The Roles of Gut Microbiome and Plasma Metabolites in the Associations between ABO Blood Groups and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES)
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Population
2.2. ABO Haplotype and Diplotype
2.3. Insulin Homeostasis Measurements
2.4. Sample Extraction and Metabolite Profiling
2.5. Metagenomics Measurements
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goumidi, L.; Thibord, F.; Wiggins, K.L.; Li-Gao, R.; Brown, M.R.; van Hylckama Vlieg, A.; Souto, J.C.; Soria, J.M.; Ibrahim-Kosta, M.; Saut, N.; et al. Association between ABO haplotypes and the risk of venous thrombosis: Impact on disease risk estimation. Blood 2021, 137, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Ewald, D.R.; Sumner, S.C. Blood type biochemistry and human disease. Wiley Interdiscip Rev. Syst. Biol. Med. 2016, 8, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Groot, H.E.; Villegas Sierra, L.E.; Said, M.A.; Lipsic, E.; Karper, J.C.; van der Harst, P. Genetically Determined ABO Blood Group and its Associations with Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Schooling, C.M. A phenome-wide association study of ABO blood groups. BMC Med. 2020, 18, 334. [Google Scholar] [CrossRef] [PubMed]
- Liumbruno, G.M.; Franchini, M. Beyond immunohaematology: The role of the ABO blood group in human diseases. Blood Transfus 2013, 11, 491–499. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Gusto, G.; Clavel-Chapelon, F.; Balkau, B.; Bonnet, F. ABO and Rhesus blood groups and risk of type 2 diabetes: Evidence from the large E3N cohort study. Diabetologia 2015, 58, 519–522. [Google Scholar] [CrossRef]
- Li-Gao, R.; Carlotti, F.; de Mutsert, R.; van Hylckama Vlieg, A.; de Koning, E.J.P.; Jukema, J.W.; Rosendaal, F.R.; Willems van Dijk, K.; Mook-Kanamori, D.O. Genome-Wide Association Study on the Early-Phase Insulin Response to a Liquid Mixed Meal: Results From the NEO Study. Diabetes 2019, 68, 2327–2336. [Google Scholar] [CrossRef]
- Ruhlemann, M.C.; Hermes, B.M.; Bang, C.; Doms, S.; Moitinho-Silva, L.; Thingholm, L.B.; Frost, F.; Degenhardt, F.; Wittig, M.; Kassens, J.; et al. Genome-wide association study in 8956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat. Genet. 2021, 53, 147–155. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Yang, H.; Wu, J.; Huang, X.; Zhou, Y.; Zhang, Y.; Liu, M.; Liu, Q.; Ke, S.; He, M.; Fu, H.; et al. ABO genotype alters the gut microbiota by regulating GalNAc levels in pigs. Nature 2022, 606, 358–367. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Menni, C.; Zhu, J.; Le Roy, C.I.; Mompeo, O.; Young, K.; Rebholz, C.M.; Selvin, E.; North, K.E.; Mohney, R.P.; Bell, J.T.; et al. