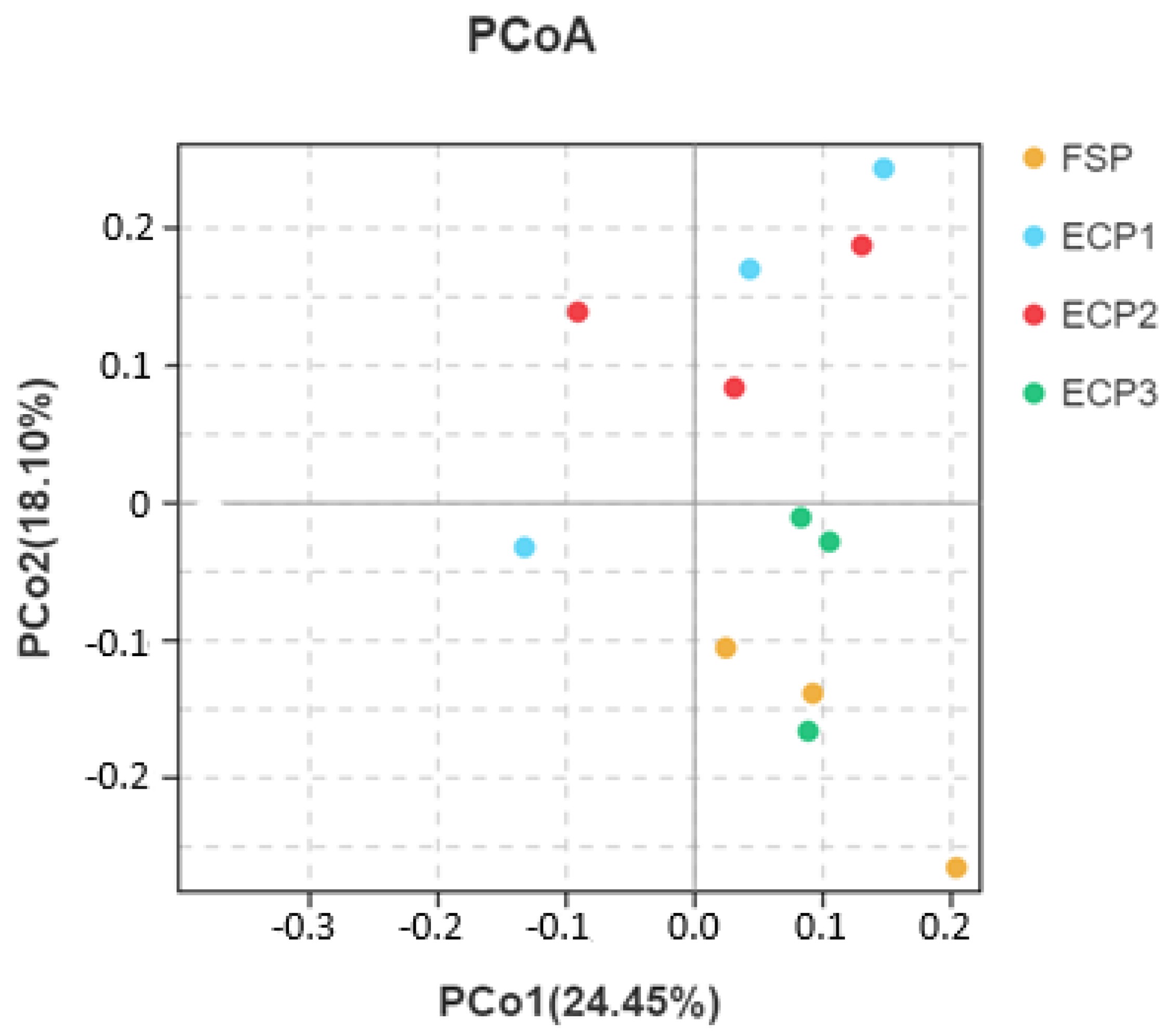
3.5.3. Beta Diversity
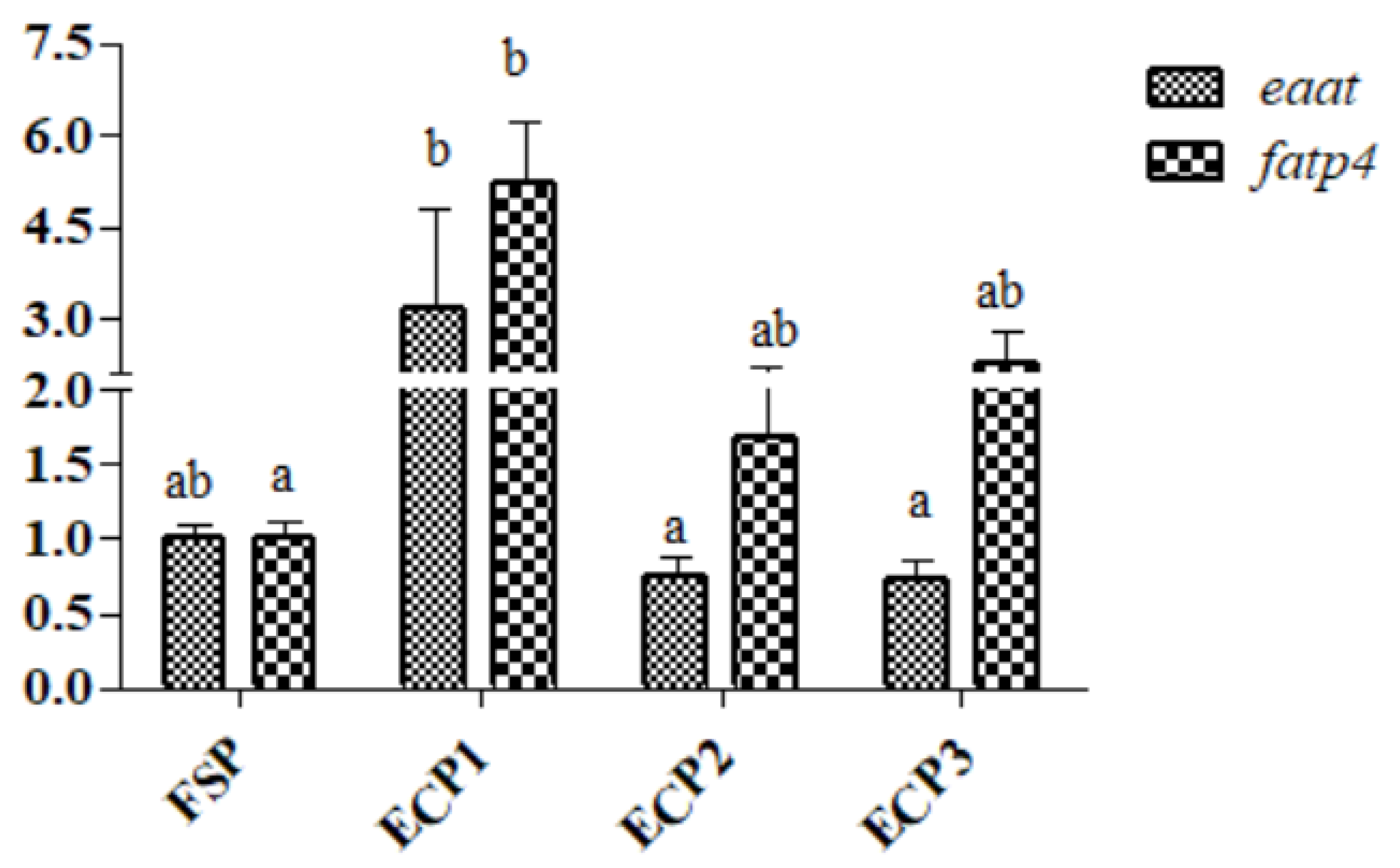
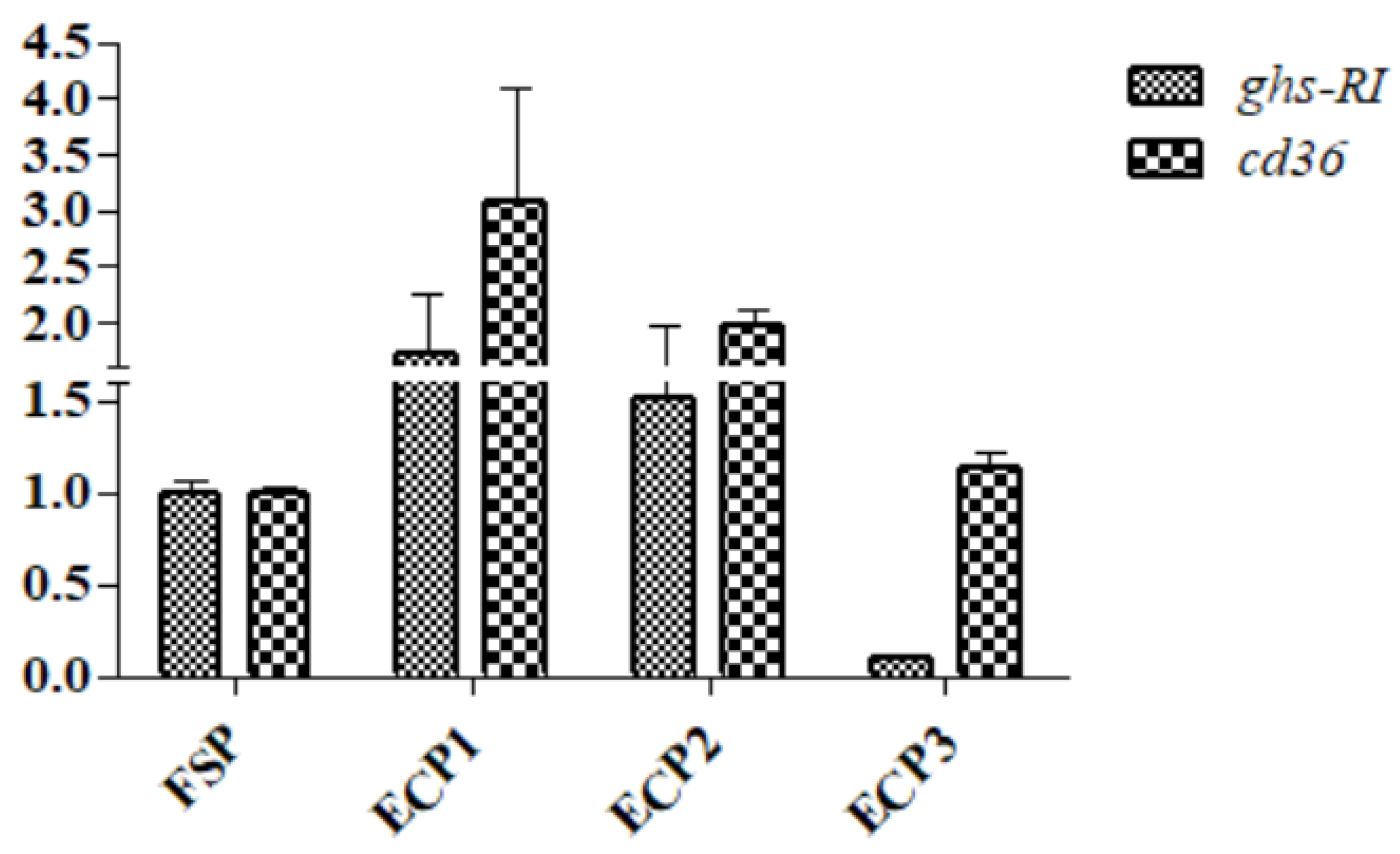
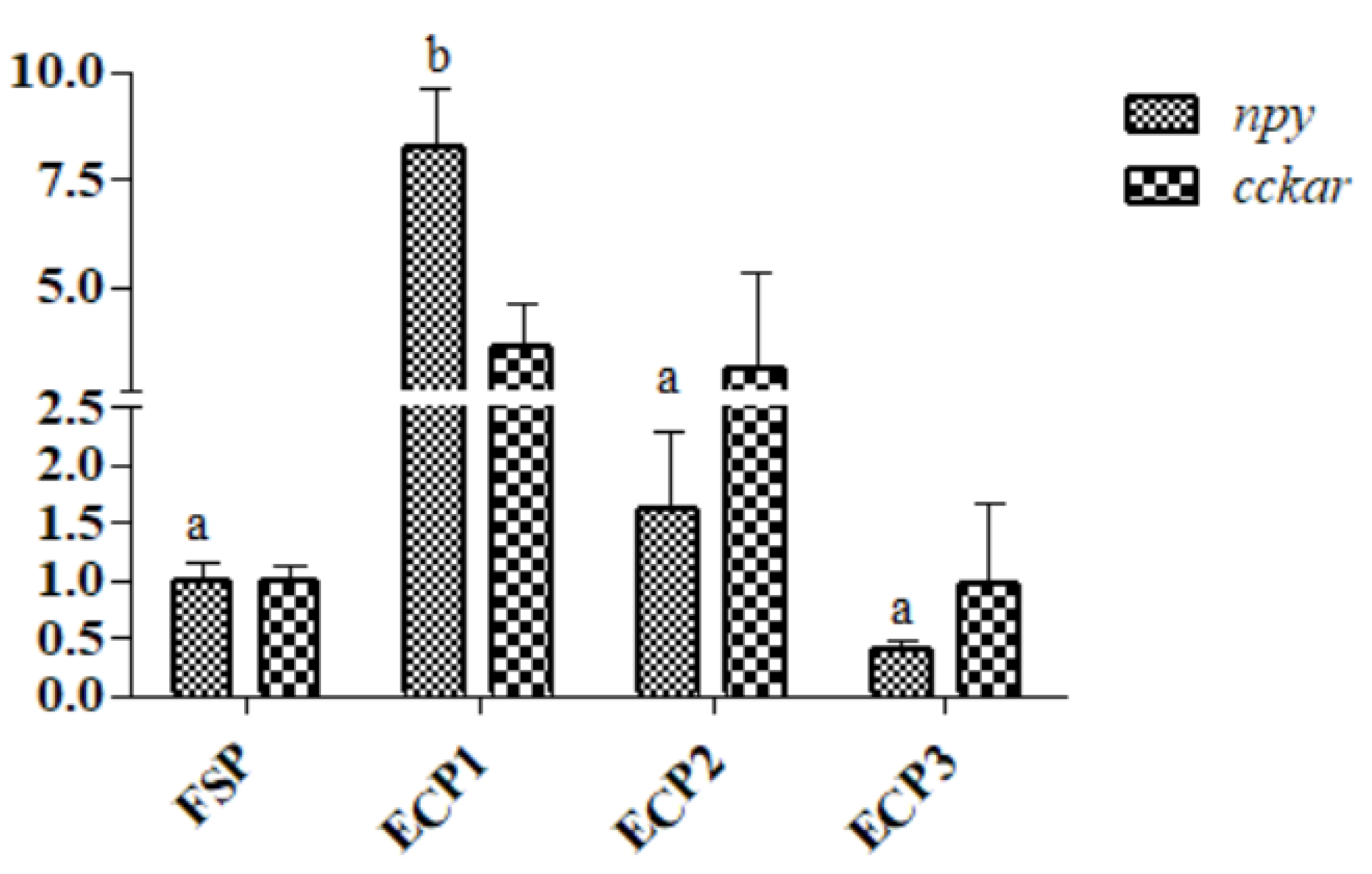
3.6. Gene mRNA Expression in the Hepatopancreas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Quebec City, QC, Canada, 2020. [Google Scholar]
- Omosowone, O.O.; Ogunrinde, A.M. Effect of partial or total supplementation of soybean meal with fluted pumpkin (Telfairia occidentalis) seed meal in the diet of hybrid Catfish (Heteroclarias) fingerlings. Food Sci. Nutr. 2018, 6, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Gunben, E.M.; Senoo, S.; Yong, A.; Shapawi, R. High potential of poultry by-product meal as a main protein source in the formulated feeds for a commonly cultured grouper in Malaysia (Epinephelus fuscoguttatus). Sains Malays. 2014, 43, 399–405. [Google Scholar]
- Hlordzi, V.; Wang, J.; Kuebutornye, F.K.A.; Yang, X.; Tan, B.; Li, T.; Cui, Z.; Lv, S.; Lao, T.; Shuyan, C. Hydrolysed fish protein powder is better at the growth performance, hepatopancreas and intestinal development of Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 23, 101025. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Tlusty, M.F.; Hardy, R.; Cross, S.F. Limiting size of fish fillets at the center of the plate improves the sustainability of aquaculture production. Sustainability 2011, 3, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R. Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi, Lates calcarifer. PLoS ONE 2020, 15, e0242079. [Google Scholar] [CrossRef]
- Ray, G.W.; Liang, D.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S.; Rimei, L. Effects of replacing fishmeal with dietary soybean protein concentrate (SPC) on growth, serum biochemical indices, and antioxidative functions for juvenile shrimp Litopenaeus vannamei. Aquaculture 2020, 516, 734630. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Babbitt, J.K.; Dong, F.M.; Hardy, R.W. Utilization of fish and animal by-product meals in low-pollution feeds for rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Res. 2000, 31, 585–593. [Google Scholar] [CrossRef]
