Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome
Abstract
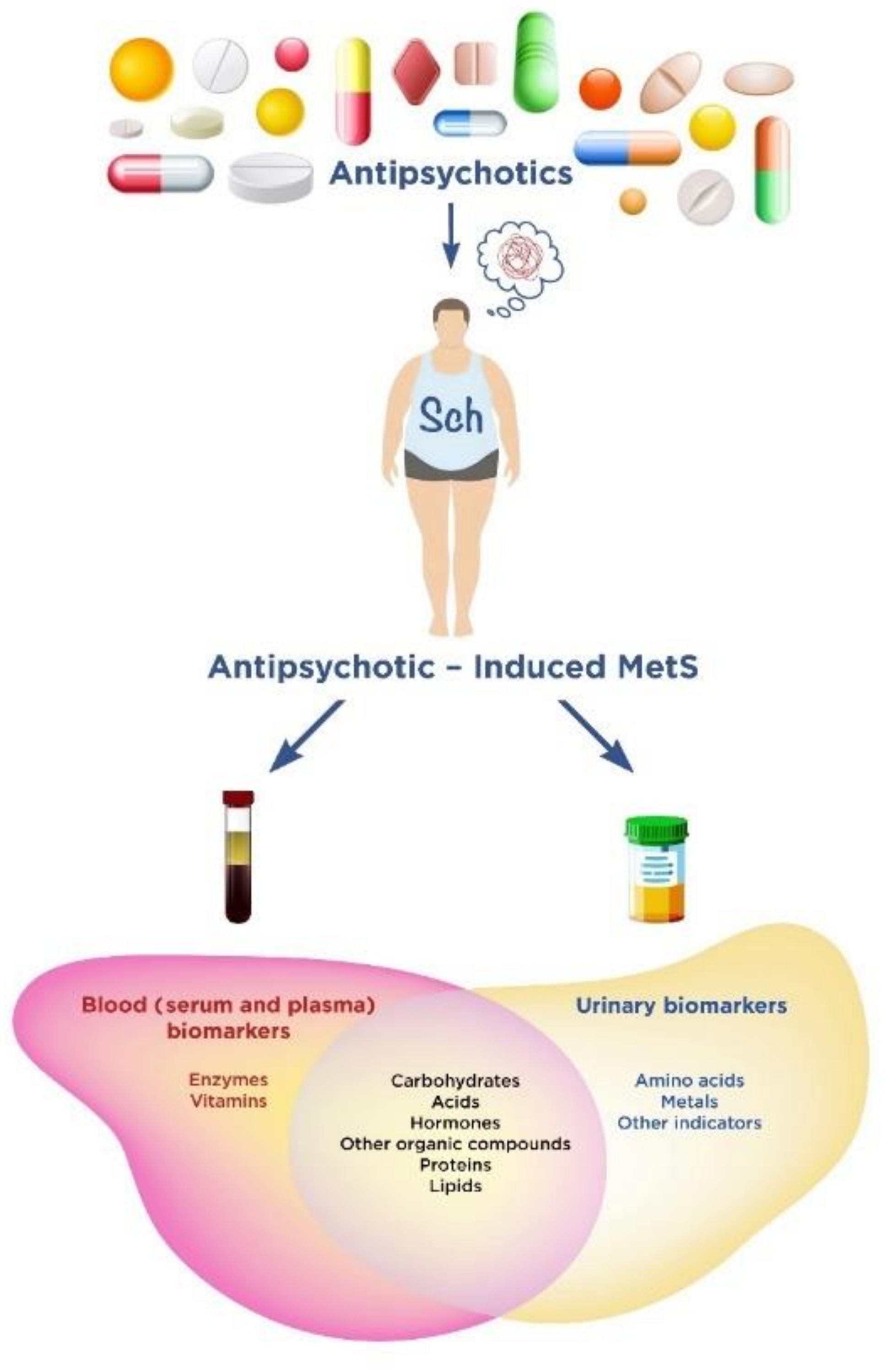
:1. Introduction
2. Blood (Serum and Plasma) Biomarkers of Antipsychotic-Induced Metabolic Syndrome
2.1. Carbohydrates
Glucose
2.2. Acids
2.2.1. Sialic Acid
2.2.2. Uric Acid
2.3. Hormones
2.3.1. Adiponectin
2.3.2. Aldosterone
2.3.3. Chemerin
2.3.4. Ghrelin
2.3.5. Insulin
2.3.6. Leptin
2.3.7. Omentin
2.3.8. Parathyroid Hormone
2.3.9. Testosterone
2.3.10. Thyroid-Stimulating Hormone
2.4. Other Organic Compounds
Bilirubin
2.5. Proteins
2.5.1. Adipocyte Fatty Acid-Binding Protein
2.5.2. C-Peptide
2.5.3. Ligand CD40
2.5.4. Cystatin C
2.5.5. Ferritin
2.5.6. Fibrinogen
2.5.7. Fibroblast Growth Factor-21
2.5.8. Monocyte Chemoattractant Protein-1
2.5.9. Plasminogen Activator Inhibitor-1
2.5.10. Retinol-Binding Protein 4
2.5.11. Tumor Necrosis Factor-Alpha
2.6. Lipids
2.6.1. Oxidized Low Density Lipoprotein
2.6.2. Apolipoprotein A1
2.6.3. Apolipoprotein B
2.6.4. Free Fatty Acids
2.7. Enzymes
2.7.1. Superoxide Dismutase
2.7.2. Gamma-Glutamyltransferase
2.7.3. Glutathione Peroxidase
2.7.4. Lipoprotein Associated Phospholipase A2
2.8. Vitamins
2.8.1. 25-Hydroxyvitamin D
2.8.2. Alpha Tocopherol
3. Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome
3.1. Carbohydrates
3.1.1. Glucose
3.1.2. Maltitol
3.2. Amino Acids
3.2.1. Aromatic Amino Acids
3.2.2. Histidine
3.2.3. Tryptophan
3.3. Acids
3.3.1. P-Cresol Sulfate
3.3.2. Salicyluric Acid
3.3.3. 4-hydroxyphenylpyruvic Acid
3.3.4. Trigonelline
3.4. Hormones
3.4.1. Epinephrine
3.4.2. Norepinephrine
3.5. Other Organic Compounds
3.5.1. Albumin
3.5.2. Imidazole
3.5.3. Trimethylamine N-oxide
3.6. Metals
3.6.1. Cadmium
3.6.2. Lead
3.6.3. Mercury
3.7. Other Indicators
Potential of Hydrogen (pH)
4. Discussion
- -
- the presence of three or more clinical criteria of MetS according to classification criteria (ATPIII, ATPIII-A or IDF) after 3 or more months of taking APs;
- -
- prolonged use of AP therapy (mono- or polytherapy) for 3 or more months;
- -
- the presence of three or more blood (plasma and serum) and three or more urinary biomarkers AIMetS.
- -
- the presence of one to three clinical criteria of MetS according to classifications (ATPIII, ATPIII-A or IDF) after 3 or more months of taking APs;
- -
- prolonged use of AP therapy (mono- or polytherapy) for 3 or more months;
- -
- the presence of one to three blood (plasma and serum) or one to three urinary biomarkers AIMetS.
- -
- the absence of clinical criteria of MetS by classification (ATPIII, ATPIII-A or IDF) after 3 or more months of taking APs;
- -
- prolonged use of AP therapy (mono- or polytherapy) for 3 or more months;
- -
- the presence of single blood (plasma and serum) or single urinary biomarkers AIMetS or the absence thereof.