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes 2020, 11, 1632–1642. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Liu, J.; Semiz, S.; van der Lee, S.J.; van der Spek, A.; Verhoeven, A.; van Klinken, J.B.; Sijbrands, E.; Harms, A.C.; Hankemeier, T.; van Dijk, K.W.; et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 2017, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Liggi, S.; Griffin, J.L. Metabolomics applied to diabetes—lessons from human population studies. Int. J. Biochem. Cell B 2017, 93, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Li-Gao, R.; de Mutsert, R.; Rensen, P.C.N.; van Klinken, J.B.; Prehn, C.; Adamski, J.; van Hylckama Vlieg, A.; den Heijer, M.; le Cessie, S.; Rosendaal, F.R.; et al. Postprandial metabolite profiles associated with type 2 diabetes clearly stratify individuals with impaired fasting glucose. Metabolomics 2018, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Bertoni, A.G.; Crago, O.L.; Hoffman, K.L.; Wood, A.C.; Arzumanyan, Z.; Lam, L.K.; Roll, K.; Sandow, K.; Wu, M.; et al. Rationale, design and baseline characteristics of the Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes Obes. Metab. 2020, 22, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.B.; Bridgewater, B.R.; Liu, Q.; Mitchell, M.W.; Robinson, R.J.; Dai, H.; Stewart, S.J.; DeHaven, C.D.; Miller, L.A.D. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in HighThroughput Profiling Metabolomics. Metabolomics 2014, 4, 132. [Google Scholar] [CrossRef]
- Ford, L.; Kennedy, A.D.; Goodman, K.D.; Pappan, K.L.; Evans, A.M.; Miller, L.A.D.; Wulff, J.E.; Wiggs, B.R.; Lennon, J.J.; Elsea, S.; et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J. Appl. Lab. Med. 2020, 5, 342–356. [Google Scholar] [CrossRef]
- Segata, N.; Waldron, L.; Ballarini, A.; Narasimhan, V.; Jousson, O.; Huttenhower, C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 2012, 9, 811–814. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of microbial compositions: A review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes 2020, 6, 60. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sanchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Schaid, D.J.; Rowland, C.M.; Tines, D.E.; Jacobson, R.M.; Poland, G.A. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am. J. Hum. Genet. 2002, 70, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Das, S.K.; Ainsworth, H.C.; Dimitrov, L.; Okut, H.; Comeau, M.E.; Sharma, N.; Ng, M.C.Y.; Norris, J.M.; Chen, Y.I.; Wagenknecht, L.E.; et al. Metabolomic architecture of obesity implicates metabolonic lactone sulfate in cardiometabolic disease. Mol. Metab. 