α/β-Hydrolase Domain-Containing 6 (ABHD6)— A Multifunctional Lipid Hydrolase
Abstract
:1. Introduction
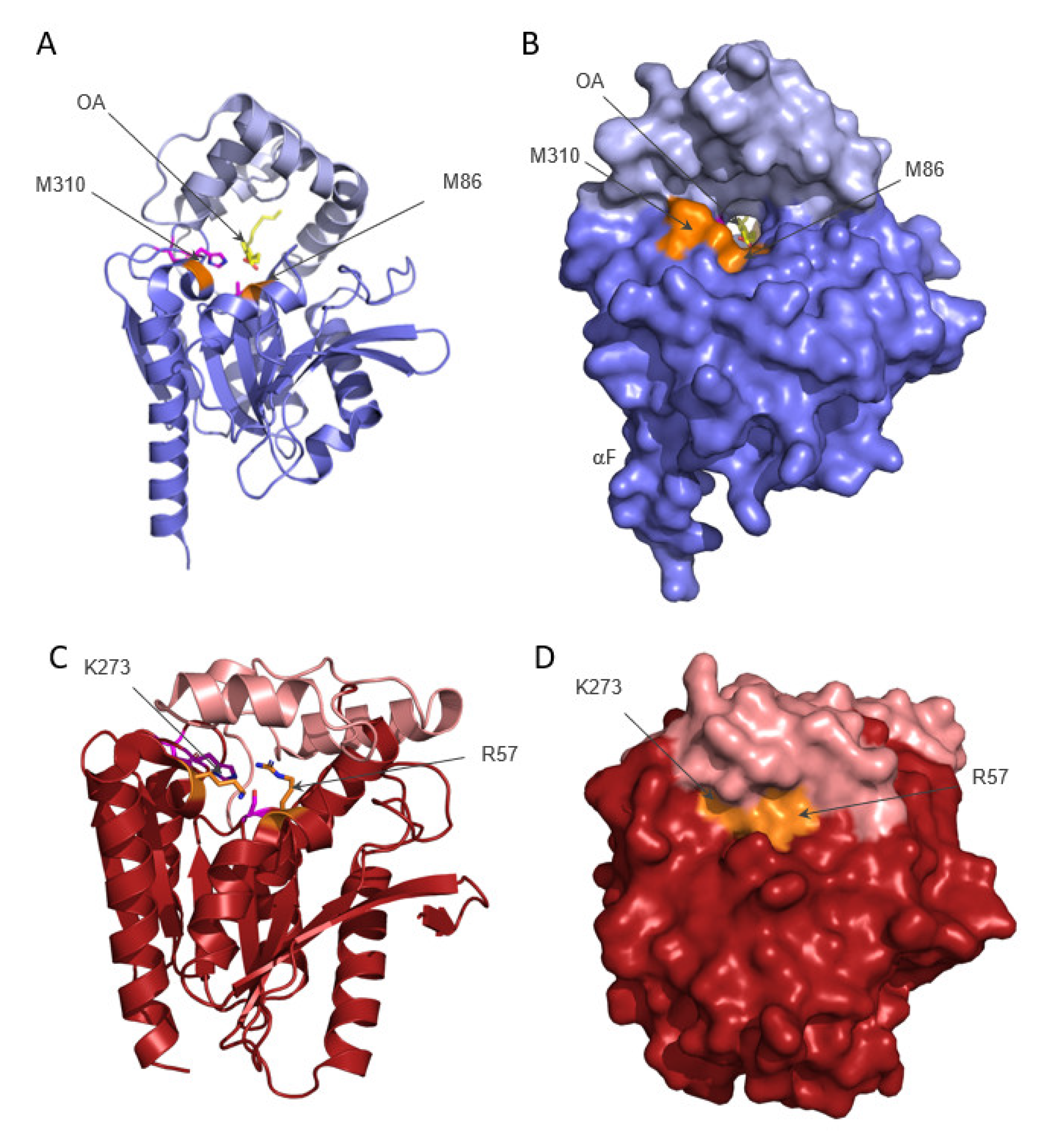
2. Structure of ABHD6
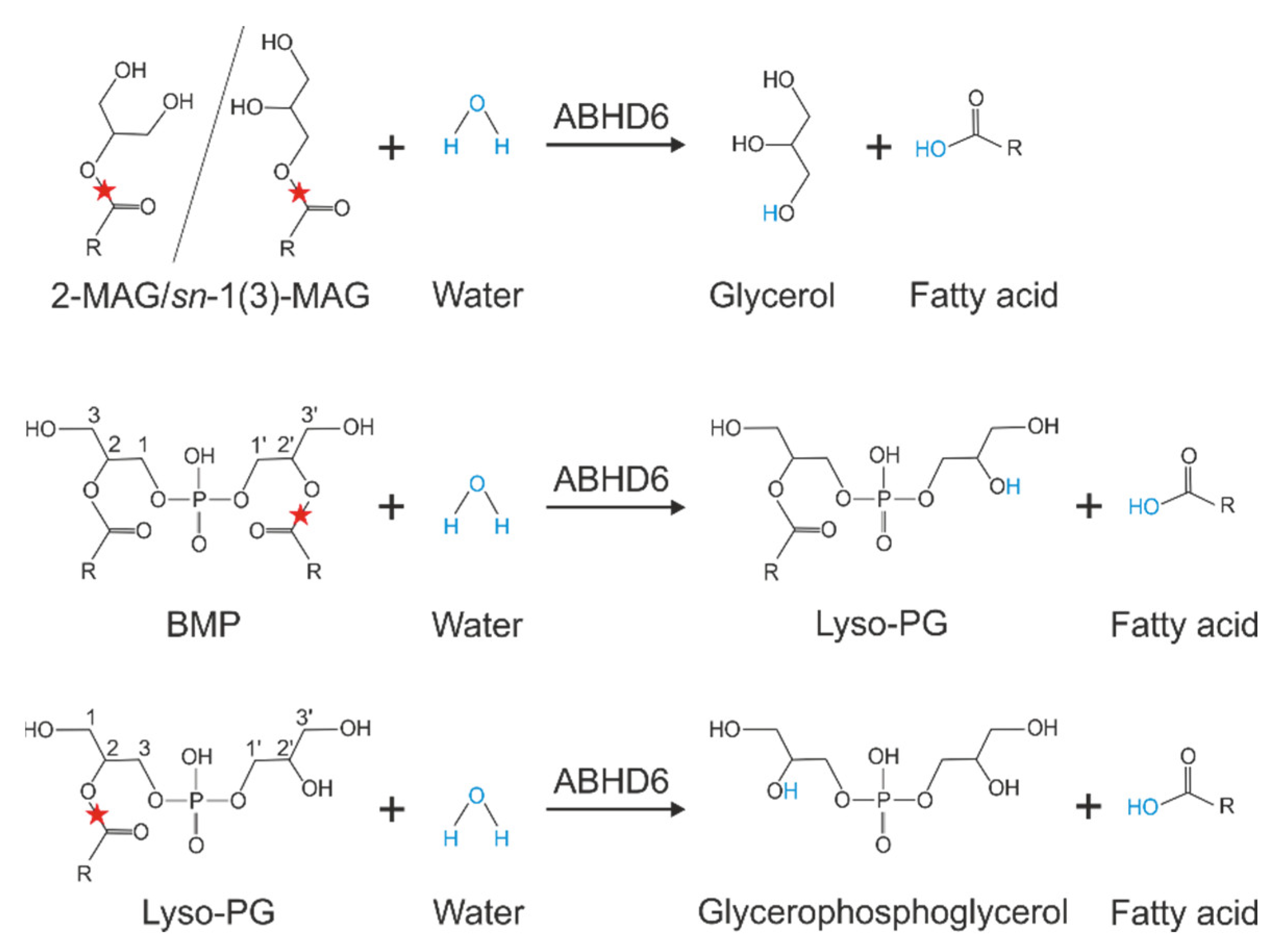
3. Substrate Specificity of ABHD6
4. Tools for the Investigation of ABHD6 Function
5. The Role of ABHD6 in Endocannabinoid Signaling
6. The Role of ABHD6 in Inflammation and Neurological Diseases
7. ABHD6 Controls Surface Delivery of AMPA-Type Glutamate Receptors
8. ABHD6 Affects Insulin Secretion
9. The Role of ABHD6 in BMP Metabolism
10. The Role of ABHD6 in Metabolic Syndrome
11. ABHD6 and Cancer
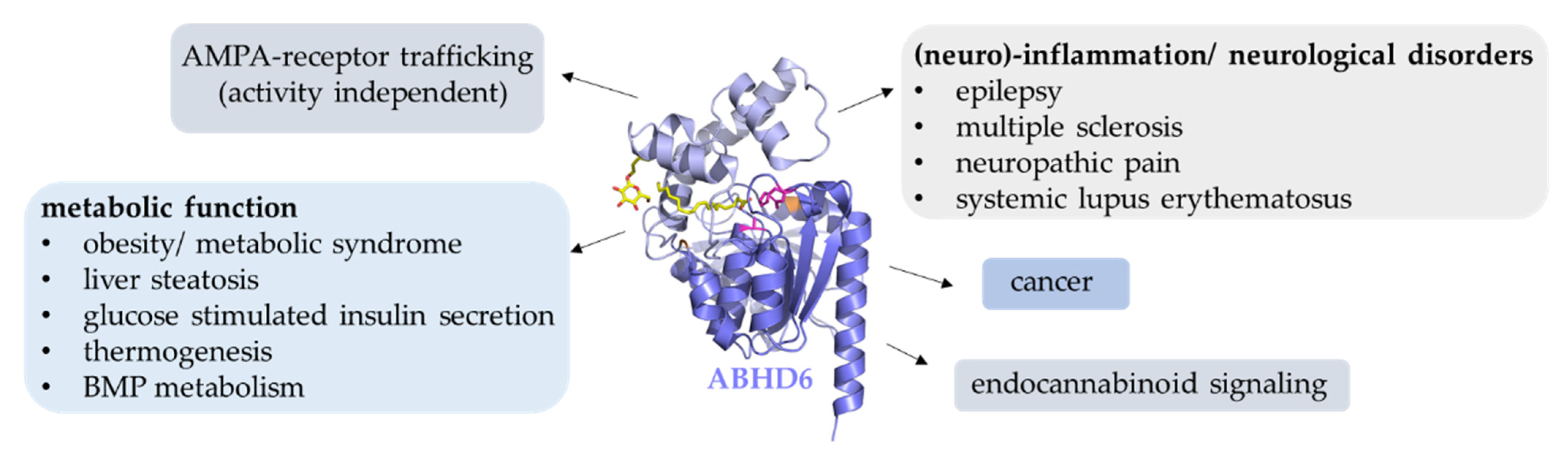
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nardini, M.; Dijkstra, B.W. Alpha/Beta Hydrolase Fold Enzymes: The Family