- Zapata, D.B.; Lazo, J.P.; Herzka, S.Z.; Viana, M.T. The effect of substituting fishmeal with poultry by-product meal in diets for Totoaba macdonaldi juveniles. Aquac. Res. 2016, 47, 1778–1789. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Wubshet, S.G.; Wold, J.P.; Afseth, N.K.; Böcker, U.; Lindberg, D.; Ihunegbo, F.N.; Måge, I. Feed-forward prediction of product qualities in enzymatic protein hydrolysis of poultry by-products: A spectroscopic approach. Food Bioprocess Technol. 2018, 11, 2032–2043. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; Nieto-López, M.; Guajardo-Barbosa, C.; Tapia-Salazar, M.; Scholz, U.; Ricque-Marie, D. Replacement of fish meal with poultry by-product meal in practical diets for Litopenaeus vannamei, and digestibility of the tested ingredients and diets. Aquaculture 2007, 272, 466–476. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, W.; Zheng, J.; Tang, Z.; Li, X.; Cao, X.; Zhang, L.; Xu, W.; Mai, K.; Ai, Q. Effects of enzymatic hydrolysis chicken by-product in high plant-based protein diet on growth performance, digestive capacity, antioxidant capacity and non-specific immunity of juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr 2021, 27, 1578–1589. [Google Scholar] [CrossRef]
- Thompson, K.R.; Metts, L.S.; Muzinic, L.A.; Dasgupta, S.; Webster, C.D.; Brady, Y.J. Use of turkey meal as a replacement for menhaden fish meal in practical diets for sunshine bass grown in cages. N. Am. J. Aquac. 2007, 69, 351–359. [Google Scholar] [CrossRef]
- Rawles, S.D.; Riche, M.; Gaylord, T.G.; Webb, J.; Freeman, D.W.; Davis, M. Evaluation of poultry by-product meal in commercial diets for hybrid striped bass (Morone chrysops♀ × M. saxatilis♂) in recirculated tank production. Aquaculture 2006, 259, 377–389. [Google Scholar] [CrossRef]
- Yu, Y. Replacement of fishmeal with poultry byproduct meal and meat and bone meal in shrimp, tilapia and trout diets. In A vances En Nutrición Acuicola. Memorias del Septimo Simposium Internacional de Nutrición Acuícola; Cruz Suárez, M., Ricque Marie, L.E., Nieto López, D., Villarreal, M.G., Scholz, D., y González, U., Eds.; Nutrición Acuícola: Hermosillo, Sonora, Mexico, 2004; pp. 16–19. [Google Scholar]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; García, V.; Fuertes, J.B. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L.). Aquac. Res. 2016, 47, 1612–1621. [Google Scholar] [CrossRef]