2021, 54, 101342. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Palmer, N.D.; Okut, H.; Hsu, F.C.; Ng, M.C.Y.; Chen, Y.I.; Goodarzi, M.O.; Taylor, K.D.; Norris, J.M.; Lorenzo, C.; Rotter, J.I.; et al. Metabolomics Identifies Distinctive Metabolite Signatures for Measures of Glucose Homeostasis: The Insulin Resistance Atherosclerosis Family Study (IRAS-FS). J. Clin. Endocrinol. Metab. 2018, 103, 1877–1888. [Google Scholar] [CrossRef]
- Meo, S.A.; Rouq, F.A.; Suraya, F.; Zaidi, S.Z. Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur. Rev. Med. Pharm. Sci. 2016, 20, 237–242. [Google Scholar]
- Hoglund, J.; Karlsson, T.; Johansson, T.; Ek, W.E.; Johansson, A. Characterization of the human ABO genotypes and their association to common inflammatory and cardiovascular diseases in the UK Biobank. Am. J. Hematol. 2021, 96, 1350–1362. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes 2020, 69, 2086–2093. [Google Scholar] [CrossRef]
- Jansen, R.S.; Addie, R.; Merkx, R.; Fish, A.; Mahakena, S.; Bleijerveld, O.B.; Altelaar, M.; IJlst, L.; Wanders, R.J.; Borst, P.; et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl. Acad. Sci. USA 2015, 112, 6601–6606. [Google Scholar] [CrossRef]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.; Lyu, X.; Zushin, P.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef]
- Cobb, J.; Gall, W.; Adam, K.P.; Nakhle, P.; Button, E.; Hathorn, J.; Lawton, K.; Milburn, M.; Perichon, R.; Mitchell, M.; et al. A novel fasting blood test for insulin resistance and prediabetes. J. Diabetes Sci. Technol. 2013, 7, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
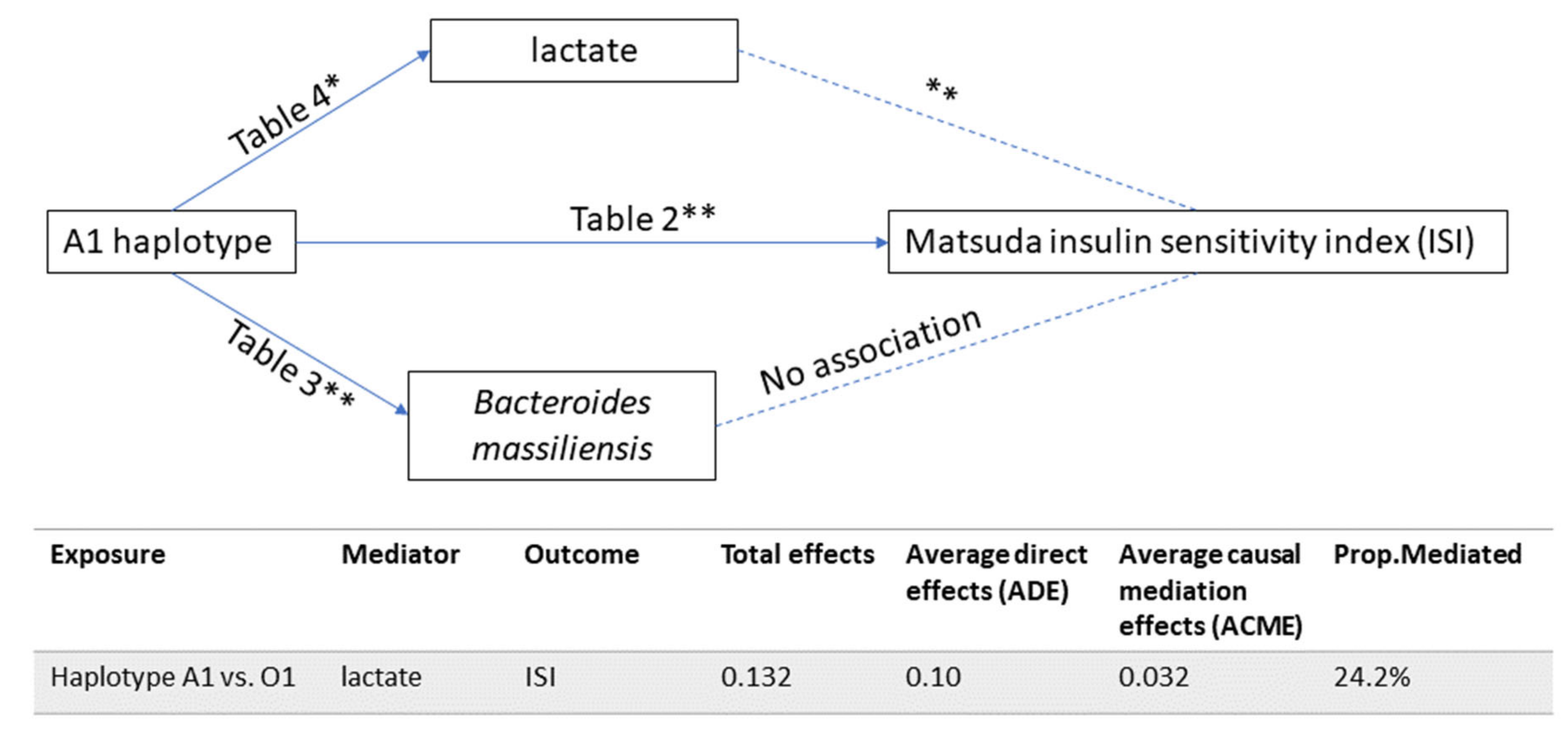


| Non-Hispanic Whites | African Americans | |
|---|---|---|
| N | 210 | 109 |
| Age (years) | 60 (9) | 57 (8) |
| Male, n (%) | 89 (42%) | 36 (33%) |
| BMI (kg/m2) | 27.3 (5.8) | 32.5 (8.9) |
| rs41302905 (for O2 haplotype) | ||
| MAF | 0.021 | 0.0091 |
| HWE p-value | 1 | 1 |
| Imputation quality | 0.92 | |
| rs8176743 (for B haplotype) | ||
| MAF | 0.069 | 0.11 |
| HWE p-value | 1 | 1 |
| Imputation quality | 0.97 | |
| rs1053878 (for A2 haplotype) | ||
| MAF | 0.078 | 0.21 |
| HWE p-value | 0.12 | 1 |
| Imputation quality | 0.95 | |
| rs8176645 β (tagging for rs8176719, for O1 haplotype) | ||
| MAF | 0.29 | 0.32 |
| HWE p-value | 0.32 | 0.67 |
| Imputation quality | 0.82 | |
| rs2519093 (A1 haplotype) | ||
| MAF | 0.15 | 0.091 |
| HWE p-value | 0.097 | 1 |
| Imputation quality | 0.99 | |
| rs600038 β (tagging for rs579459, for A1 haplotype) | ||
| MAF | 0.18 | 0.11 |
| HWE p-value | 0.052 | 1 |
| Imputation quality | 1.00 | |