5. Limitation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Expert Panel on Detection and Evaluation of Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Hanley, A.J.; Karter, A.J.; Williams, K.; Festa, A.; D’Agostino, R.B., Jr.; Wagenknecht, L.E.; Haffner, S.M. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: The Insulin Resistance Atherosclerosis Study. Circulation 2005, 112, 3713–3721. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.; Gordon, T. A general cardiovascular risk profile: The Framingham Study. Am. J. Cardiol. 1976, 38, 46–51. [Google Scholar] [CrossRef]
- Correll, C.U.; Frederickson, A.M.; Kane, J.M.; Manu, P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J. Clin. Psychiatry 2006, 67, 575–583. [Google Scholar] [CrossRef]
- Van Winkel, R.; Rutten, B.P.; Peerbooms, O.; Peuskens, J.; van Os, J.; De Hert, M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2010, 121, 193–198. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; McGrath, J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 2007, 64, 1123–1131. [Google Scholar] [CrossRef]
- Laursen, T.M. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr. Res. 2011, 131, 101–104. [Google Scholar] [CrossRef]
- Brown, S. Excess mortality of schizophrenia. A meta-analysis. Br. J. Psychiatry 1997, 171, 502–508. [Google Scholar] [CrossRef]
- Chesney, E.; Goodwin, G.M.; Fazel, S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry 2014, 13, 153–160. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incidence cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Ryan, M.C.; Collins, P.; Thakore, J.H. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry 2003, 160, 284–289. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; De Hert, M. Prevalence of Metabolic Syndrome and Metabolic Abnormalities in Schizophrenia and Related Disorders—A Systematic Review and Meta-Analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef]
- Nasrallah, H.A.; Meyer, J.M.; Goff, D.C.; McEvoy, J.P.; Davis, S.M.; Stroup, T.S.; Lieberman, J.A. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr. Res. 2005, 80, 19–32. [Google Scholar]
- Molina, J.D.; Avila, S.; Rubio, G.; López-Muñoz, F. Metabolomic Connections between Schizophrenia, Antipsychotic Drugs and Metabolic Syndrome: A Variety of Players. Curr. Pharm. Des. 2021, 27, 4049–4061. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Mansoub, S.; Chan, M.K.; Adeli, K. Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin. Biochem. 2006, 39, 569–587. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lin, S.Y.; Liu, P.H.; Cheung, B.M.; Lai, W.A. Association between hematological parameters and metabolic syndrome components in a Chinese population. J. Diabetes Complicat. 2004, 18, 322–327. [Google Scholar] [CrossRef]
- Lann, D.; LeRoith, D. Insulin resistance as the underlying cause for the metabolic syndrome. Med. Clin. N. Am. 2007, 91, 1063–1077. [Google Scholar]
- Hajer, G.R.; van der Graaf, Y.; Olijhoek, J.K.; Verhaar, M.C.; Visseren, F.L. Levels of homocysteine are increased in metabolic syndrome patients but are not associated with an increased cardiovascular risk, in contrast to patients without the metabolic syndrome. Heart 2007, 93, 216–220. [Google Scholar] [CrossRef]
- Servais, A.; Giral, P.; Bernard, M.; Bruckert, E.; Deray, G.; IsnardBagnis, C. Is serum cystatin-C a reliable marker for metabolic syndrome? Am. J. Med. 2008, 121, 426–432. [Google Scholar] [CrossRef]
- Onat, A.; Uyarel, H.; Hergenç, G.; Karabulut, A.; Albayrak, S.; Sari, I.; Yazici, M.; Keleş, I. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am. J. Hypertens. 2006, 19, 1055–1062. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Dyslipidemia and the metabolic syndrome. Diabetes Care 2004, 27, 3009–3016. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological role of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Jacobs, M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Ferreira, I.; Blaak, E.E.; Feskens, E.J.; Jansen, E.H.; Schalkwijk, C.G.; Stehouwer, C.D. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: The CODAM study. Eur. J. Clin. Investig. 2009, 39, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Eaton, S.E.; Cotter, M.A.; Tesfaye, S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001, 44, 1973–1988. [Google Scholar] [CrossRef]
- Tarquini, R.; Lazzeri, C.; Pala, L.; Rotella, C.M.; Gensini, G.F. The diabetic cardiomyopathy. Acta Diabetol. 2011, 48, 173–181. [Google Scholar] [CrossRef]
- Newsome, C.A.; Shiell, A.W.; Fall, C.H.; Phillips, D.I.; Shier, R.; Law, C.M. Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet. Med. 2003, 20, 339–348. [Google Scholar] [CrossRef]
- Smith, G.N.; Flynn, S.W.; McCarthy, N.; Meistrich, B.; Ehmann, T.S.; MacEwan, G.W.; Altman, S.; Kopala, L.C.; Honer, W.G. Low birthweight in schizophrenia: Prematurity or poor fetal growth? Schizophr. Res. 2001, 47, 177–184. [Google Scholar] [CrossRef]
- Susser, E.S.; Lin, S.P. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–45. Arch. Gen. Psychiatry 1992, 49, 983–988. [Google Scholar] [CrossRef]
- Lindenmayer, J.P.; Czobor, P.; Volavka, J.; Citrome, L.; Sheitman, B.; McEvoy, J.P.; Cooper, T.B.; Chakos, M.; Lieberman, J.A. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am. J. Psychiatry 2003, 160, 290–296. [Google Scholar] [CrossRef]
- Van Winkel, R.; De Hert, M.; Wampers, M. Major changes in glucose metabolism including new-onset diabetes within 3 months after initiation or switch of atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J. Clin. Psychiatry 2008, 69, 472–479. [Google Scholar] [CrossRef]
- Shahid, S.M.; Nawab, S.N.; Shaikh, R.; Mahboob, T. Glycemic control, dyslipidemia and endothelial dysfunction in coexisted diabetes, hypertension and nephropathy. Pak. J. Pharm. Sci. 2012, 25, 123–129. [Google Scholar]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet 1999, 353, 1649–1652. [Google Scholar] [CrossRef]
- Nayak, S.B.; Bhaktha, G. Relationship between sialic acid and metabolic variables in Indian type 2 diabetic patients. Lipids Health Dis. 2005, 4, 15. [Google Scholar] [CrossRef]
- Hammad, I.K.; Abed, B.A.; Rashid, N.F. The relationship between serum total sialic acid and the presence of metabolic syndrome in type 2 diabetes mellitus. Iraqi J. Community Med. 2013, 11, 37–41. [Google Scholar]
- Ovist, R.; Ismail, I.S.; Muniandy, S. Correlation of plasma C-reactive protein levels to sialic acid and lipid concentration in the normal population. J. Med. Sci. 2007, 7, 1049–1053. [Google Scholar]
- Gavella, M.; Lipovac, V.; Car, A.; Vucić, M.; Sokolić, L.; Rakos, R. Serum sialic acid in subjects with impaired glucose tolerance and in newly diagnosed type 2 diabetic patients. Acta Diabetol. 2003, 40, 95–100. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Liu, Y.; Xu, D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019, 18, 173. [Google Scholar] [CrossRef]
- Sato, C.; Hane, M.; Kitajima, K. Role of Polysialic Acid in Schizophrenia. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2021; pp. 276–286. [Google Scholar]
- Varma, R.; Hoshino, A.Y.; Vercellotti, J.R. Serum glycoproteins in schizophrenia. Carbohydr. Res. 1980, 82, 343–351. [Google Scholar] [CrossRef]
- Cirillo, P.; Sato, W.; Reungjui, S.; Heinig, M.; Gersch, M.; Sautin, Y.; Nakagawa, T.; Johnson, R.J. Uric acid, the metabolic syndrome, and renal disease. J. Am. Soc. Nephrol. 2006, 17, 165–168. [Google Scholar] [CrossRef]
- Maxwell, S.R.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M.A.; Thorpe, G.H.; Jones, A.F.; Barnett, A.H. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1997, 27, 484–490. [Google Scholar] [CrossRef]
- Matsuura, F.; Yamashita, S.; Nakamura, T.; Nishida, M.; Nozaki, S.; Funahashi, T.; Matsuzawa, Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998, 47, 929–933. [Google Scholar] [CrossRef]
- Hara, S.; Tsuji, H.; Ohmoto, Y.; Amakawa, K.; Hsieh, S.D.; Arase, Y.; Nakajima, H. High serum uric acid level and low urine pH as predictors of metabolic syndrome: A retrospective cohort study in a Japanese urban population. Metabolism 2012, 61, 281–288. [Google Scholar] [CrossRef]
- Soukup, M.; Biesiada, I.; Henderson, A.; Idowu, B.; Rodeback, D.; Ridpath, L.; Bridges, E.G.; Nazar, A.M.; Bridges, K.G. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol. Metab. Syndr. 2012, 4, 14. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol. 2006, 290, 625–631. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. High plasma uric acid concentration: Causes and consequences. Diabetol. Metab. Syndr. 2012, 4, 12. [Google Scholar] [CrossRef]
- Salehidoost, R.; Aminorroaya, A.; Zare, M.; Amini, M. Is uric acid an indicator of metabolic syndrome in the first-degree relatives of patients with type 2 diabetes? J. Res. Med. Sci. 2012, 17, 1005–1010. [Google Scholar]