Keeps Growing. Curr. Opin. Struct. Biol. 1999, 9, 732–737. [Google Scholar] [CrossRef]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J.; et al. The α/β Hydrolase Fold. Protein Eng. Des. Sel. 1992, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Schrag, J.D.; Cygler, M. Lipases and Alpha/Beta Hydrolase Fold. Methods Enzym. 1997, 284, 85–107. [Google Scholar]
- Holmquist, M. Alpha/Beta-Hydrolase Fold Enzymes: Structures, Functions and Mechanisms. Curr. Protein Pept. Sci. 2000, 1, 209–235. [Google Scholar] [CrossRef]
- Ollis, D.L.; Carr, P.D. Alpha/Beta Hydrolase Fold: An Update. Protein Pept. Lett. 2009, 16, 1137–1148. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A Comprehensive Profile of Brain Enzymes that Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian Alpha Beta Hydrolase Domain (ABHD) Proteins: Lipid Metabolizing Enzymes at the Interface of Cell Signaling and Energy Metabolism. Biochim. Biophys. Acta —Mol. Cell Biol. Lipids 2013, 1831, 792–802. [Google Scholar] [CrossRef]
- Thomas, G.; Betters, J.L.; Lord, C.C.; Brown, A.L.; Marshall, S.; Ferguson, D.; Sawyer, J.; Davis, M.A.; Melchior, J.T.; Blume, L.C.; et al. The Serine Hydrolase ABHD6 Is a Critical Regulator of the Metabolic Syndrome. Cell Rep. 2013, 5, 508–520. [Google Scholar] [CrossRef]
- Nawrotek, A.; Talagas, A.; Vuillard, L.; Miallau, L. Crystal Structure of Human Monoacylglycerol Lipase ABHD6 in Complex with Oleic Acid and Octyl Glucoside. PDB, 23 June 2021. Available online: https://www.rcsb.org/structure/7OTS (accessed on 1 May 2022).