| Haplotype | rs41302905 | rs8176743 | rs1053878 | rs8176645 ψ | rs2519093 | rs600038 ψ | frequency | ISI β (95% CI) | AUC-Ins30/AUC-Glu30β (95% CI) | AUC-Cpep/AUC-Insβ (95% CI) | DI30 β (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Whites | |||||||||||
| O1 | C | C | G | delG | C | T | 0.68 | Reference | |||
| A1 | C | C | G | G | T | C | 0.13 | 0.132 (0.049, 0.216) ** | −0.069 (−0.14, 0.003) | 0.037 (−0.005, 0.078) | 0.062 (−0.003, 0.127) |
| A2 | C | C | A | G | C | T | 0.068 | 0.137 (0.023, 0.251) * | −0.055 (−0.152, 0.043) | 0.066 (0.01, 0.123) * | 0.082 (−0.007, 0.171) |
| B | C | T | G | G | C | T | 0.060 | −0.079 (−0.209, 0.051) | 0.043 (−0.068, 0.155) | −0.032 (−0.097, 0.032) | −0.035 (−0.137, 0.066) |
| African Americans | |||||||||||
| O1 | C | C | G | delG | C | T | 0.52 | Reference | |||
| unknown | C | C | G | G | C | T | 0.061 | 0.092 (−0.144, 0.329) | −0.035 (−0.225, 0.156) | 0.097 (−0.018, 0.213) | 0.07 (−0.113, 0.253) |
| A1 | C | C | G | G | T | C | 0.053 | 0.062 (−0.146, 0.269) | 0.028 (−0.159, 0.216) | 0.002 (−0.114, 0.118) | 0.104 (−0.065, 0.272) |
| A2/O1 | C | C | A | delG | C | T | 0.083 | 0.037 (−0.121, 0.195) | −0.089 (−0.249, 0.07) | 0.036 (−0.054, 0.127) | −0.033 (−0.187, 0.122) |
| A2 | C | C | A | G | C | T | 0.10 | 0.039 (−0.1, 0.178) | 0.036 (−0.087, 0.159) | 0.037 (−0.044, 0.119) | 0.07 (−0.053, 0.194) |
| B | C | T | G | G | C | T | 0.064 | −0.044 (−0.218, 0.13) | 0.01 (−0.151, 0.171) | −0.088 (−0.195, 0.019) | −0.028 (−0.19, 0.134) |
| African Americans | Whites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype O1 | Haplotype unknown β (95% CI) | Haplotype A1 β (95% CI) | Haplotype A2/O1 β (95% CI) | Haplotype A2 β (95% CI) | Haplotype B β (95% CI) | Haplotype A1 β (95% CI) | Haplotype A2 β (95% CI) | Haplotype B β (95% CI) | ||
| Bacteroides | Caccae β (95% CI) | Reference | 0.13 (−3.79, 4.04) | −0.62 (−3.96, 2.72) | −0.2 (−3.35, 2.94) | 0.64 (−1.93, 3.21) | −0.32 (−3.63, 2.99) | 0.07 (−1.34, 1.48) | −0.2 (−2.14, 1.74) | 0.53 (−1.71, 2.77) |
| Cellulosilyticus β (95% CI) | −3.07 (−6.12, −0.01) * | −1.27 (−4.24, 1.7) | −0.15 (−2.8, 2.5) | 0.9 (−1.43, 3.23) | 0.03 (−2.98, 3.05) | −0.14 (−1.59, 1.3) | −0.37 (−2.37, 1.63) | −0.12 (−2.42, 2.18) | ||
| dorei β (95% CI) | −1.06 (−4.9, 2.79) | −0.74 (−4.05, 2.58) | 1.11 (−1.92, 4.14) | 2.77 (0.22, 5.33) * | −1.54 (−4.79, 1.71) | 0.49 (−0.97, 1.95) | 0.64 (−1.37, 2.65) | 0.43 (−1.9, 2.75) | ||
| Faecis β (95% CI) | −2.04 (−5.3, 1.21) | −0.4 (−3.55, 2.75) | 1.89 (−0.98, 4.77) | 0.49 (−1.79, 2.77) | −0.34 (−3.23, 2.54) | −0.21 (−1.43, 1.01) | −0.51 (−2.18, 1.15) | −0.02 (−1.97, 1.93) | ||