- Yao, J.K.; Reddy, R.; van Kammen, D.P. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998, 80, 29–39. [Google Scholar] [CrossRef]
- He, Q.; You, Y.; Yu, L.; Yao, L.; Lu, H.; Zhou, X.; Wu, S.; Chen, L.; Chen, Y.; Zhao, X. Uric Acid Levels in Subjects with Schizophrenia: A Systematic Review and Meta-analysis. Psychiatry Res. 2020, 292, 113305. [Google Scholar] [CrossRef]
- Nakano, Y.; Tobe, T.; Choi-Miura, N.H. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 1996, 120, 803–812. [Google Scholar] [CrossRef]
- Betowski, J. Adiponectin and resistin—New hormones of white adipose tissue. Med. Sci. Monit. 2003, 9, 55–61. [Google Scholar]
- Choi, K.M.; Lee, J.; Lee, K.W.; Seo, J.A.; Oh, J.H.; Kim, S.G.; Kim, N.H.; Choi, D.S.; Baik, S.H. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin. Endocrinol. 2004, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.B.; Yoon, J.; Kim, J.Y.; Yoo, B.S.; Lee, S.H.; Park, J.K.; Choe, K.H. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei Med. J. 2011, 52, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Elshaari, F.A.; Alshaari, A.A.; Ali, S. Adiponectin/leptin ratio as a biomarker of acute metabolic stress. Int. J. Biol. Med. Res. 2013, 4, 3278–3283. [Google Scholar]
- Mi, J.; Munkonda, M.N.; Li, M.; Zhang, M.X.; Zhao, X.Y.; Fouejeu, P.C.; Cianflone, K. Adiponectin and leptin metabolic biomarkers in Chinese children and adolescents. J. Obes. 2010, 2010, 892081. [Google Scholar] [CrossRef]
- Choi, K.M.; Ryu, O.H.; Lee, K.W.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, D.S.; Baik, S.H. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res. Clin. Pract. 2007, 75, 235–240. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, S.V.; Yoon, J.H. Prospective study of serum adiponectin and incident metabolic syndrome. The ARIRANG study. Diabetes Care 2013, 36, 1547–1553. [Google Scholar] [CrossRef]
- Fujikawa, R.; Ito, C.; Tsuboi, A. Is the screening of metabolic syndrome using adiponectin possible? Diabetol. Int. 2015, 6, 313–320. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Kahn, C.R. Adiponectin: Linking the fat cell to insulin sensitivity. Lancet 2003, 362, 1431–1432. [Google Scholar] [CrossRef]
- Bartoli, F.; Lax, A.; Crocamo, C.; Clerici, M.; Carra, G. Plasma adiponectin levels in schizophrenia and role of second-generation antipsychotics: A meta-analysis. Psychoneuroendocrinology 2015, 56, 179–189. [Google Scholar] [CrossRef]
- Tay, Y.H.; Lee, J. The relationship between serum adiponectin levels, cardiometabolic indices and metabolic syndrome in schizophrenia. Asian J. Psychiatry 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Perez-Iglesias, R.; Vazquez-Barquero, J.L.; Amado, J.A.; Berja, A.; Garcia-Unzueta, M.T.; Pelayo-Terán, J.M.; Carrasco-Marín, E.; Mata, I.; Crespo-Facorro, B. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J. Clin. Psychopharmacol. 2008, 28, 289–295. [Google Scholar] [CrossRef]
- Wampers, M.; Hanssens, L.; van Winkel, R.; Heald, A.; Collette, J.; Peuskens, J.; Reginster, J.Y.; Scheen, A.; De Hert, M. Differential effects of olanzapine and risperidone on plasma adiponectin levels over time: Results from a 3-month prospective open-label study. Eur. Neuropsychopharmacol. 2012, 22, 17–26. [Google Scholar] [CrossRef]
- Lu, M.L.; Wang, T.N.; Lin, T.Y.; Shao, W.C.; Chang, S.H.; Chou, J.Y.; Ho, Y.F.; Liao, Y.T.; Chen, V.C. Differential effects of olanzapine and clozapine on plasma levels of adipocytokines and total ghrelin. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 58, 47–50. [Google Scholar] [CrossRef]
- Vidarsdottir, S.; Vlug, P.; Roelfsema, F.; Frolich, M.; Pijl, H. Orally disintegrating and oral standard olanzapine tablets similarly elevate the homeostasis model assessment of insulin resistance index and plasma triglyceride levels in 12 healthy men: A randomized crossover study. J. Clin. Psychiatry 2010, 71, 1205–1211. [Google Scholar] [CrossRef]
- Bai, Y.M.; Chen, J.Y.; Yang, W.S.; Chi, Y.C.; Liou, Y.J.; Lin, C.C.; Wang, Y.C.; Lin, C.Y.; Su, T.P.; Chou, P. Adiponectin as a potential biomarker for the metabolic syndrome in Chinese patients taking clozapine for schizophrenia. J. Clin. Psychiatry 2007, 68, 1834–1839. [Google Scholar] [CrossRef]
- Musani, S.K.; Vasan, R.S.; Bidulescu, A. Aldosterone, C-reactive protein, and plasma B-type natriuretic peptides are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components. Findings from the Jackson Heart Study. Diabetes Care 2013, 36, 3084–3092. [Google Scholar] [CrossRef]
- Kidambi, S.; Kotchen, J.M.; Grim, C.E.; Raff, H.; Mao, J.; Singh, R.J.; Kotchen, T.A. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 2007, 49, 704–711. [Google Scholar] [CrossRef]
- Barcelo, A.; Pierola, J.; Esquinas, C. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: Effect of continuous positive airway pressure treatment. PLoS ONE 2014, 9, 84362. [Google Scholar] [CrossRef]
- Jungmann, E.; Wächtler, M.; Schwedes, U.; Usadel, K.H.; Schöffling, K. The effect of metoclopramide and haloperidol on plasma renin activity and aldosterone levels in rats. Res. Exp. Med. 1983, 183, 133–138. [Google Scholar] [CrossRef]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef]
- Fatima, S.S.; Bozaoglu, K.; Rehman, R.; Alam, F.; Memon, A.S. Elevated chemerin levels in Pakistani men: An interrelation with metabolic syndrome phenotypes. PLoS ONE 2013, 8, 57113. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, G.Y.; Wang, X.Z.; Jia, J.; Di, L.L.; Yang, L.; Chen, X.; Qian, F.F.; Chen, J.J. Plasma chemerin level in metabolic syndrome. Genet. Mol. Res. 2013, 12, 5986–5991. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Li, S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: A meta-analysis. PLoS ONE 2014, 9, 113915. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, M.K.; Ahn, K.Y.; Im, J.A.; Park, M.S.; Lee, D.C.; Jeon, J.Y.; Lee, J.W. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE 2012, 7, 34710. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, K.K.; Hoo, R.L.; Siu, P.M.; Yau, S. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- Samy, D.M.; Mostafa, D.K.; Abdelmonsif, D.A.; Ismail, C.A.; Hassaan, P.S. Crosstalk of hypothalamic chemerin, histamine, and AMPK in diet-and olanzapine-induced obesity in rats. Life Sci. 2021, 284, 119897. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; Hermans, N. Biomarkers of Metabolic Syndrome: Biochemical Background and Clinical Significance. Metab. Syndr. Relat. Disord. 2016, 14, 47–93. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Arvat, E.; Benso, A.; Papotti, M.; Muccioli, G.; Deghenghi, R.; Ghigo, E. Ghrelin: Endocrine and non-endocrine actions. J. Pediatr. Endocrinol. Metab. 2002, 15, 1219–1227. [Google Scholar] [CrossRef]
- De Vriese, C.; Delporte, C. Ghrelin: A new peptide regulating growth hormone release and food intake. Int. J. Biochem. Cell Biol. 2008, 40, 1420–1424. [Google Scholar] [CrossRef]
- Muccioli, G.; Tschöp, M.; Papotti, M.; Deghenghi, R.; Heiman, M.; Ghigo, E. Neuroendocrine and peripheral activities of ghrelin: Implications in metabolism and obesity. Eur. J. Pharmacol. 2002, 440, 235–254. [Google Scholar] [CrossRef]
- Becker, A.E.; Grinspoon, S.K.; Klibanski, A.; Herzog, D.B. Eating disorders. N. Engl. J. Med. 1999, 340, 1092–1098. [Google Scholar]
- Mokhort, T. Ghrelin basal levels in metabolic syndrome. Endocr. Abstr. 2007, 14, 230. [Google Scholar]
- Ukkola, O. Ghrelin and metabolic disorders. Curr. Protein Pept. Sci. 2009, 10, 2–7. [Google Scholar] [CrossRef]
- Chedraui, P.; Perez-Lopez, F.R.; Escobar, G.S. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas 2014, 79, 86–90. [Google Scholar] [CrossRef]
- Sentissi, O.; Epelbaum, J.; Olie, J.P.; Poirier, M.F. Leptin and Ghrelin Levels in Patients With Schizophrenia During Different Antipsychotics Treatment, A Re-view. Schizophr. Bull. 2008, 34, 1189–1199. [Google Scholar]
- Thevis, M.; Thomas, A.; Schänzer, W. Insulin. Handb. Exp. Pharmacol. 2010, 195, 209–226. [Google Scholar]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Ebenbichler, C.F.; Laimer, M.; Eder, U.; Mangweth, B.; Weiss, E.; Hofer, A.; Hummer, M.; Kemmler, G.; Lechleitner, M.; Patsch, J.R.; et al. Olanzapine induces insulin resistance: Results from a prospective study. J. Clin. Psychiatry 2003, 64, 1436–1439. [Google Scholar] [CrossRef]
- McDonagh, M.; Peterson, K.; Carson, S.; Fu, R.; Thakurta, S. Drug Class Review: Atypical Antipsychotic Drugs: Final Update 3 Report; Oregon Health & Science University: Portland, OR, USA, 2010. [Google Scholar]
- Hakami, A.Y.; Felemban, R.; Ahmad, R.G.; Al-Samadani, A.H.; Salamatullah, H.K.; Baljoon, J.M.; Alghamdi, L.J.; RamadaniSindi, M.H.; Ahmed, M.E. The Association Between Antipsychotics and Weight Gain and the Potential Role of Metformin Concomitant Use: A Retrospective Cohort Study. Front. Psychiatry 2022, 13, 914165. [Google Scholar] [CrossRef]