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Kind, L.; Kursula, P. Structural Properties and Role of the Endocannabinoid Lipases ABHD6 and ABHD12 in Lipid Signalling and Disease. Amino Acids 2019, 51, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Pribasnig, M.A.; Mrak, I.; Grabner, G.F.; Taschler, U.; Knittelfelder, O.; Scherz, B.; Eichmann, T.O.; Heier, C.; Grumet, L.; Kowaliuk, J.; et al. α/β Hydrolase Domain-Containing 6 (ABHD6) Degrades the Late Endosomal/Lysosomal Lipid Bis(Monoacylglycero)Phosphate. J. Biol. Chem. 2015, 290, 29869–29881. [Google Scholar] [CrossRef] [PubMed]
- Rauwerdink, A.; Kazlauskas, R.J. How the Same Core Catalytic Machinery Catalyzes 17 Different Reactions: The Serine-Histidine-Aspartate Catalytic Triad of α/β-Hydrolase Fold Enzymes. ACS Catal. 2015, 5, 6153–6176. [Google Scholar] [CrossRef]
- Labar, G.; Bauvois, C.; Borel, F.; Ferrer, J.-L.L.; Wouters, J.; Lambert, D.M. Crystal Structure of the Human Monoacylglycerol Lipase, a Key Actor in Endocannabinoid Signaling. Chembiochem 2010, 11, 218–227. [Google Scholar] [CrossRef]
- Rengachari, S.; Bezerra, G.A.G.A.; Riegler-Berket, L.; Gruber, C.C.C.C.; Sturm, C.; Taschler, U.; Boeszoermenyi, A.; Dreveny, I.; Zimmermann, R.; Gruber, K.; et al. The Structure of Monoacylglycerol Lipase from Bacillus Sp. H257 Reveals Unexpected Conservation of the Cap Architecture between Bacterial and Human Enzymes. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2012, 1821, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, P.; Zimmermann, R.; Breinbauer, R.; Pavkov-Keller, T.; Oberer, M. The Crystal Structure of Monoacylglycerol Lipase from M. Tuberculosis Reveals the Basis for Specific Inhibition. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Wennberg, C.L.; van der Spoel, D.; Hub, J.S. Large Influence of Cholesterol on Solute Partitioning into Lipid Membranes. J. Am. Chem. Soc. 2012, 134, 5351–5361. [Google Scholar] [CrossRef]
- Hub, J.S.; Winkler, F.K.; Merrick, M.; de Groot, B.L. Potentials of Mean Force and Permeabilities for Carbon Dioxide, Ammonia, and Water Flux across a Rhesus Protein Channel and Lipid Membranes. J. Am. Chem. Soc. 2010, 132, 13251–13263. [Google Scholar] [CrossRef]
- Navia-Paldanius, D.; Savinainen, J.; Laitinen, J. Biochemical and Pharmacological Characterization of Human α/β-Hydrolase Domain Containing 6 (ABHD6) and 12 (ABHD12). J. Lipid Res. 2012, 6, 1–36. [Google Scholar] [CrossRef]
- Farah, S.I.; Hilston, S.; Tran, N.; Zvonok, N.; Makriyannis, A. 1-, 2- and 3-AG as Substrates of the Endocannabinoid Enzymes and Endogenous Ligands of the Cannabinoid Receptor 1. Biochem. Biophys. Res. Commun. 2022, 591, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Van Esbroeck, A.C.M.; Kantae, V.; Di, X.; van der Wel, T.; den Dulk, H.; Stevens, A.F.; Singh, S.; Bakker, A.T.; Florea, B.I.; Stella, N.; et al. Identification of α,β-Hydrolase Domain Containing Protein 6 as a Diacylglycerol Lipase in Neuro-2a Cells. Front. Mol. Neurosci. 2019, 12, 286. [Google Scholar] [CrossRef]
- Zhao, S.; Mugabo, Y.; Iglesias, J.; Xie, L.; Delghingaro-Augusto, V.; Lussier, R.; Peyot, M.-L.; Joly, E.; Taïb, B.; Davis, M.A.; et al. α/β-Hydrolase Domain-6-Accessible Monoacylglycerol Controls Glucose-Stimulated Insulin Secretion. Cell Metab. 2014, 6, 993–1007. [Google Scholar] [CrossRef]
- Zhao, S.; Mugabo, Y.; Ballentine, G.; Attane, C.; Iglesias, J.; Poursharifi, P.; Zhang, D.; Nguyen, T.A.; Erb, H.; Prentki, R.; et al. α/β-Hydrolase Domain 6 Deletion Induces Adipose Browning and Prevents Obesity and Type 2 Diabetes. Cell Rep. 2016, 14, 2872–2888. [Google Scholar] [CrossRef]