| Finegoldii β (95% CI) | −0.51 (−3.55, 2.54) | 1.31 (−1.45, 4.06) | 2.14 (−0.38, 4.65) | −2.94 (−5.01, −0.86) * | 0.43 (−2.11, 2.98) | −0.33 (−1.7, 1.05) | 0.13 (−1.78, 2.03) | −0.97 (−3.16, 1.23) | ||
| Fragilis β (95% CI) | 0.72 (−2.8, 4.24) | −1.15 (−4.29, 1.98) | −0.61 (−3.38, 2.17) | 1.28 (−1.04, 3.59) | 0.46 (−2.57, 3.49) | 1.14 (−0.21, 2.48) | 1.4 (−0.45, 3.25) | 1.1 (−1.05, 3.25) | ||
| Galacturonicus β (95% CI) | −1.81 (−3.71, 0.08) | −0.97 (−2.77, 0.83) | −1.57 (−3.23, 0.09) | −0.69 (−2.08, 0.71) | 0.77 (−1.1, 2.63) | 0.5 (−0.39, 1.38) | −0.11 (−1.32, 1.1) | −0.23 (−1.64, 1.18) | ||
| Massiliensis β (95% CI) | −1.53 (−4.82, 1.76) | 1.29 (−1.57, 4.14) | 3.37 (0.77, 5.97) * | −2.07 (−4.33, 0.19) | −1.15 (−3.99, 1.69) | −2.02 (−3.3, −0.75) ** | −0.57 (−2.34, 1.19) | −1.29 (−3.34, 0.75) | ||
| Ovatus β (95% CI) | −1.67 (−4.59, 1.25) | −1.73 (−4.33, 0.88) | −0.94 (−3.27, 1.39) | 0.85 (−1.09, 2.8) | 0.33 (−2.24, 2.9) | −0.4 (−1.17, 0.37) | 0.39 (−0.67, 1.44) | −1.39 (−2.62, −0.15) * | ||
| Stercoris β (95% CI) | 2.88 (−1.25, 7.01) | 2.47 (−0.97, 5.91) | 1.02 (−2.26, 4.29) | −2.49 (−5.2, 0.22) | 3.92 (0.26, 7.58) * | −0.57 (−2.1, 0.96) | −0.52 (−2.63, 1.59) | −0.15 (−2.58, 2.28) | ||
| Thetaiotaomicron β (95% CI) | 1.04 (−2.1, 4.19) | 1.8 (−1.24, 4.84) | −2.31 (−5.37, 0.75) | 1.22 (−0.89, 3.32) | −0.81 (−3.57, 1.95) | −0.15 (−1.35, 1.05) | 1.35 (−0.3, 2.99) | −0.37 (−2.26, 1.52) | ||
| Uniformis β (95% CI) | 0.71 (−2.42, 3.85) | 1.97 (−0.84, 4.78) | 1.56 (−0.96, 4.08) | 0.41 (−1.72, 2.54) | −0.45 (−3.21, 2.32) | −0.67 (−1.57, 0.24) | 0.52 (−0.71, 1.76) | 1.12 (−0.31, 2.55) | ||
| Vulgatus β (95% CI) | −2.53 (−5.76, 0.71) | −0.86 (−3.6, 1.88) | −2.66 (−4.97, −0.35) * | −1.14 (−3.21, 0.94) | −1.06 (−3.47, 1.35) | 0.08 (−0.93, 1.08) | −0.28 (−1.66, 1.11) | −0.49 (−2.08, 1.09) | ||
| Xylanisolvens β (95% CI) | −0.81 (−3.76, 2.14) | 0.44 (−2.33, 3.22) | 2.94 (0.46, 5.42) * | −1.68 (−3.72, 0.36) | 1.36 (−1.42, 4.14) | 0.65 (−0.59, 1.9) | 0.9 (−0.81, 2.62) | 0.6 (−1.39, 2.58) | ||
| Roseburia | Faecis β (95% CI) | −0.94 (−4.44, 2.56) | 0.94 (−2, 3.88) | −0.47 (−3.3, 2.36) | 0.44 (−1.87, 2.75) | −3.7 (−6.81, −0.59) * | −0.19 (−1.46, 1.08) | 0.47 (−1.28, 2.23) | −0.14 (−2.16, 1.87) | |
| Hominis β (95% CI) | 0.59 (−2.08, 3.25) | −0.35 (−2.71, 2.01) | −3.1 (−5.24, −0.96) * | 0.45 (−1.26, 2.16) | −0.09 (−2.33, 2.15) | 0.47 (−0.46, 1.4) | −1.48 (−2.75, −0.2) * | −0.18 (−1.66, 1.29) | ||
| Intestinalis β (95% CI) | −3.09 (−6.17, −0.01) * | −0.18 (−2.97, 2.61) | −0.04 (−2.36, 2.28) | −1.27 (−3.21, 0.67) | −0.92 (−3.38, 1.53) | 0.17 (−0.89, 1.23) | 0.53 (−0.92, 1.97) | −1.76 (−3.45, −0.08) * | ||