- Dobrodeeva, V.S.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Personalized approach to antipsychotic-induced weight gain prognosis. Pers. Psychiatry Neurol. 2021, 1, 3–10. [Google Scholar] [CrossRef]
- Howard, J.M.; Pidgeon, G.P.; Reynolds, J.V. Leptin and gastrointestinal malignancies. Obes. Rev. 2010, 11, 863–874. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Cohen, B.; Novick, D.; Rubinstein, M. Modulation of insulin activities by leptin. Science 1996, 274, 1185–1188. [Google Scholar] [CrossRef]
- Naveed, B.; Weiden, M.D.; Kwon, S.; Gracely, E.J.; Comfort, A.L.; Ferrier, N.; Kasturiarachchi, K.J.; Cohen, H.W.; Aldrich, T.K.; Rom, W.N.; et al. Metabolic syndrome biomarkers predict lung function impairment: A nested case-control study. Am. J. Respir. Crit. Care Med. 2012, 185, 392–399. [Google Scholar] [CrossRef]
- Chiu, F.H.; Chuang, C.H.; Li, W.C.; Weng, Y.M.; Fann, W.C.; Lo, H.Y.; Sun, C.; Wang, S.H. The association of leptin and C-reactive protein with the cardiovascular risk factors and metabolic syndrome score in Taiwanese adults. Cardiovasc. Diabetol. 2012, 11, 40. [Google Scholar] [CrossRef]
- Dobrodeeva, V.S.; Shnayder, N.A.; Novitsky, M.A.; Asadullin, A.R.; Vaiman, E.E.; Petrova, M.M.; Limankin, O.V.; Neznanov, N.G.; Garganeeva, N.P.; Nasyrova, R.F. Association of a Single-Nucleotide Variant rs11100494 of the NPY5R Gene with Antipsychotic-Induced Metabolic Disorders. Pharmaceutics 2022, 14, 222. [Google Scholar] [CrossRef]
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- De Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Pan, H.Y.; Guo, L.; Li, Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 88, 29–33. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Takahashi, R.; Terakura, Y.; Ohashi, K.; Ikeda, N.; Higuchi, A.; Terasaki, H.; Kihara, S.; Murohara, T. Omentin as a novel biomarker of metabolic risk factors. Diabetol. Metab. Syndr. 2012, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Castro, A.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity 2011, 19, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, O.; Canan, F.; Tosun, M.; Kayka, N.; Tuman, T.C.; Alhan, C.; Alcelik, A. Plasma Omentin Levels in Drug-Free Patients with Schizophrenia. Neuropsychobiology 2014, 69, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; von Mühlen, D.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care 2007, 30, 1549–1555. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar]
- George, J.A.; Norris, S.A.; van Deventer, H.E.; Crowther, N.J. The association of 25 hydroxyvitamin D and parathyroid hormone with metabolic syndrome in two ethnic groups in South Africa. PLoS ONE 2013, 8, 61282. [Google Scholar] [CrossRef]
- Cheng, S.P.; Liu, C.L.; Liu, T.P.; Hsu, Y.C.; Lee, J.J. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediat. Inflamm. 2014, 2014, 709024. [Google Scholar] [CrossRef]
- Saab, G.; Whaley-Connell, A.; Bombeck, A.; Kurella Tamura, M.; Li, S.; Chen, S.C.; McFarlane, S.I.; Sowers, J.R.; Norris, K.; Bakris, G.L.; et al. The association between parathyroid hormone levels and the cardiorenal metabolic syndrome in non-diabetic chronic kidney disease. Cardiorenal Med. 2011, 1, 123–130. [Google Scholar] [CrossRef]
- Lee, D.M.; Rutter, M.K.; O’Neill, T.W.; Boonen, S.; Vanderschueren, D.; Bouillon, R.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; Forti, G.; et al. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur. J. Endocrinol. 2009, 161, 947–954. [Google Scholar] [CrossRef]
- Milovanovic, D.R.; Stanojevic Pirkovic, M.; Zivancevic Simonovic, S.; Matovic, M.; Djukic Dejanovic, S.; Jankovic, S.M.; Ravanic, D.; Petronijevic, M.; Ignjatovic Ristic, D.; Mladenovic, V.; et al. Parameters of Calcium Metabolism Fluctuated during Initiation or Changing of Antipsychotic Drugs. Psychiatry Investig. 2016, 13, 89–101. [Google Scholar] [CrossRef]
- Gooren, L.; Meryn, S.; Shabsigh, R. Introduction: Testosterone and the metabolic syndrome. J. Mens Health 2008, 55, 2–6. [Google Scholar] [CrossRef]
- Stanworth, R.D.; Jones, T.H. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front. Horm. Res. 2009, 37, 74–90. [Google Scholar]
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Arver, S. Testosterone and the metabolic syndrome. J. Mens Health 2008, 5, 7–10. [Google Scholar] [CrossRef]
- Han, Y.; Ji, H.; Liu, L.; Zhu, Y.; Jiang, X. The Relationship of Functional Status of Cortisol, Testosterone, and Parameters of Metabolic Syndrome in Male Schizophrenics. BioMed Res. Int. 2020, 2020, 9124520. [Google Scholar] [CrossRef]
- Konarzewska, B.; Galińska-Skok, B.; Waszkiewicz, N.; Łazarczyk-Kirejczyk, J.; Malus, A.; Simonienko, K.; Szulc, A. Association between serum testosterone levels, body mass index (BMI) and insulin in male patients with schizophrenia treated with atypical antipsychotics--olanzapine or risperidone. Neuro. Endocrinol. Lett. 2014, 35, 50–57. [Google Scholar]
- Asvold, B.O.; Vatten, L.J.; Nilsen, T.I.; Bjøro, T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur. J. Endocrinol. 2007, 156, 181–186. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; Jørgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Heima, N.E.; Eekhoff, E.M.; Oosterwerff, M.M.; Lips, P.T.; van Schoor, N.M.; Simsek, S. Thyroid function and the metabolic syndrome in older persons: A population-based study. Eur. J. Endocrinol. 2012, 168, 59–65. [Google Scholar] [CrossRef]
- Saleem, M.S.; Shirwany, T.A.; Khan, K.A. Relationship of thyroid-stimulating hormone with metabolic syndrome in a sample of euthyroid Pakistani population. J. Ayub Med. Coll. Abbottabad 2011, 23, 63–68. [Google Scholar]
- Waring, A.C.; Rodondi, N.; Harrison, S.; Kanaya, A.M.; Simonsick, E.M.; Miljkovic, I.; Satterfield, S.; Newman, A.B.; Bauer, D.C.; Health, Ageing, and Body Composition (Health ABC) Study. Thyroid function and prevalent and incident metabolic syndrome in older adults: The Health, Ageing and Body Composition Study. Clin. Endocrinol. 2012, 76, 911–918. [Google Scholar] [CrossRef]
- Newcomer, J.W. Second-generation (atypical) antipsychotics and metabolic effects: A comprehensive literature review. CNS Drugs. 2005, 19, 1–93. [Google Scholar] [CrossRef]
- Scheen, A.J.; De Hert, M. Drug induced diabetes mellitus: The example of atypical antipsychotics. Rev. Med. Liege 2005, 60, 455–460. [Google Scholar] [PubMed]
- Kornetova, E.G.; Kornetov, A.N.; Mednova, I.A.; Lobacheva, O.A.; Gerasimova, V.I.; Dubrovskaya, V.V.; Tolmachev, I.V.; Semke, A.V.; Loonen, A.; Bokhan, N.A.; et al. Body Fat Parameters, Glucose and Lipid Pro-files, and Thyroid Hormone Levels in Schizophrenia Patients with or without Metabolic Syndrome. Diagnostics 2020, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Baldessarini, R.J.; Vitrani, G.; Bonfitto, I.; Cecere, A.C.; Rinaldi, A.; Bellomo, A. Metabolic Syndrome in Psychotic Disorder Patients Treated With Oral and Long-Acting Injected Antipsychotics. Front. Psychiatry 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.L.; Rigato, I.; Ostrow, J.D.; Bossi, F.; Bortoluzzi, A.; Sukowati, C.H.; Tedesco, F.; Tiribelli, C. Bilirubin inhibits the TNF alpha-related induction of three endothelial adhesion molecules. Biochem. Biophys. Res. Commun. 2009, 386, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, M.; Xu, M.; Bi, Y.; Li, X.; Chen, Y.; Ning, G.; Wang, W. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J. Diabetes 2011, 3, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, B.T.; Park, S.B.; Cho, D.Y.; Je, S.H.; Kim, K.N. Serum total bilirubin concentration is inversely correlated with Framingham risk score in Koreans. Arch. Med. Res. 2012, 43, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Deetman, P.E.; Bakker, S.J.L.; Dullaart, R.P.F. High sensitive C-reactive protein and serum amyloid A are inversely related to serum bilirubin: Effect-modification by metabolic syndrome. Cardiovasc. Diabetol. 2013, 12, 166. [Google Scholar] [CrossRef]
- Lin, L.Y.; Kuo, H.K.; Hwang, J.J.; Lai, L.P.; Chiang, F.T.; Tseng, C.D.; Lin, J.L. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis 2009, 203, 563–568. [Google Scholar] [CrossRef]
- Karadag, F.; Sengul, C.B.; En-li, Y.; Karakulah, K.; Alacam, H.; Kaptanoglu, B.; Kalkanci, O.; Herken, H. Relationship between Serum Bilirubin Levels and Metabolic Syndrome in Patients with Schizophrenia Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2017, 15, 153–162. [Google Scholar] [CrossRef]
- Jenko-Pražnikar, Z.; Petelin, A.; Jurdana, M.; Žiberna, L. Serum bilirubin levels are lower in over-weight asymptomatic middle-aged adults: An early indicator of metabolic syn-drome? Metabolism 2013, 62, 976–985. [Google Scholar] [CrossRef]