- Dawson, M.R.; Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Seaton, P.J. Evaluation of poultry by-product meal as an alternative to fish meal in the diet of juvenile Black Sea Bass reared in a recirculating aquaculture system. N. Am. J. Aquac. 2018, 80, 74–87. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Fasakin, E.A.; Serwata, R.D.; Davies, S.J. Comparative utilization of rendered animal derived products with or without composite mixture of soybean meal in hybrid tilapia (Oreochromis niloticus × Oreochromis mossambicus) diets. Aquaculture 2005, 249, 329–338. [Google Scholar] [CrossRef]
- Udoinyang, E.P.; Amali, O.; Iheukwumere, C.C.; Ukpatu, J.E. Length-weight relationship and condition factor of seven shrimp species in the artisanal shrimp fishery of Iko river estuary, southeastern Nigeria. Int. J. Fish. Aquat. Stud. 2016, 4, 109–114. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Shi, B.; Jin, M.; Jiao, L.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Effects of dietary zinc level on growth performance, lipolysis and expression of genes involved in the calcium/calmodulin-dependent protein kinase kinase-β/AMP-activated protein kinase pathway in juvenile Pacific white shrimp. Br. J. Nutr. 2020, 124, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, S.; Lei, W.; Zhu, X.; Yang, Y. Effect of replacement of fish meal by meat and bone meal and poultry by-product meal in diets on the growth and immune response of Macrobrachium nipponense. Fish Shellfish Immunol. 2004, 17, 105–114. [Google Scholar] [CrossRef]
- Bae, J.; Azad, A.K.; Won, S.; Hamidoghli, A.; Seong, M.; Bai, S.C. Effects of enzymatically hydrolyzed fish by-products in diet of juvenile rainbow trout (Oncorhynchus mykiss). Fish. Aquat. Sci. 2019, 22, 1. [Google Scholar] [CrossRef] [Green Version]
- Dios, D. Fishmeal replacement with feather-enzymatic hydrolyzates co-extruded with soya-bean meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2001, 7, 143–151. [Google Scholar]