- Fisette, A.; Tobin, S.; Décarie-Spain, L.; Bouyakdan, K.; Peyot, M.-L.; Madiraju, S.R.M.; Prentki, M.; Fulton, S.; Alquier, T. α/β-Hydrolase Domain 6 in the Ventromedial Hypothalamus Controls Energy Metabolism Flexibility. Cell Rep. 2016, 17, 1217–1226. [Google Scholar] [CrossRef]
- Grabner, G.F.; Fawzy, N.; Pribasnig, M.A.; Trieb, M.; Taschler, U.; Holzer, M.; Schweiger, M.; Wolinski, H.; Kolb, D.; Horvath, A.; et al. Metabolic Disease and ABHD6 Alter the Circulating Bis(Monoacylglycerol)Phosphate Profile in Mice and Humans. J. Lipid Res. 2019, 60, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Poursharifi, P.; Attané, C.; Mugabo, Y.; Al-Mass, A.; Ghosh, A.; Schmitt, C.; Zhao, S.; Guida, J.; Lussier, R.; Erb, H.; et al. Adipose ABHD6 Regulates Tolerance to Cold and Thermogenic Programs. JCI Insight 2020, 5, e140294. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Kadekawa, K.; Nishijima, S.; Sakanashi, M.; Okitsu-Sakurayama, S.; Higa-Nakamine, S.; Yamamoto, H.; Sugaya, K. Phenotypic Characterization of the Endocannabinoid-Degrading Enzyme Alpha/Beta-Hydrolase Domain 6 Knockout Rat. Cannabis Cannabinoid Res. 2022, 7, 179–187. [Google Scholar] [CrossRef]
- Bononi, G.; Tuccinardi, T.; Rizzolio, F.; Granchi, C. α/β-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases. J. Med. Chem. 2021, 64, 9759–9785. [Google Scholar] [CrossRef]
- Malamas, M.S.; Lamani, M.; Farah, S.I.; Mohammad, K.A.; Miyabe, C.Y.; Rajarshi, G.; Wu, S.; Zvonok, N.; Chandrashekhar, H.; Wood, J.A.; et al. Design and Synthesis of Highly Potent and Specific ABHD6 Inhibitors. ChemMedChem 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain Monoglyceride Lipase Participating in Endocannabinoid Inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef] [PubMed]
- Marrs, W.R.; Blankman, J.L.; Horne, E.A.; Thomazeau, A.; Lin, Y.H.; Coy, J.; Bodor, A.L.; Muccioli, G.G.; Hu, S.S.-J.; Woodruff, G.; et al. The Serine Hydrolase ABHD6 Controls the Accumulation and Efficacy of 2-AG at Cannabinoid Receptors. Nat. Neurosci. 2010, 13, 951–957. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Xu, C.; Odah, E.; Cudaback, E.; Cisneros, J.A.; Lambert, D.M.; Lopez Rodriguez, M.L.; Bajjalieh, S.; Stella, N. Identification of a Novel Endocannabinoid-Hydrolyzing Enzyme Expressed by Microglial Cells. J. Neurosci. 2007, 27, 2883–2889. [Google Scholar] [CrossRef]
- Marrs, W.R.; Horne, E.A.; Ortega-Gutierrez, S.; Cisneros, J.A.; Xu, C.; Lin, Y.H.; Muccioli, G.G.; Lopez-Rodriguez, M.L.; Stella, N. Dual Inhibition of Alpha/Beta-Hydrolase Domain 6 and Fatty Acid Amide Hydrolase Increases Endocannabinoid Levels in Neurons. J. Biol. Chem. 2011, 286, 28723–28728. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic Monoacylglycerol Lipase Blockade Causes Functional Antagonism of the Endocannabinoid System. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef]
- Taschler, U.; Radner, F.P.; Heier, C.; Schreiber, R.; Schweiger, M.; Schoiswohl, G.; Preiss-Landl, K.; Jaeger, D.; Reiter, B.; Koefeler, H.C.; et al. Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-Induced Insulin Resistance. J. Biol. Chem. 2011, 286, 17467–17477. [Google Scholar] [CrossRef]
- Chanda, P.K.; Gao, Y.; Mark, L.; Btesh, J.; Strassle, B.W.; Lu, P.; Piesla, M.J.; Zhang, M.-Y.Y.; Bingham, B.; Uveges, A.; et al. Monoacylglycerol Lipase Activity Is a Critical Modulator of the Tone and Integrity of the Endocannabinoid System. Mol. Pharmacol. 2010, 78, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Taschler, U.; Eichmann, T.O.; Radner, F.P.W.; Grabner, G.F.; Wolinski, H.; Storr, M.; Lass, A.; Schicho, R.; Zimmermann, R. Monoglyceride Lipase-Deficiency Causes Desensitization of Intestinal Cannabinoid Receptor Type 1 and Increased Colonic μ-Opioid Receptor Sensitivity. Br. J. Pharmacol. 2015, 172, 4419–4429. [Google Scholar] [CrossRef]