- Fruebis, J.; Tsao, T.S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Lopes-Virella, M.F.; Garvey, W.T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 2002, 165, 259–269. [Google Scholar] [CrossRef]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M. Adipocyte fatty acid binding protein in a Caucasian population: A new marker of metabolic syndrome? Eur. J. Clin. Investig. 2006, 36, 621–625. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Cabré, A.; Lázaro, I.; Cofán, M.; Jarauta, E.; Plana, N.; Garcia-Otín, A.L.; Ascaso, J.F.; Ferré, R.; Civeira, F.; Ros, E.; et al. FABP4 plasma levels are increased in familial combined hyperlipidemia. J. Lipid Res. 2010, 51, 1173–1178. [Google Scholar] [CrossRef]
- Milner, K.L.; van der Poorten, D.; Xu, A.; Bugianesi, E.; Kench, J.G.; Lam, K.S.; Chisholm, D.J.; George, J. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1926–1934. [Google Scholar] [CrossRef]
- Mankowska-Cyl, A.; Krintus, M.; Rajewski, P.; Sypniewska, G. A-FABP and its association with atherogenic risk profile and insulin resistance in young overweight and obese women. Biomark. Med. 2013, 7, 723–730. [Google Scholar] [CrossRef]
- Chow, W.S.; Tso, A.W.K.; Xu, A. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: A 12-year prospective study. J. Am. Heart Assoc. 2013, 2, 4176. [Google Scholar] [CrossRef]
- Baessler, A.; Lamounier-Zepter, V.; Fenk, S.; Strack, C.; Lahmann, C.; Loew, T.; Schmitz, G.; Blüher, M.; Bornstein, S.R.; Fischer, M. Adipocyte fatty acid-binding protein levels are associated with left ventricular diastolic dysfunction in morbidly obese subjects. Nutr. Diabetes 2014, 4, 106. [Google Scholar] [CrossRef]
- Xu, A.; Tso, A.W.; Cheung, B.M.; Wang, Y.; Wat, N.M.; Fong, C.H.; Yeung, D.C.; Janus, E.D.; Sham, P.C.; Lam, K.S. Circulating adipocytefatty acid binding protein levels predict the development of the metabolic syndrome: A 5-year prospective study. Circulation 2007, 115, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, T.N.; Yoo, H.J.; Lee, K.W.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin. Endocrinol. 2009, 70, 569–574. [Google Scholar] [CrossRef]
- Rendell, M. The expanding clinical use of C-peptide radioimmunoassay. Acta Diabetol. Latina 1983, 20, 105–113. [Google Scholar] [CrossRef]
- Banu, S.; Jabir, N.R.; Manjunath, C.N.; Shakil, S.; Kamal, M.A. C-peptide and its correlation to parameters of insulin resistance in the metabolic syndrome. CNS Neurol. Disord. Drug Targets 2011, 10, 921–927. [Google Scholar] [CrossRef]
- Shimajiri, Y.; Tsunoda, K.; Furuta, M.; Kadoya, Y.; Yamada, S.; Nanjo, K.; Sanke, T. Prevalence of metabolic syndrome in Japanese type 2 diabetic patients and its significance for chronic vascular complications. Diabetes Res. Clin. Pract. 2008, 79, 310–317. [Google Scholar] [CrossRef]
- Abdullah, A.; Hasan, H.; Raigangar, V.; Bani-Issa, W. C-Peptide versus insulin: Relationships with risk biomarkers of cardiovascular disease in metabolic syndrome in young arab females. Int. J. Endocrinol. 2012, 2012, 420792. [Google Scholar]
- Wu, R.R.; Zhao, J.P.; Liu, Z.N.; Zhai, J.G.; Guo, X.F.; Guo, W.B.; Tang, J.S. Effects of typical and atypical antipsychotics on glucose–insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology 2006, 186, 572–578. [Google Scholar] [CrossRef]
- Balõtšev, R.; Haring, L.; Koido, K.; Leping, V.; Kriisa, K.; Zilmer, M.; Vasar, V.; Piir, A.; Lang, A.; Vasar, E. Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: A 7-month follow-up study. Early Interv. Psychiatry 2019, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- El-Shahhat, N.; Ramadan, M.M.; El-Malkey, N. Soluble CD40 ligand, interleukin (IL)-6, and hemostatic parameters in metabolic syndrome patients with and without overt ischemic heart disease. Egypt Heart J. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M. Inflammation and atherothrombosis: From population biology and bench research to clinical practice. Am. J. Coll Cardiol. 2006, 48, 33–46. [Google Scholar] [CrossRef]
- Turk, V.; Bode, W. The cystatins: Protein inhibitors of cysteine proteinases. FEBS Lett. 1991, 285, 213–219. [Google Scholar] [CrossRef]
- Surendar, J.; Anuradha, S.; Ashley, B.; Balasubramanyam, M.; Aravindhan, V.; Rema, M.; Mohan, V. Cystatin C and cystatin glomerular filtration rate as markers of early renal disease in Asian Indian subjects with glucose intolerance (CURES-32). Metab. Syndr. Relat. Disord. 2009, 7, 419–425. [Google Scholar] [CrossRef]
- Chen, J.; Gu, D.; Chen, C.S.; Wu, X.; Hamm, L.L.; Muntner, P.; Batuman, V.; Lee, C.H.; Whelton, P.K.; He, J. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol. Dial. Transplant. 2007, 22, 1100–1106. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef]
- Asefy, Z.; Mirinejad, M.; Amirrasooli, H.; Tagikhani, M. Assessing validity of serum cystatin C for predicting metabolic syndrome. Pak. J. Biol. Sci. 2014, 17, 582–585. [Google Scholar] [CrossRef]
- Surendar, J.; Indulekha, K.; Aravindhan, V.; Ganesan, A.; Mohan, V. Association of cystatin-C with metabolic syndrome in normal glucose tolerant subjects (CURES-97). Diabetes Technol. Ther. 2010, 12, 907–912. [Google Scholar] [CrossRef]
- Liu, P.; Sui, S.; Xu, D.; Xing, X.; Liu, C. Clinical analysis of the relationship between cystatin C and metabolic syndrome in the elderly. Rev. Port. Cardiol. 2014, 33, 411–416. [Google Scholar] [CrossRef]
- Wang, G.N.; Sun, K.; Hu, D.L.; Wu, H.H.; Wang, X.Z.; Zhang, J.S. Serum cystatin C levels are associated with coronary artery disease and its severity. Clin. Biochem. 2014, 47, 176–181. [Google Scholar] [CrossRef]
- Andrews, N.C.; Levy, J.E. Iron is hot: An update on the pathophysiology of hemochromatosis. Blood 1998, 92, 1845–1851. [Google Scholar] [CrossRef]
- Martinelli, N.; Traglia, M.; Campostrini, N.; Biino, G.; Corbella, M.; Sala, C.; Busti, F.; Masciullo, C.; Manna, D.; Previtali, S.; et al. Increased serum hepcidin levels in subjects with the metabolic syndrome: A population study. PLoS ONE 2012, 7, 48250. [Google Scholar] [CrossRef]
- Mateo-Gallego, R.; Calmarza, P.; Jarauta, E. Serum ferritin is a major determinant of lipid phenotype in familiar combined hyperlipidemia and familial hypertriglyceridemia. Metabolism 2010, 59, 154–158. [Google Scholar] [CrossRef]
- Bouvier, M.L.; Fehsel, K.; Schmitt, A.; Meisenzahl-Lechner, E.; Gaebel, W.; von Wilmsdorff, M. Sex-dependent effects of long-term clozapine or haloperidol medication on red blood cells and liver iron metabolism in Sprague Dawley rats as a model of metabolic syndrome. BMC Pharm. Toxicol 2022, 23, 8. [Google Scholar] [CrossRef]
- Calarge, C.A.; Murry, D.J.; Ziegler, E.E.; Arnold, L.E.; Ziegler, L. Eugene Arnold. J. Child. Adolesc. Psychopharmacol. 2016, 26, 471–477. [Google Scholar] [CrossRef]
- Palomo, I.G.; Gutiérrez, C.L.; Alarcón, M.L.; Jaramillo, J.C.; Segovia, F.M.; Leiva, E.M.; Mujica, V.E.; Icaza, G.N.; Díaz, N.S.; Moore-Carrasco, R. Increased concentration of plasminogen activator inhibitor-1 and fibrinogen in individuals with metabolic syndrome. Mol. Med. Rep. 2009, 2, 253–257. [Google Scholar] [CrossRef]
- Imperatore, G.; Riccardi, G.; Iovine, C.; Rivellese, A.A.; Vaccaro, O. Plasma fibrinogen: A new factor of the metabolic syndrome. A population-based study. Diabetes Care 1998, 21, 649–654. [Google Scholar] [CrossRef]
- Válek, J.; Válková, L.; Vlasáková, Z.; Topinka, V. Increased fibrinogen levels in the offspring of hypertensive men. Relation with hyperinsulinemia and the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2229–2233. [Google Scholar] [CrossRef]
- Rahamon, S.K.; Akinlade, K.S.; Arinola, O.G.; Kakako, S.L.; Lasebikan, V.O. Impact of type and duration of use of antipsychotic drugs on plasma levels of selected acute-phase proteins in patients with major mental illnesses. Biomed Res. J. 2020, 7, 12–16. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Novotny, D.; Vaverkova, H.; Karasek, D.; Lukes, J.; Slavik, L.; Malina, P.; Orsag, J. Evaluation of total adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in individuals with metabolic syndrome. Physiol. Res. 2014, 63, 219–228. [Google Scholar] [CrossRef]
- Reinehr, T.; Woelfle, J.; Wunsch, R.; Roth, C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2143–2150. [Google Scholar] [CrossRef]
- Chow, W.S.; Xu, A.; Woo, Y.C.; Tso, A.W.; Cheung, S.C.; Fong, C.H.; Tse, H.F.; Chau, M.T.; Cheung, B.M.; Lam, K.S. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2454–2459. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, A.F.; Bakker, S.C.; Kahn, R.S. Fibroblast growth factors in schizophrenia. Schizophr. Bull. 2010, 36, 1157–1166. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Chensue, S.W.; Kunkel, S.L.; Farese, R.V., Jr.; Broxmeyer, H.E.; Charo, I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 1997, 100, 2552–2561. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Loughrey, B.V.; McGinty, A.; Young, I.S.; McCance, D.R.; Powell, L.A. Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low-dose atorvastatin treatment: Evidence from a randomized controlled trial. Clin. Endocrinol. 2013, 79, 800–806. [Google Scholar] [CrossRef]