- Leduc, A.; Zatylny-Gaudin, C.; Robert, M.; Corre, E.; le Corguille, G.; Castel, H.; Lefevre-Scelles, A.; Fournier, V.; Gisbert, E.; Andree, K.B. Dietary aquaculture by-product hydrolysates: Impact on the transcriptomic response of the intestinal mucosa of European seabass (Dicentrarchus labrax) fed low fish meal diets. BMC Genom. 2018, 19, 396. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, W.; Wen, H.; Zhou, Y.; Wu, J. Animal by-products with or without enzymatic hydrolysis completely replacement of fish meal in genetically improved farmed tilapia diets (Oreochromis niloticus). Aquac. Res. 2021, 52, 291–301. [Google Scholar] [CrossRef]
- Barreto-Curiel, F.; Parés-Sierra, G.; Correa-Reyes, G.; Durazo-Beltrán, E.; Viana, M.T. Total and partial fishmeal substitution by poultry by-product meal (petfood grade) and enrichment with acid fish silage in aquafeeds for juveniles of rainbow trout Oncorhynchus mykiss. Lat. Am. J. Aquat. Res. 2016, 44, 327–335. [Google Scholar] [CrossRef]
- Shapawi, R.; Ng, W.-K.; Mustafa, S. Replacement of fish meal with poultry by-product meal in diets formulated for the humpback grouper, Cromileptes altivelis. Aquaculture 2007, 273, 118–126. [Google Scholar] [CrossRef]
- Hatlen, B.; Jakobsen, J.-V.; Crampton, V.; Alm, M.; Langmyhr, E.; Espe, M.; Hevrøy, E.; Torstensen, B.; Liland, N.; Waagbø, R. Growth, feed utilization and endocrine responses in Atlantic salmon (Salmo salar) fed diets added poultry by-product meal and blood meal in combination with poultry oil. Aquac. Nutr. 2014, 21, 714–725. [Google Scholar] [CrossRef]
- Hevroy, E.; Espe, M.; Waagbo, R.; Sandnes, K.; Ruud, M.; Hemre, G.-I. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 2005, 11, 301–313. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmad, A.B.; Abol-Munafi, A.B. The selection of viscerosomatic and hepatosomatic indices for the measurement and analysis of Oreochromis niloticus condition fed with varying dietary maltose levels. Int. J. Fauna Biol. Stud. 2014, 1, 18–20. [Google Scholar]
- Yang, Y.; Xie, S.; Cui, Y.; Zhu, X.; Lei, W.; Yang, Y. Partial and total replacement of fishmeal with poultry by-product meal in diets for gibel carp, Carassius auratus gibelio Bloch. Aquac. Res. 2006, 37, 40–48. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish Immunol. 2016, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Lee, S.; Yun, H.; Hong, J.; Moniruzzaman, M.; Bai, S.C.; Park, G.; Chee, S.; Jeon, T. Evaluation of dietary fishmeal analogue with addition of shrimp soluble extract on growth and nonspecific immune response of rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2017, 48, 583–591. [Google Scholar] [CrossRef]