| Inulinivorans β (95% CI) | −2.03 (−4.97, 0.92) | −0.18 (−3.13, 2.77) | −1.16 (−3.26, 0.94) | −0.34 (−2.31, 1.64) | −0.75 (−3.04, 1.55) | 0.08 (−0.94, 1.1) | −1.71 (−3.11, −0.32) * | −0.68 (−2.3, 0.94) | ||
| sp_CAG_471 β (95% CI) | −1.8 (−3.47, −0.14) * | 1.49 (−0.2, 3.18) | −1.68 (−3.18, −0.17) * | −0.25 (−1.52, 1.01) | −1.46 (−3.08, 0.15) | −0.46 (−1.2, 0.29) | −1.01 (−2.03, 0.01) | −0.26 (−1.45, 0.92) | ||
| Faecalibacterium | Prausnitzii β (95% CI) | 0.55 (−1.05, 2.16) | 0.3 (−1.14, 1.74) | −0.85 (−2.27, 0.56) | 0.31 (−0.89, 1.51) | −0.36 (−1.81, 1.09) | 0.33 (−0.23, 0.89) | 0.55 (−0.21, 1.32) | −0.26 (−1.16, 0.63) | |
| Akkermansia | Muciniphila β (95% CI) | −2.09 (−5.32, 1.14) | 4.44 (1.7, 7.17) ** | 2.31 (−0.34, 4.97) | 1.98 (−0.06, 4.01) | 3.55 (0.86, 6.24) * | 0.69 (−0.56, 1.94) | 0.92 (−0.78, 2.63) | 0.55 (−1.44, 2.54) | |
| Whites | African Americans | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Haplotype O1 | Haplotype A1 β (95% CI) | Haplotype A2 β (95% CI) | Haplotype B β (95% CI) | Haplotype unknown β (95% CI) | Haplotype A1 β (95% CI) | Haplotype A2/O1 β (95% CI) | Haplotype A2 β (95% CI) | Haplotype B β (95% CI) | |
| isoleucine | reference | −0.006 (−0.063, 0.05) | 0.005 (−0.072, 0.082) | 0.047 (−0.041, 0.136) | −0.118 (−0.251, 0.015) | 0.005 (−0.12, 0.13) | −0.124 (−0.238, −0.011) * | −0.018 (−0.115, 0.08) | −0.081 (−0.204, 0.043) |
| leucine | 0.015 (−0.035, 0.065) | 0.018 (−0.05, 0.087) | 0.042 (−0.036, 0.12) | −0.075 (−0.198, 0.049) | 0.035 (−0.076, 0.145) | −0.102 (−0.207, 0.003) | 0.002 (−0.083, 0.087) | −0.069 (−0.184, 0.046) | |
| lactate | −0.092 (−0.181, −0.002) * | −0.033 (−0.155, 0.089) | −0.043 (−0.183, 0.096) | −0.002 (−0.253, 0.248) | 0.02 (−0.232, 0.272) | −0.06 (−0.271, 0.15) | −0.161 (−0.34, 0.019) | 0.03 (−0.197, 0.257) | |
| valine | 0.007 (−0.051, 0.066) | 0.012 (−0.068, 0.093) | 0.048 (−0.044, 0.139) | −0.1 (−0.229, 0.029) | −0.007 (−0.134, 0.12) | −0.105 (−0.216, 0.005) | 0.032 (−0.06, 0.123) | −0.092 (−0.211, 0.027) | |
| glucose | −0.024 (−0.056, 0.008) | −0.035 (−0.079, 0.009) | −0.01 (−0.06, 0.04) | 0.076 (−0.011, 0.162) | −0.069 (−0.162, 0.024) | −0.03 (−0.107, 0.047) | −0.026 (−0.092, 0.041) | 0.059 (−0.023, 0.141) | |
| 1,5-anhydroglucitol | −0.027 (−0.124, 0.069) | 0.002 (−0.129, 0.133) | −0.004 ( −0.155, 0.147) | 0.068 (−0.177, 0.313) | 0.047 (−0.164, 0.259) | −0.07 (−0.266, 0.125) | −0.181 (−0.346, −0.016) * | 0.083 (−0.127, 0.294) | |
| 2-hydroxybutyrate | −0.03 (−0.136, 0.075) | −0.085 (−0.229, 0.059) | 0.035 (−0.13, 0.199) | −0.168 (−0.489, 0.153) | −0.206 (−0.496, 0.084) | −0.079 (−0.345, 0.188) | −0.166 (−0.382, 0.05) | −0.157 (−0.439, 0.125) | |