- Ma, J.; Yan, L.; Guo, T.; Yang, S.; Ni, D.; Liu, Y.; Wang, J. A Pilot Study of Biomarkers of Oxidative Stress in Serum and Schizophrenia. Psychiatry Res. 2020, 284, 112757. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.M.; Madsen, J.B.; Kristensen, T.; Bodker, J.S.; Blouse, G.E.; Wind, T.; Andreasen, P.A. Biochemical properties of plasminogen activator inhibitor-1. Front. Biosci. 2009, 14, 1337–1361. [Google Scholar] [CrossRef] [PubMed]
- Tjärnlund-Wolf, A.; Brogren, H.; Lo, E.H.; Wang, X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke 2012, 43, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, A.K.; Jain, A.; Goswami, B. Imbalance between protective (adiponectin) and prothrombotic (plasminogen activator inhibitor-1) adipokines in metabolic syndrome. Diabetes Metab. Syndr. 2014, 8, 152–155. [Google Scholar] [CrossRef]
- Al-Hamodi, Z.; Ismail, I.S.; Saif-Ali, R.; Ahmed, K.A.; Muniandy, S. Association of plasminogen activator inhibitor-1 and tissue plasminogen activator with type 2 diabetes and metabolic syndrome in Malaysian subjects. Cardiovasc. Diabetol. 2011, 10, 23. [Google Scholar] [CrossRef]
- Devaraj, S.; Xu, D.Y.; Jialal, I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells. Implications for the metabolic syndrome and atherothrombosis. Circulation 2003, 107, 398–404. [Google Scholar] [CrossRef]
- Esmat, S.; Abd Al Salam, R.F.; Rashed, L. Effect of exercise on plasminogen activator inhibitor-1 (PAI-1) level in patients with metabolic syndrome. J. Am. Sci. 2010, 6, 1374–1380. [Google Scholar]
- Folsom, A.R.; Qamhieh, H.T.; Wing, R.R.; Jeffery, R.W.; Stinson, V.L.; Kuller, L.H.; Wu, K.K. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler. Thromb. 1993, 13, 162–169. [Google Scholar] [CrossRef]
- Herman, M.A.; Kahn, B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Investig. 2006, 116, 1767–1775. [Google Scholar] [CrossRef]
- Qi, Q.; Yu, Z.; Ye, X.; Zhao, F.; Huang, P.; Hu, F.B.; Franco, O.H.; Wang, J.; Li, H.; Liu, Y.; et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J. Clin. Endocrinol. Metab. 2007, 92, 4827–4834. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Li, D. Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol. Metab. Syndr. 2014, 6, 25–32. [Google Scholar] [CrossRef]
- Lee, J.W.; Im, J.A.; Lee, H.R.; Shim, J.Y.; Youn, B.S.; Lee, D.C. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity 2007, 15, 2225–2232. [Google Scholar] [CrossRef]
- Boyraz, M.; Cekmez, F.; Karaoğlu, A.; Cinaz, P.; Durak, M.; Bideci, A. Relationship of adipokines (adiponectin, resistin and RBP4) with metabolic syndrome components in pubertal obese children. Biomark. Med. 2013, 7, 423–428. [Google Scholar] [CrossRef]
- Tschoner, A.; Sturm, W.; Engl, J.; Kaser, S.; Laimer, M.; Laimer, E.; Weiss, H.; Patsch, J.R.; Ebenbichler, C.F. Retinol-binding protein 4, visceral fat, and the metabolic syndrome: Effects of weight loss. Obesity 2008, 16, 2439–2444. [Google Scholar] [CrossRef]
- Arner, E.; Rydén, M.; Arner, P. Tumor necrosis factor alpha and regulation of adipose tissue. N. Engl. J. Med. 2010, 362, 1151–1153. [Google Scholar] [CrossRef]
- Beumer, W.; Drexhage, R.C.; De Wit, H. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology 2012, 37, 1901–1911. [Google Scholar] [CrossRef]
- Paredes, R.M.; Quinones, M.; Marballi, K.; Gao, X.; Valdez, C.; Ahuja, S.S.; Walss-Bass, C. Metabolomic profiling of schizophrenia patients at risk for metabolic syndrome. Int. J. Neuropsychopharmacol. 2014, 17, 1139–1148. [Google Scholar] [CrossRef]
- Ma, R.C.W.; Kong, A.P.S.; Chan, N.; Tong, P.C.Y.; Chan, J.C.N. Drug-Induced Endocrine and Metabolic Disorders. Drug Saf. 2007, 30, 215–245. [Google Scholar] [CrossRef]
- Bernal-Lopez, M.R.; Garrido-Sanchez, L.; Gomez-Carrillo, V.; Gallego-Perales, J.L.; Llorente-Cortes, V.; Calleja, F.; Gomez-Huelgas, R.; Badimon, L.; Tinahones, F.J. Antioxidized LDL antibodies are associated with different metabolic pathways in patients with atherosclerotic plaque and type 2 diabetes. Diabetes Care 2013, 36, 1006–1011. [Google Scholar] [CrossRef]
- Knopp, R.H.; Paramsothy, P. Oxidized LDL and abdominal obesity: A key to understanding the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1–2. [Google Scholar] [CrossRef]
- Leiva, E.; Mujica, V.; Sepúlveda, P.; Guzmán, L.; Núñez, S.; Orrego, R.; Palomo, I.; Andrews, M.; Arredondo, M.A. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol. Trace Elem. Res. 2013, 151, 1–8. [Google Scholar] [CrossRef]
- Andarzi, S.; Hajinejad, S.; Miri, H.R. Correlation of Metabolic Syndrome with IL-27 in the Patients with Schizophrenia. J. Nutr. Fasting Health 2019, 7, 1–10. [Google Scholar]
- Yin, Q.; Chen, X.; Li, L.; Zhou, R.; Huang, J.; Yang, D. Apolipoprotein B/apolipoprotein A1 ratio is a good predictive marker of metabolic syndrome and pre-metabolic syndrome in Chinese adolescent women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2013, 39, 203–209. [Google Scholar] [CrossRef]
- Huang, F.; Yang, Z.; Xu, B.; Bi, Y.; Xu, M.; Xu, Y.; Lu, J.; Liu, Y.; Dai, M.; Zhou, W.; et al. Both serum apolipoprotein B and the apolipoprotein B/apolipoprotein A-I ratio are associated with carotid intima-media thickness. PLoS ONE 2013, 8, 54628. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Boiko, A.S.; Mednova, I.A.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Apolipoprotein serum levels related to metabolic syndrome in patients with schizophrenia. Heliyon 2019, 5, 02033. [Google Scholar] [CrossRef]
- Scott, J.; Wallis, S.C.; Pease, R.J.; Knott, T.J.; Powell, L. Apolipoprotein B: A novel mechanism for deriving two proteins from one gene. Agents Actions Suppl. 1988, 26, 27–51. [Google Scholar]
- Shapiro, M.D.; Fazio, S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Research 2017, 6, 134. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef]
- Norman, A.W.; Henry, H.L. Chapter 10: Adrenal corticoids. In Hormones, 3rd ed.; Norman, A.W., Henry, H.L., Eds.; Academic Press: Cambridge, UK, 2015; pp. 223–238. [Google Scholar]
- Bermúdez, V.; Rojas, J.; Salazar, J.; Bello, L.; Añez, R.; Toledo, A.; Chacín, M.; Aguirre, M.; Villalobos, M.; Chávez, M.; et al. Variations of lipoprotein(a) levels in the metabolic syndrome: A report from the Maracaibo City Metabolic Syndrome Prevalence Study. J. Diabetes Res. 2013, 2013, 416451. [Google Scholar] [CrossRef] [PubMed]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and I kappa B-alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sjögren, P.; Ärnlöv, J.; Cederholm, T.; Lind, L.; Stenvinkel, P.; Lindholm, B.; Risérus, U.; Carrero, J.J. Serum fatty acid patterns, insulin sensitivity and the metabolic syndrome in individuals with chronic kidney disease. J. Intern. Med. 2014, 275, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.L.; Chao, C.L.; Kuo, C.H.; Lin, H.J.; Liu, P.H.; Chen, P.R.; Hsu, H.C.; Lee, B.C.; Lee, Y.T.; Chen, M.F. Plasma fatty acids and the risk of metabolic syndrome in ethnic Chinese adults in Taiwan. Lipids Health Dis. 2011, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Aro, A. Fatty acid composition of serum lipids: Is this marker of fat intake still relevant for identifying metabolic and cardiovascular disorders? Nutr. Metab. Cardiovasc. Dis. 2003, 13, 253–255. [Google Scholar] [CrossRef]
- Vessby, B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr. Opin. Lipidol. 2003, 14, 15–19. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, C.M.; Ha, E.; Park, S.; Jung, J.; Ryoo, J.H. Association between metabolic syndrome and incidence of cholelithiasis in the Korean population. J. Gastroenterol. Hepatol. 2021, 36, 3524–3531. [Google Scholar] [CrossRef]
- Wang, C.J.; Zhang, Z.J.; Sun, J.; Zhang, X.B.; Mou, X.D.; Zhang, X.R.; Shang, X.F.; Zhang, T.Q. Serum Free Fatty Acids and Glucose Metabolism, Insulin Resistance in Schizophrenia with Chronic Antipsychotics. Biol. Psychiatry 2006, 60, 1309–1313. [Google Scholar] [CrossRef]
- Xu, H.; Zhuang, X. Atypical antipsychotics-induced metabolic syndrome and nonalcoholic fatty liver disease: A critical review. Neuropsychiatr. Dis. Treat. 2019, 15, 2087–2099. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Feoli, A.M.P.; Macagnan, F.E.; Piovesan, C.H. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: Effects of a single exercise session. Oxidative Med. Cell. Longev. 2014, 2014, 587083. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, D.F.; Qi, L.Y.; Chen, S.; Cao, L.Y.; Chen, D.C.; Xiu, M.H.; Wang, F.; Wu, G.Y.; Lu, L.; et al. Superoxide dismutase and cytokines in chronic patients with schizophrenia: Association with psychopathology and response to antipsychotics. Psychopharmacology 2009, 204, 177–184. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, D.F.; Cao, L.Y.; Zhang, P.Y.; Wu, G.Y.; Shen, Y.C. The effect of risperidone treatment on superoxide dismutase in schizophrenia. J. Clin. Psychopharmacol. 2003, 23, 128–131. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Gamma-glutamyl transferase and cardiovascular disease. Ann. Transl. Med. 2016, 4, 481. [Google Scholar] [CrossRef]