- Alhouayek, M.; Masquelier, J.; Cani, P.D.; Lambert, D.M.; Muccioli, G.G. Implication of the Anti-Inflammatory Bioactive Lipid Prostaglandin D2-Glycerol Ester in the Control of Macrophage Activation and Inflammation by ABHD6. Proc. Natl. Acad. Sci. USA 2013, 110, 17558–17563. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ribeiro, R.; Tanaka, M.; Zhang, Y. Activation of CB2 Receptor Is Required for the Therapeutic Effect of ABHD6 Inhibition in Experimental Autoimmune Encephalomyelitis. Neuropharmacology 2015, 99, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.J.; Rouzer, C.A.; Morgan, A.J.; Patel, S.; Marnett, L.J. Aspects of Prostaglandin Glycerol Ester Biology. Adv. Exp. Med. Biol. 2019, 1161, 77–88. [Google Scholar] [CrossRef]
- Masquelier, J.; Alhouayek, M.; Terrasi, R.; Bottemanne, P.; Paquot, A.; Muccioli, G.G. Lysophosphatidylinositols in Inflammation and Macrophage Activation: Altered Levels and Anti-Inflammatory Effects. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2018, 1863, 1458–1468. [Google Scholar] [CrossRef]
- Taschler, U.; Hasenoehrl, C.; Storr, M.; Schicho, R. Cannabinoid receptors in regulating the GI Tract: Experimental evidence and therapeutic relevance. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 239, pp. 343–362. ISBN 978-3-319-29806-1. [Google Scholar]
- Kurano, M.; Kobayashi, T.; Sakai, E.; Tsukamoto, K.; Yatomi, Y. Lysophosphatidylinositol, Especially Albumin-bound Form, Induces Inflammatory Cytokines in Macrophages. FASEB J. 2021, 35, e21673. [Google Scholar] [CrossRef]
- Li, W.; Blankman, J.L.; Cravatt, B.F. A Functional Proteomic Strategy to Discover Inhibitors for Uncharacterized Hydrolases. J. Am. Chem. Soc. 2007, 129, 9594–9595. [Google Scholar] [CrossRef]
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Alhouayek, M.; Muccioli, G.G. The α/β–Hydrolase Domain 6 Inhibitor WWL70 Decreases Endotoxin-Induced Lung Inflammation in Mice, Potential Contribution of 2-Arachidonoylglycerol, and Lysoglycerophospholipids. FASEB J. 2019, 33, 7635–7646. [Google Scholar] [CrossRef]
- Bachovchin, D.A.; Ji, T.; Li, W.; Simon, G.M.; Blankman, J.L.; Adibekian, A.; Hoover, H.; Niessen, S.; Cravatt, B.F. Superfamily-Wide Portrait of Serine Hydrolase Inhibition Achieved by Library-versus-Library Screening. Proc. Natl. Acad. Sci. USA 2010, 107, 20941–20946. [Google Scholar] [CrossRef]
- Naydenov, A.V.; Horne, E.A.; Cheah, C.S.; Swinney, K.; Hsu, K.-L.; Cao, J.K.; Marrs, W.R.; Blankman, J.L.; Tu, S.; Cherry, A.E.; et al. ABHD6 Blockade Exerts Antiepileptic Activity in PTZ-Induced Seizures and in Spontaneous Seizures in R6/2 Mice. Neuron 2014, 83, 361–371. [Google Scholar] [CrossRef]
- Tchantchou, F.; Zhang, Y. Selective Inhibition of Alpha/Beta-Hydrolase Domain 6 Attenuates Neurodegeneration, Alleviates Blood Brain Barrier Breakdown, and Improves Functional Recovery in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2013, 30, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Manterola, A.; Bernal-Chico, A.; Cipriani, R.; Canedo-Antelo, M.; Moreno-García, Á.; Martín-Fontecha, M.; Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Ortega-Gutiérrez, S.; Brown, J.M.; et al. Deregulation of the Endocannabinoid System and Therapeutic Potential of ABHD6 Blockade in the Cuprizone Model of Demyelination. Biochem. Pharmacol. 