- Sullivan, K.B. Replacement of Fish Meal by Alternative Protein Sources in Diets for Juvenile Black Sea Bass. Master’s Thesis, University of North Carolina Wilmington, Wilmington, NC, USA, 2008. [Google Scholar]
- Banavreh, A.; Soltani, M.; Kamali, A.; Yazdani-Sadati, M.A.; Shamsaie, M. Immuno-physiological and antioxidant responses of Siberian sturgeon (Acipenser baerii) fed with different levels of olive pomace. Fish Physiol. Biochem. 2019, 45, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Mohanty, P.K. Comparative haematological and serum biochemical analysis of catfishes Clarias batrachus (Linnaeus, 1758) and Heteropneustes fossilis (Bloch, 1794) with respect to sex. J. Entomol. Zool. Stud. 2014, 2, 191–197. [Google Scholar]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? npj Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Ayiku, S.; Shen, J.; Tan, B.; Dong, X.; Liu, H. Effects of reducing dietary fishmeal with yeast supplementations on Litopenaeus vannamei growth, immune response and disease resistance against Vibrio harveyi. Microbiol. Res. 2020, 239, 126554. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.; Dong, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2020, 518, 734563. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, H.; Liang, M. Amino acid absorption and protein synthesis responses of turbot Scophthalmus maximus to lysine and leucine in free, dipeptide and tripeptide forms. Aquac. Nutr. 2020, 26, 358–367. [Google Scholar] [CrossRef]
- Chi, S.Y.; Zhong, J.L.; He, Y.F.; Li, J.M.; Luo, Y. Cloning and tissue expression of amino acid transporter B0AT1 gene in Epinephelus coioides. J. Guangdong Ocean Univ. 2017, 37, 1–6. [Google Scholar]
- Duan, Y.; Li, F.; Liu, H.; Li, Y.; Liu, Y.; Kong, X.; Zhang, Y.; Deng, D.; Tang, Y.; Feng, Z. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front. Biosci. 2015, 20, 796–813. [Google Scholar]