- Lee, D.S.; Evans, J.C.; Robins, S.J. Atherosclerosis and lipoproteins. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk. The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 127–133. [Google Scholar] [CrossRef]
- Lüdtke, A.; Genschel, J.; Brabant, G.; Bauditz, J.; Taupitz, M.; Koch, M.; Wermke, W.; Worman, H.J.; Schmidt, H.H. Hepatic steatosis in Dunnigan-type familial partial lipodystrophy. Am. J. Gastroenterol. 2005, 100, 2218–2224. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Onat, A.; Hergenç, G.; Karabulut, A.; Türkmen, S.; Doğan, Y.; Uyarel, H.; Can, G.; Sansoy, V. Serum gamma glutamyltransferase as a marker of metabolic syndrome and coronary disease likelihood in nondiabetic middleaged and elderly adults. Prev. Med. 2006, 43, 136–139. [Google Scholar] [CrossRef]
- Stranges, S.; Trevisan, M.; Dorn, J.M.; Dmochowski, J.; Donahue, R.P. Body fat distribution, liver enzymes, and risk of hypertension: Evidence from the Western New York Study. Hypertension 2005, 46, 1186–1193. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Whincup, P.H. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care 2005, 28, 2913–2918. [Google Scholar] [CrossRef]
- Nakagawa, H.; Isogawa, A.; Tateishi, R.; Tani, M.; Yoshida, H.; Yamakado, M.; Koike, K. Serum gamma-glutamyltransferase level is associated with serum superoxide dismutase activity and metabolic syndrome in a Japanese population. J. Gastroenterol. 2012, 47, 187–194. [Google Scholar] [CrossRef]
- Atasoy, N.; Erdogan, A.; Yalug, I.; Ozturk, U.; Konuk, N.; Atik, L.; Ustundag, Y. A review of liver function tests during treatment with atypical antipsychotic drugs: A chart review study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1255–1260. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, A.Y.; Choi, J.W.; Kim, M.; Yasue, S.; Son, H.J.; Masuzaki, H.; Park, K.S.; Kim, J.B. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008, 22, 2176–2189. [Google Scholar] [CrossRef]
- Vidović, B.; Dorđević, B.; Milovanović, S.; Škrivanj, S.; Pavlović, Z.; Stefanović, A.; Kotur-Stevuljević, J. Selenium, zinc, and copper plasma levels in patients with schizophrenia: Relationship with metabolic risk factors. Biol. Trace Elem. Res. 2013, 156, 22–28. [Google Scholar] [CrossRef]
- Arnaud, J.; de Lorgeril, M.; Akbaraly, T.; Salen, P.; Arnout, J.; Cappuccio, F.P.; van Dongen, M.C.; Donati, M.B.; Krogh, V.; Siani, A.; et al. Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome European population—The IMMIDIET study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 517–524. [Google Scholar] [CrossRef]
- Pizent, A.; Pavlovic, M.; Jurasovic, J.; Dodig, S.; Pasalic, D.; Mujagic, R. Antioxidants, trace elements and metabolic syndrome in elderly subjects. J. Nutr. Health Aging 2010, 14, 866–871. [Google Scholar] [CrossRef]
- Harrington, J.R. The role of MCP-1 in atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. Effects of antipsychotics on antioxidant defense system in patients with schizophrenia: A meta-analysis. Psychiatry Res. 2022, 309, 114429. [Google Scholar] [CrossRef]
- Persson, M.; Nilsson, J.A.; Nelson, J.J.; Hedblad, B.; Berglund, G. The epidemiology of Lp-PLA (2): Distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis 2007, 190, 388–396. [Google Scholar] [CrossRef]
- Tsimikas, S.; Willeit, J.; Knoflach, M. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: Results from the Bruneck study. Eur. Heart J. 2009, 30, 107–115. [Google Scholar] [CrossRef]
- Gong, H.P.; Du, Y.M.; Zhong, L.N.; Dong, Z.Q.; Wang, X.; Mao, Y.J.; Lu, Q.H. Plasma lipoprotein associated phospholipase A2 in patients with metabolic syndrome and carotid atherosclerosis. Lipids Health Dis. 2011, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E.; Schwarz, M.; Myint, A.M. The metabolic syndrome in schizophrenia: Is inflammation a contributing cause? J. Psychopharmacol. 2012, 26, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Valipour, G.; Saneei, P.; Esmaillzadeh, A. Serum vitamin D levels in relation to schizophrenia: A systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2014, 99, 3863–3872. [Google Scholar] [CrossRef] [PubMed]
- Bruins, J.; Jörg, F.; van den Heuvel, E.R.; Bartels-Velthuis, A.A.; Corpeleijn, E.; Muskiet, F.A.J.; Pijnenborg, G.H.M.; Bruggeman, R. The relation of vitamin D, metabolic risk and negative symptom severity in people with psychotic disorders. Schizophr. Res. 2018, 195, 513–518. [Google Scholar] [CrossRef]
- Sempértegui, F.; Estrella, B.; Tucker, K.L.; Hamer, D.H.; Narvaez, X.; Sempértegui, M.; Griffiths, J.K.; Noel, S.E.; Dallal, G.E.; Selhub, J.; et al. Metabolic syndrome in the elderly living in marginal peri-urban communities in Quito, Ecuador. Public Health Nutr. 2011, 14, 758–767. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Ma, A.; Li, Y.; Han, X.; Liang, H. Effects of vitamin E on plasma lipid status and oxidative stress in Chinese women with metabolic syndrome. Int. J. Vitam. Nutr. Res. 2010, 80, 178–187. [Google Scholar] [CrossRef]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1345–1370. [Google Scholar] [CrossRef]
- Bruzzone, C.; Gil-Redondo, R.; Seco, M.; Barragán, R.; de la Cruz, L.; Cannet, C.; Millet, O. A molecular signature for the metabolic syndrome by urine metabolomics. Cardiovasc. Diabetol. 2021, 20, 155. [Google Scholar] [CrossRef]
- Cohn, T.A.; Sernyak, M.J. Metabolic Monitoring for Patients Treated with Antipsychotic Medications. Can. J. Psychiatry 2006, 51, 492–501. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Maltitol: Analytical determination methods, applications in the food industry, metabolism and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 5227. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Echeta, K.C. Current developments in sugar alcohols: Chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int. J. Adv. Acad. Res. 2019, 5, 1–33. [Google Scholar]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut microbial metabolites of aromatic amino acids as signals in host–microbe interplay. Trends Endocrinol. Metab. 2020, 31, 818–834. [Google Scholar] [CrossRef]
- Peddinti, G.; Cobb, J.; Yengo, L.; Froguel, P.; Kravić, J.; Balkau, B. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia 2017, 60, 1740–1750. [Google Scholar] [CrossRef]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.-C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Oh, J.S.; Seo, H.S.; Kim, K.H.; Pyo, H.; Chung, B.C.; Lee, J. Urinary profiling of tryptophan and its related metabolites in patients with metabolic syndrome by liquid chromatography-electrospray ionization/mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5501–5512. [Google Scholar] [CrossRef]
- Schepers, E.; Glorieux, G.; Vanholder, R. The gut: The forgotten organ in uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef]
- Meijers, B.K.; Claes, K.; Bammens, B.; de Loor, H.; Viaene, L.; Verbeke, K.; Kuypers, D.; Vanrenterghem, Y.; Evenepoel, P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1182–1189. [Google Scholar] [CrossRef]
- James, T.; Collins, S.; Amlôt, R.; Marczylo, T. Analysis of chemical simulants in urine: A useful tool for assessing emergency decontamination efficacy in human volunteer studies. Prehospital Disaster Med. 2020, 35, 482–487. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, W.; Lü, C.; Huang, J.; Hu, S.; Yao, S.; Mei, J. Biosynthesis of 4-hydroxyphenylpyruvic acid from l-tyrosine using recombinant Escherichia coli cells expressing membrane bound l-amino acid deaminase. Chin. J. Chem. Eng. 2018, 26, 380–385. [Google Scholar] [CrossRef]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2014, 14, 765–798. [Google Scholar] [CrossRef]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef]
- Savransky, A.; Chiappelli, J.; Du, X.; Carino, K.; Kvarta, M.; Bruce, H.; Kochunov, P.; Goldwaser, E.; Tan, Y.; Hare, S.; et al. Association of working memory and elevated overnight urinary norepinephrine in patients with schizophrenia. J. Psychiatr. Res. 2021, 137, 89–95. [Google Scholar] [CrossRef]
- Lee, Z.S.; Critchley, J.A.; Tomlinson, B.; Young, R.P.; Thomas, G.N.; Cockram, C.S.; Chan, Y.K.; Chan, J.C. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Miscellaneous 2001, 50, 135–143. [Google Scholar] [CrossRef]