2018, 157, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Manterola, A.; Bernal-Chico, A.; Cipriani, R.; Ruiz, A.; Pérez-Samartín, A.; Moreno-Rodríguez, M.; Hsu, K.L.; Cravatt, B.F.; Brown, J.M.; Rodríguez-Puertas, R.; et al. Re-Examining the Potential of Targeting ABHD6 in Multiple Sclerosis: Efficacy of Systemic and Peripherally Restricted Inhibitors in Experimental Autoimmune Encephalomyelitis. Neuropharmacology 2018, 141, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Chico, A.; Canedo, M.; Manterola, A.; Victoria Sánchez-Gómez, M.; Pérez-Samartín, A.; Rodríguez-Puertas, R.; Matute, C.; Mato, S. Blockade of Monoacylglycerol Lipase Inhibits Oligodendrocyte Excitotoxicity and Prevents Demyelination in Vivo. Glia 2015, 63, 163–176. [Google Scholar] [CrossRef]
- Wen, J.; Jones, M.; Tanaka, M.; Selvaraj, P.; Symes, A.J.; Cox, B.; Zhang, Y. WWL70 Protects against Chronic Constriction Injury-Induced Neuropathic Pain in Mice by Cannabinoid Receptor-Independent Mechanisms. J. Neuroinflamm. 2018, 15, 9. [Google Scholar] [CrossRef]
- Oparina, N.Y.; Delgado-Vega, A.M.; Martinez-Bueno, M.; Magro-Checa, C.; Fernández, C.; Castro, R.O.; Pons-Estel, B.A.; D’Alfonso, S.; Sebastiani, G.D.; Witte, T.; et al. PXK Locus in Systemic Lupus Erythematosus: Fine Mapping and Functional Analysis Reveals Novel Susceptibility Gene ABHD6. Ann. Rheum. Dis. 2015, 74, e14. [Google Scholar] [CrossRef]
- Rahaman, O.; Bhattacharya, R.; Liu, C.S.C.; Raychaudhuri, D.; Ghosh, A.R.; Bandopadhyay, P.; Pal, S.; Goswami, R.P.; Sircar, G.; Ghosh, P.; et al. Cutting Edge: Dysregulated Endocannabinoid-Rheostat for Plasmacytoid Dendritic Cell Activation in a Systemic Lupus Endophenotype. J. Immunol. 2019, 202, 1674–1679. [Google Scholar] [CrossRef]
- Tanaka, M.; Moran, S.; Wen, J.; Affram, K.; Chen, T.; Symes, A.J.; Zhang, Y. WWL70 Attenuates PGE2 Production Derived from 2-Arachidonoylglycerol in Microglia by ABHD6-Independent Mechanism. J. Neuroinflammation 2017, 14, 7. [Google Scholar] [CrossRef]
- Schwenk, J.; Harmel, N.; Brechet, A.; Zolles, G.; Berkefeld, H.; Müller, C.S.; Bildl, W.; Baehrens, D.; Hüber, B.; Kulik, A.; et al. High-Resolution Proteomics Unravel Architecture and Molecular Diversity of Native AMPA Receptor Complexes. Neuron 2012, 74, 621–633. [Google Scholar] [CrossRef]
- Wei, M.; Jia, M.; Zhang, J.; Yu, L.; Zhao, Y.; Chen, Y.; Ma, Y.; Zhang, W.; Shi, Y.; Zhang, C. The Inhibitory Effect of α/β-Hydrolase Domain-Containing 6 (ABHD6) on the Surface Targeting of GluA2- and GluA3-Containing AMPA Receptors. Front. Mol. Neurosci. 2017, 10, 55. [Google Scholar] [CrossRef]
- Zhao, S.; Poursharafi, P.; Mugabo, Y.; Levens, E.J.; Vivot, K.; Attane, C.; Iglesias, J.; Peyot, M.; Joly, E.; Madiraju, S.R.M.; et al. A/Β-Hydrolase Domain-6 and Saturated Long Chain Monoacylglycerol Regulate Insulin Secretion Promoted by Both Fuel and Non-Fuel Stimuli. Mol. Metab. 2015, 4, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Berdan, C.A.; Erion, K.A.; Burritt, N.E.; Corkey, B.E.; Deeney, J.T. Inhibition of Monoacylglycerol Lipase Activity Decreases Glucose-Stimulated Insulin Secretion in INS-1 (832/13) Cells and Rat Islets. PLoS ONE 2016, 11, e0149008. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Beuchat, M.H.; Chevallier, J.; Makino, A.; Mayran, N.; Escola, J.M.; Lebrand, C.; Cosson, P.; Kobayashi, T.; Gruenberg, J. Separation and Characterization of Late Endosomal Membrane Domains. J. Biol. Chem. 2002, 277, 32157–32164. [Google Scholar] [CrossRef]
- Locatelli-Hoops, S.; Remmel, N.; Klingenstein, R.; Breiden, B.; Rossocha, M.; Schoeniger, M.; Koenigs, C.; Saenger, W.; Sandhoff, K. Saposin A Mobilizes Lipids from Low Cholesterol and High Bis(Monoacylglycerol)Phosphate-Containing Membranes: Patient Variant Saposin a Lacks Lipid Extraction Capacity. J. Biol. Chem. 2006, 281, 32451–32460. [Google Scholar] [CrossRef] [PubMed]