- Bakke, S.; Jordal, A.-E.O.; Gómez-Requeni, P.; Verri, T.; Kousoulaki, K.; Aksnes, A.; Rønnestad, I. Dietary protein hydrolysates and free amino acids affect the spatial expression of peptide transporter PepT1 in the digestive tract of Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, G.; Zhao, X.; Dong, X.; Chi, S.; Tan, B. Addition of hydrolysed porcine mucosa to low-fishmeal feed improves intestinal morphology and the expressions of intestinal amino acids and small peptide transporters in hybrid groupers (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Aquaculture 2021, 535, 736389. [Google Scholar] [CrossRef]
- Zhou, P.; Luo, Y.; Zhang, L.; Li, J.; Zhang, B.; Xing, S.; Zhu, Y.; Gao, F.; Zhou, G. Effects of cysteamine supplementation on the intestinal expression of amino acid and peptide transporters and intestinal health in finishing pigs. Anim. Sci. J. 2017, 88, 314–321. [Google Scholar] [CrossRef]
- Carr, W.E.S.; Netherton, I.J.C.; Gleeson, A.R.; Derby, C.D. Stimulants of Feeding Behavior in Fish: Analyses of Tissues of Diverse Marine Organisms. Biol. Bull. 1996, 190, 149–160. [Google Scholar] [CrossRef]
- Basto-Silva, C.; Enes, P.; Oliva-Teles, A.; Balbuena-Pecino, S.; Navarro, I.; Capilla, E.; Guerreiro, I. Dietary protein source and protein/carbohydrate ratio affects appetite regulation-related genes expression in gilthead seabream (Sparus aurata). Aquaculture 2021, 533, 736142. [Google Scholar] [CrossRef]


| Ingredients | FSP | ECP1 | ECP2 | ECP3 |
|---|---|---|---|---|
| Brown fish meal | 20 | 20 | 20 | 20 |
| Fish soluble pulp | 4 | 0.00 | 0.00 | 0.00 |
| ECP 1 | 0 | 4 | 0 | 0 |
| ECP 2 | 0 | 0 | 4 | 0 |
| ECP 3 | 0 | 0 | 0 | 4 |
| Soybean meal | 15 | 15 | 15 | 15 |
| Cottonseed protein | 11 | 11 | 11 | 12.5 |
| Wheat flour | 24 | 24 | 24 | 24 |
| Peanut meal | 14 | 14 | 14 | 14 |
| Shrimp shell meal | 3 | 3 | 3 | 3 |
| Fish oil | 1.6 | 1.6 | 1.6 | 1.6 |
| Soy lecithin | 1.5 | 1.5 | 1.5 | 1.5 |
| Soybean oil | 1.5 | 1.5 | 1.5 | 1.3 |
| Calcium monophosphate | 1 | 1 | 1 | 1 |
| Premix (Vitamin + Mineral) | 1 | 1 | 1 | 1 |
| Cholesterol | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidant | 0.45 | 0.45 | 0.45 | 0.45 |
| Microcrystalline cellulose | 1.3 | 1.3 | 1.3 | 0 |
| Choline Chloride | 0.4 | 0.4 | 0.4 | 0.4 |
| Attractant | 0.1 | 0.1 | 0.1 | 0.1 |
| Total % | 100 | 100 | 100 | 100 |
| Nutrient composition (%) | ||||
| Moisture | 9.34 | 9.55 | 9.32 | 9.21 |
| Crude protein (dry matter) | 38.34 | 38.09 | 38.29 | 38.13 |
| Crude lipid (dry matter) | 7.14 | 6.77 | 6.79 | 6.55 |