- Wu, J.; Tomsa, D.; Zhang, M.; Komenda, P.; Tangri, N.; Rigatto, C.; Lin, F. A Passive Mixing Microfluidic Urinary Albumin Chip for Chronic Kidney Disease Assessment. ACS Sens. 2018, 3, 2191–2197. [Google Scholar] [CrossRef]
- Brantsma, A.H.; Bakker, S.J.L.; Hillege, H.L.; de Zeeuw, D.; de Jong, P.E.; Gansevoort, R.T. Urinary Albumin Excretion and Its Relation With C-Reactive Protein and the Metabolic Syndrome in the Prediction of Type 2 Diabetes. Diabetes Care 2005, 28, 2525–2530. [Google Scholar] [CrossRef]
- Saadi, M.M.; Roy, M.N.; Haque, R.; Tania, F.A.; Mahmood, S.; Ali, N. Association of microalbuminuria with metabolic syndrome: A cross-sectional study in Bangladesh. BMC Endocr. Disord. 2020, 20, 153. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ntzouvani, A.; Nomikos, T.; Panagiotakos, D.; Fragopoulou, E.; Pitsavos, C.; McCann, A.; Ueland, P.M.; Antonopoulou, S. Amino acid profile and metabolic syndrome in a male Mediterranean population: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shu, X.; Rivera, E.S.; Zhang, X.; Cai, Q.; Calcutt, M.W.; Xiang, Y.B.; Li, H.; Gao, Y.T.; Wang, T.J.; et al. Urinary Levels of Trimethylamine-N-Oxide and Incident Coronary Heart Disease: A Prospective Investigation Among Urban Chinese Adults. J. Am. Heart Assoc. 2019, 8, e010606. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Zong, G.; Seely, E.W.; Weisskopf, M.; James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ. Int. 2018, 121, 349–356. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, Y. Association of blood cadmium with hypertension in the Korean general population: Analysis of the 2008–2010 Korean national health and nutrition examination survey data. Am. J. Ind. Med. 2012, 55, 1060–1067. [Google Scholar] [CrossRef]
- Gallagher, C.M.; Meliker, J.R. Blood and Urine Cadmium, Blood Pressure, and Hypertension: A Systematic Review and Meta-analysis. Environ. Health Perspect. 2010, 118, 1676–1684. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, P.C.; Wu, C.; Sung, F.C.; Su, T.C. Association between urine lead levels and cardiovascular disease risk factors, carotid intima-media thickness and metabolic syndrome in adolescents and young adults. Int. J. Hyg. Environ. Health 2019, 223, 248–255. [Google Scholar] [CrossRef]
- Joy, A.; Qureshi, A. Mercury in Dental Amalgam, Online Retail, and the Minamata Convention on Mercury. Environ. Sci. Technol. 2020, 54, 14139–14142. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Popova, E.V.; Sinitskii, A.I.; Nemereshina, O.N.; Gatiatulina, E.R.; Nikonorov, A.A.; Skalny, A.V. Mercury and metabolic syndrome: A review of experimental and clinical observations. BioMetals 2015, 28, 231–254. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Cameron, M.A.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Low Urine pH: A Novel Feature of the Metabolic Syndrome. Clin. J. Am. Soc. Nephrol. 2007, 2, 883–888. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]


| Biomarker | Reference Values | Change in MetS | Symptom of MetS | References |
|---|---|---|---|---|
| Carbohydrates | ||||
| Glucose | >100 mg/dL | High | Insulin resistance | [32,36,39,40] |
| Acids | ||||
| Sialic acid | 2–2.33 mmol/L | High | CHD Acute inflammation | [41,43,48] |
| Uric acid | M 202.3–416.5 µmol/L, F 142.8–339.2 µmol/L | High | Obesity | [50,53,54,59] |
| Hormones | ||||
| Adiponectin | 0.6–1.33 g/L | Low | Insulin resistance | [64,71] |
| Aldosterone | 25–315 pg/mL | High | AH | [77] |
| Chemerin | N/A | High | BMI, CHD | [81,87] |
| Ghrelin | 0–100 ng/L | Low | Obesity, BMI | [89,90,91] |
| Insulin | 2.6–24.9 mcIU/mL | High | Insulin resistance | [97,98,101] |
| Leptin | M 2–5.6 ng/mL, F 3.7–11.1 ng/mL | High | Insulin resistance, leptin resistance | [103,109] |
| Omentin | N/A | Low | Obesity, endothelial dysfunction | [112,115] |
| Parathyroid hormone | 15–65 pg/mL | High | CVD | [116,118,119] |
| Testosterone | M 8.64–29 nmol/L (18–55 y.o.), F 0.29–1.67 (18– 55 y.o.) nmol/L | Low | Obesity | [123,127,128] |
| Thyroid stimulating hormone | 0.27–4.2 µIU/mL | High | CVD | [129,130,133] |
| Other organic compounds | ||||
| Bilirubin direct and total | 2.5–550 µmol/L | Low | Oxidative stress | [138,139,144] |
| Proteins | ||||
| Adipocyte fatty acid-binding protein | <6.2 ng/mL | High | Obesity, cardiometabolic disorders | [149,150,152] |
| C-peptide | 1.1–4.4 ng/mL | High | Insulin-related diseases | [159,160,161] |
| CD40 ligand | N/A | High | CHD | [164,166] |
| Cystatin C | 0.5–1.2 mg/L | High | AH | [168,171] |
| Ferritin | M 20–250 µg/L, F 10–120 µg/L | Controversial | Oxidative stress | [177,178] |
| Fibrinogen | 1.8–3.5 g/L | High | AH | [180,183] |
| Fibroblast Growth Factor 21 | M 3.6–1021.4 pg/mL, F 65.3–1209.8 pg/mL | High | Obesity, carotid atherosclerosis | [185,186,187,188] |
| Monocyte chemoattractant protein-1 | N/A | High | CHD | [191,192,193] |
| Plasminogen activator inhibitor-1 | N/A | High | CVD | [194,197,199] |
| Retinol-binding protein 4 | N/A | High | Waist-to-hip ratio, visceral fat areas | [201,202,203,206] |
| Tumor necrosis factor-a | <8.1 pg/mL | High | CHD | [207,208] |
| Lipids | ||||
| Oxidized low density lipoprotein | 26–117 IU/L | High | Oxidative stress, inflammation | [213] |
| Apolipoprotein A1 | M > 1.2 g/L, F 1.4 g/L | Low | Insulin resistance, dyslypidemia, obesity | [215,218] |
| Apolipoprotein B | 0.6–1.33 g/L | High | Insulin resistance, dyslypidemia, obesity | [220,221] |
| Free fatty acids | M 8.3–10.9 ng/mL, F 11.4–13.6 ng/mL | High | Insulin resistance | [224,231] |
| High density lipoprotein | 0.7–1.7 mmol/L | Low | Insulin resistance | [88] |
| Low-density lipoprotein cholesterol | <2.6 mmol/L | High | Dyslypidemia, obesity | [88] |
| Triglycerides | <1.7 mmol/L | High | Dyslypidemia, obesity | [88] |
| Enzymes | ||||
| Erythrocyte superoxide dismutase Er | 1200–2000 U/g | Low | Oxidativestress, inflammation | [232,233,235] |
| Gamma-glutamyl transferase | M 10–71 U/L, F 6–42 U/L | High | Oxidative stress, inflammation | [237,244] |
| Lipoprotein-associated phospholipase A | <200 ng/mL | High | CVD | [251] |
| Vitamins | ||||
| 25-Hydroxyvitamin D | 30–100 ng/mL | Low | CVD | [121,255,256] |
| Vitamin E | 5.00–18.00 µg/mL | Low | Oxidative stress | [258] |
| Biomarker | Reference Values | Change in MetS | Symptom of MetS | References |
|---|---|---|---|---|
| Carbohydrates | ||||
| Glucose | 0–0.8 mmol/L | High | Insulin resistance | [260] |
| Maltitol | None | High | Insulin resistance | [258] |
| Amino acids | ||||
| Aromatic amino acids | None | High | DM 2 | [265,291] |
| Histidine | 52–162 µmol/mmol | Low | AH | [260] |
| Tryptophan | 0.4–1.4 mg | High | CVD | [268] |
| Acids | ||||
| P-cresol sulfate | None | High | Insulin resistance | [260] |
| Salicyluric acid | None | High | Obesity | [260] |
| 4-hydroxyphenylpyruvic acid (4-HPPA) | None | High | Insulin resistance | [260] |
| Trigonelline | None | Low | Dyslypidemia, obesity | [260] |
| Hormones | ||||
| Epinephrine | 0–20 mcg/24-h | Low | Obesity | [276] |
| Norepinephrine | 15–80 mcg/24-h | High | Obesity | [276] |
| Other organic compounds | ||||
| Albumin | <30 mg/g | High | AH, DM 2 | [260,278] |
| Imidazole | None | Low | AH | [260] |
| Trimethylamine N-oxide (TMAO) | None | Low | Obesity | [283,291] |
| Metals | ||||
| Cadmium | 0.59–0.77 microgram/L | High | AH and low HDL | [284] |
| Lead | None | High | BMI, insulin resistance | [287] |
| Mercury | <10 mcg/L | High | Dyslipidemia | [289] |
| Other indicators | ||||
| pH | 4.6–8.0 | Low | Insulin resistance | [290] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khasanova, A.K.; Dobrodeeva, V.S.; Shnayder, N.A.; Petrova, M.M.; Pronina, E.A.; Bochanova, E.N.; Lareva, N.V.; Garganeeva, N.P.; Smirnova, D.A.; Nasyrova, R.F. Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome. Metabolites 2022, 12, 726. https://doi.org/10.3390/metabo12080726
Khasanova AK, Dobrodeeva VS, Shnayder NA, Petrova MM, Pronina EA, Bochanova EN, Lareva NV, Garganeeva NP, Smirnova DA, Nasyrova RF. Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome. Metabolites. 2022; 12(8):726. https://doi.org/10.3390/metabo12080726
Chicago/Turabian StyleKhasanova, Aiperi K., Vera S. Dobrodeeva, Natalia A. Shnayder, Marina M. Petrova, Elena A. Pronina, Elena N. Bochanova, Natalia V. Lareva, Natalia P. Garganeeva, Daria A. Smirnova, and Regina F. Nasyrova. 2022. "Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome" Metabolites 12, no. 8: 726. https://doi.org/10.3390/metabo12080726
APA StyleKhasanova, A. K., Dobrodeeva, V. S., Shnayder, N. A., Petrova, M. M., Pronina, E. A., Bochanova, E. N., Lareva, N. V., Garganeeva, N. P., Smirnova, D. A., & Nasyrova, R. F. (2022). Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome. Metabolites, 12(8), 726. https://doi.org/10.3390/metabo12080726