- Oninla, V.O.; Breiden, B.; Babalola, J.O.; Sandhoff, K. Acid Sphingomyelinase Activity Is Regulated by Membrane Lipids and Facilitates Cholesterol Transfer by NPC2. J. Lipid Res. 2014, 55, 2606–2619. [Google Scholar] [CrossRef]
- Schulze, H.; Sandhoff, K. Lysosomal Lipid Storage Diseases. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef]
- Müller-Calleja, N.; Hollerbach, A.; Royce, J.; Ritter, S.; Pedrosa, D.; Madhusudhan, T.; Teifel, S.; Meineck, M.; Häuser, F.; Canisius, A.; et al. Lipid Presentation by the Protein C Receptor Links Coagulation with Autoimmunity. Science 2021, 371, eabc0956. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Desprez, T.; Metna-Laurent, M.; Bellocchio, L.; Marsicano, G.; Soria-Gomez, E. Dissecting the Cannabinergic Control of Behavior: The Where Matters. BioEssays 2015, 37, 1215–1225. [Google Scholar] [CrossRef]
- Li, F.; Fei, X.; Xu, J.; Ji, C. An Unannotated Alpha/Beta Hydrolase Superfamily Member, ABHD6 Differentially Expressed among Cancer Cell Lines. Mol. Biol. Rep. 2009, 36, 691–696. [Google Scholar] [CrossRef]
- Max, D.; Hesse, M.; Volkmer, I.; Staege, M.S. High Expression of the Evolutionarily Conserved Alpha/Beta Hydrolase Domain Containing 6 (ABHD6) in Ewing Tumors. Cancer Sci. 2009, 100, 2383–2389. [Google Scholar] [CrossRef]
- Grüner, B.M.; Schulze, C.J.; Yang, D.; Ogasawara, D.; Dix, M.M.; Rogers, Z.N.; Chuang, C.-H.; McFarland, C.D.; Chiou, S.-H.; Brown, J.M.; et al. An in Vivo Multiplexed Small-Molecule Screening Platform. Nat. Methods 2016, 13, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xie, H.; Heier, C.; Huang, J.; Zheng, Q.; Eichmann, T.O.; Schoiswohl, G.; Ni, J.; Zechner, R.; Ni, S.; et al. Enhanced Monoacylglycerol Lipolysis by ABHD6 Promotes NSCLC Pathogenesis. EBioMedicine 2020, 53, 102696. [Google Scholar] [CrossRef] [PubMed]



| Organism/Strain | Knockout/Knockdown | Observation | Reference |
|---|---|---|---|
| ABHD6-ko (mouse) | global | increased glucose stimulated insulin secretion; | [24] |
| ABHD6-ko (mouse) | global | increased circulating bis(monoacylglycerol)phosphate levels; | [27] |
| ABHD6-flox/Ins1-Cre/ERT(mouse) | β-cell-specific | enhanced glucose stimulated insulin secretion in vivo and ex vivo; | [24] |
| ABHD6-flox/AdipoQ-Cre/ERT2(mouse) | adipocyte-specific | elevated energy expenditure in cold and resistance to cold-induced hypothermia; protected from diet-induced obesity; | [28] |
| ABHD6-ko (rat) | global (CRISPR/Cas9-mediated) | shorter intervals between bladder contractions, hyperalgesia and increased PGE2; | [29] |
| ABHD6-ASO (mouse) 1 | liver-specific (ASO-mediated knock down) | protected from diet induced obesity and liver steatosis; | [8] |
| VMHKO (mouse) 2 | VMH neuron-specific (AAV-mediated) | altered food intake, reduced energy expenditure, prone to diet-induced obesity; | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusch, L.-M.; Riegler-Berket, L.; Oberer, M.; Zimmermann, R.; Taschler, U. α/β-Hydrolase Domain-Containing 6 (ABHD6)— A Multifunctional Lipid Hydrolase. Metabolites 2022, 12, 761. https://doi.org/10.3390/metabo12080761
Pusch L-M, Riegler-Berket L, Oberer M, Zimmermann R, Taschler U. α/β-Hydrolase Domain-Containing 6 (ABHD6)— A Multifunctional Lipid Hydrolase. Metabolites. 2022; 12(8):761. https://doi.org/10.3390/metabo12080761
Chicago/Turabian StylePusch, Lisa-Maria, Lina Riegler-Berket, Monika Oberer, Robert Zimmermann, and Ulrike Taschler. 2022. "α/β-Hydrolase Domain-Containing 6 (ABHD6)— A Multifunctional Lipid Hydrolase" Metabolites 12, no. 8: 761. https://doi.org/10.3390/metabo12080761