| Ash (dry matter) | 10.22 | 10.24 | 10.33 | 10.11 |
| FSP | ECP1 | ECP2 | ECP3 | Cottonseed Protein | |
|---|---|---|---|---|---|
| Aspartic acid | 1.99 | 1.79 | 1.82 | 1.25 | 5.68 |
| Glutamic acid | 2.4 | 2.43 | 2.48 | 1.48 | 12.33 |
| Serine | 0.72 | 0.73 | 0.74 | 0.17 | 2.48 |
| Histidine | 1.64 | 0.82 | 0.84 | 0.16 | 1.73 |
| Arginine | 2.35 | 2.05 | 2.09 | 0.52 | 7.75 |
| Threonine | 0.98 | 0.62 | 0.63 | 0.14 | 1.93 |
| Glycine | 1.78 | 3.28 | 3.34 | 0.35 | 2.45 |
| Alanine | 2.94 | 1.66 | 1.69 | 0.71 | 2.29 |
| Proline | 1.96 | 3.19 | 3.25 | 0.41 | 2.14 |
| Tyrosine | 0.78 | 0.41 | 0.42 | 0.21 | 1.71 |
| Valine | 1.04 | 0.66 | 0.67 | 0.29 | 2.75 |
| Methionine | 0.46 | 0.4 | 0.41 | 0.12 | 0.76 |
| Cystine | 0.2 | 0.39 | 0.40 | 0.03 | 0.76 |
| Isoleucine | 0.91 | 0.67 | 0.68 | 0.28 | 1.87 |
| Leucine | 1.72 | 1.32 | 1.35 | 0.29 | 3.22 |
| Phenylalanine | 1.2 | 0.84 | 0.86 | 0.15 | 3.39 |
| Lysine | 2.05 | 1.23 | 1.25 | 0.34 | 2.62 |
| Gene | Primer | Source |
|---|---|---|
| npy | F: GGTGATGTCGAAGTGGCCGGAGTTG R: ACCTCGCCAGGGAGAAGCGGAACCA | GFRP01055388 |
| fatp4 | F:CCGACGGGCAAAGCGACTGAACCA R: TCTATTCCACCAGGTATCTTTATCG | KY271629 |
| eaat | F: GTTACAACATCAAACCCGAGACAG R:CCCGAGAAGGTGAAGATGAGGAGC | GGUK01021174 |
| ghs-RI | F: TGCGAAGGAGGAACTCTGAACATT R: CCAAGTAAGTCGCTTCCTGGCTCT | HAAW01018270 |
| cckar | F: ATCGTGTCCCTTGTGCTGTCTGTT R: GTCATCGCCGTCATCTTCTTCGTC | XM_027379811 |
| cd36 | F: AACCAAGGTCCTGACCATCAC R: AGGTGAGAGTCGACGAGGAA | [24] |
| β-actin | F: AAGATGTGTGACGACGAAGTAGC R: AGGATACCTCGCTTGCTCTG | GFRP01025709 |
| FSP | ECP1 | ECP2 | ECP3 | |
|---|---|---|---|---|
| IW | 0.26 ± 0.00 | 0.26 ± 0.00 | 0.26 ± 0.01 | 0.26 ± 0.00 |
| WGR/% | 2789.92 ± 34.42 | 2923.21 ± 17.57 | 2818.48 ± 57.17 | 2553.41 ± 46.36 |
| SGR (%/d) | 5.53 ± 0.08 | 5.87 ± 0.06 | 5.52 ± 0.17 | 5.49 ± 0.22 |
| SR,% | 80.00 ± 3.85 | 88.33.00 ± 2.15 | 82.22 ± 5.55 | 86.67 ± 6.67 |
| FCR | 1.64 ± 0.02 a | 1.58 ± 0.03 a | 1.84 ± 0.04 b | 1.70 ± 0.5 ab |
| CF, g/cm3 | 0.81 ± 0.03 | 0.87 ± 0.01 | 0.81 ± 0.03 | 0.81 ± 0.01 |
| VSI,% | 5.85 ± 0.09 | 5.55 ± 0.22 | 5.11 ± 0.34 | 5.07 ± 0.32 |
| HSI,% | 4.65 ± 0.12 | 4.11 ± 0.20 | 4.00 ± 0.28 | 3.96 ± 0.14 |
| FSP | ECP1 | ECP2 | ECP3 | |
|---|---|---|---|---|
| Moisture | 73.44 ± 0.26 | 74.59 ± 0.57 | 73.62 ± 0.19 | 74.05 ± 0.21 |
| Crude protein | 69.28 ± 2.11 | 63.76 ± 3.13 | 69.23 ± 2.08 | 64.39 ± 0.63 |
| Crude lipid | 6.83 ± 0.78 b | 5.74 ± 0.16 a | 6.41 ± 0.16 ab | 7.47 ± 0.40 b |
| Ash | 10.43 ± 0.01 a | 11.75 ± 0.04 b | 10.98 ± 0.35 ab | 11.81 ± 0.25 b |
| PER | 4.67 ± 0.80 ab | 5.41 ± 0.11 b | 4.62 ± 0.10 ab | 4.48 ± 0.27 a |
| PPV | 40.17 ± 2.09 | 40.64 ± 6.83 | 39.57 ± 2.34 | 32.08 ± 1.00 |
| AST, U/L | ALT, U/L | LZM, U/ml | CAT, U/ml | TP, g/ml | |
|---|---|---|---|---|---|
| FSP | 17.94 ± 3.58 a | 46.76 ± 4.17 ab | 275.00 ± 21.43 | 7.08 ± 3.23 | 299.67 ± 8.51 |
| ECP1 | 26.72 ± 4.20 ab | 58.42 ± 4.19 bc | 285.71 ± 19.88 | 13.28 ± 3.87 | 297.79 ± 20.72 |
| ECP2 | 41.76 ± 0.63 b | 59.38 ± 0.37 c | 291.67 ± 5.19 | 15.86 ± 6.88 | 324.81 ± 7.92 |
| ECP3 | 12.07 ± 2.93 a | 40.55 ± 1.12 a | 300.00 ± 5.46 | 10.45 ± 1.18 | 289.89 ± 13.86 |
| Lipase, U/gprot | Amylase, U/mgprot | Protease, U/mL | |
|---|---|---|---|
| FSP | 0.81 ± 0.26 | 3.07 ± 0.27 a | 1352.24 ± 116.5 a |
| ECP1 | 1.06 ± 0.05 | 5.47 ± 0.03 b | 1944.10 ± 156.08 b |
| ECP2 | 0.52 ± 0.04 | 3.05 ± 0.03 a | 2322.53 ± 53.75 b |
| ECP3 | 0.60 ± 0.01 | 3.16 ± 0.01 a | 1828.34 ± 124.36 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlordzi, V.; Tan, B.; Dong, X.; Zhang, S.; Zhu, L.; Zhang, L.; Hu, X.; Chi, S. Enzymatic Chicken Pulp Promotes Appetite, Digestive Enzyme Activity, and Growth in Litopenaeus vannamei. Metabolites 2022, 12, 698. https://doi.org/10.3390/metabo12080698
Hlordzi V, Tan B, Dong X, Zhang S, Zhu L, Zhang L, Hu X, Chi S. Enzymatic Chicken Pulp Promotes Appetite, Digestive Enzyme Activity, and Growth in Litopenaeus vannamei. Metabolites. 2022; 12(8):698. https://doi.org/10.3390/metabo12080698
Chicago/Turabian StyleHlordzi, Vivian, Beiping Tan, Xiaohui Dong, Shuang Zhang, Lin Zhu, Ling Zhang, Xiangna Hu, and Shuyan Chi. 2022. "Enzymatic Chicken Pulp Promotes Appetite, Digestive Enzyme Activity, and Growth in Litopenaeus vannamei" Metabolites 12, no. 8: 698. https://doi.org/10.3390/metabo12080698
APA StyleHlordzi, V., Tan, B., Dong, X., Zhang, S., Zhu, L., Zhang, L., Hu, X., & Chi, S. (2022). Enzymatic Chicken Pulp Promotes Appetite, Digestive Enzyme Activity, and Growth in Litopenaeus vannamei. Metabolites, 12(8), 698. https://doi.org/10.3390/metabo12080698