| N-lactoyl phenylalanine | −0.05 (−0.166, 0.065) | −0.094 (−0.251, 0.062) | 0.049 (−0.131, 0.229) | 0.001 (−0.288, 0.29) | −0.056 (−0.323, 0.211) | 0.013 (−0.223, 0.249) | −0.083 (−0.282, 0.116) | −0.176 (−0.437, 0.085) | |
| N-lactoyl tyrosine | −0.097 (−0.223, 0.028) | −0.016 (−0.189, 0.157) | 0.038 (−0.157, 0.232) | −0.049 (−0.353, 0.256) | −0.093 (−0.386, 0.2) | −0.002 (−0.261, 0.258) | −0.012 (−0.247, 0.223) | −0.004 (−0.293, 0.284) | |
| N-lactoyl valine | −0.045 (−0.192, 0.101) | 0.044 (−0.158, 0.245) | 0.115 (−0.119, 0.349) | 0.003 (−0.356, 0.363) | −0.077 (−0.401, 0.247) | −0.004 (−0.293, 0.285) | −0.001 (−0.247, 0.244) | −0.207 (−0.524, 0.11) | |
| N-lactoyl leucine | −0.105 (−0.224, 0.013) | −0.109 (−0.27, 0.053) | 0.029 (−0.156, 0.215) | −0.09 (−0.413, 0.232) | −0.075 (−0.365, 0.215) | −0.088 (−0.351, 0.174) | −0.083 (−0.305, 0.138) | −0.235 (−0.521, 0.051) | |
| N-lactoyl isoleucine | −0.048 (−0.184, 0.088) | −0.008 (−0.193, 0.177) | 0.038 (−0.173, 0.249) | −0.178 (−0.543, 0.187) | −0.152 (−0.474, 0.17) | 0.009 (−0.292, 0.309) | −0.028 (−0.273, 0.216) | −0.191 (−0.505, 0.123) | |
| metabolonic lactone sulfate | −0.145 (−0.352, 0.063) | −0.199 (−0.48, 0.081) | 0.238 (−0.084, 0.56) | −0.146 (−0.606, 0.314) | −0.123 (−0.566, 0.32) | −0.165 (−0.544, 0.214) | −0.264 (−0.576, 0.048) | −0.218 (−0.631, 0.195) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li-Gao, R.; Grubbs, K.; Bertoni, A.G.; Hoffman, K.L.; Petrosino, J.F.; Ramesh, G.; Wu, M.; Rotter, J.I.; Chen, Y.-D.I.; Evans, A.M.; et al. The Roles of Gut Microbiome and Plasma Metabolites in the Associations between ABO Blood Groups and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Metabolites 2022, 12, 787. https://doi.org/10.3390/metabo12090787
Li-Gao R, Grubbs K, Bertoni AG, Hoffman KL, Petrosino JF, Ramesh G, Wu M, Rotter JI, Chen Y-DI, Evans AM, et al. The Roles of Gut Microbiome and Plasma Metabolites in the Associations between ABO Blood Groups and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Metabolites. 2022; 12(9):787. https://doi.org/10.3390/metabo12090787
Chicago/Turabian StyleLi-Gao, Ruifang, Kirk Grubbs, Alain G. Bertoni, Kristi L. Hoffman, Joseph F. Petrosino, Gautam Ramesh, Martin Wu, Jerome I. Rotter, Yii-Der Ida Chen, Anne M. Evans, and et al. 2022. "The Roles of Gut Microbiome and Plasma Metabolites in the Associations between ABO Blood Groups and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES)" Metabolites 12, no. 9: 787. https://doi.org/10.3390/metabo12090787
APA StyleLi-Gao, R., Grubbs, K., Bertoni, A. G., Hoffman, K. L., Petrosino, J. F., Ramesh, G., Wu, M., Rotter, J. I., Chen, Y.-D. I., Evans, A. M., Robinson, R. J., Sommerville, L., Mook-Kanamori, D., Goodarzi, M. O., Michelotti, G. A., & Sheridan, P. A. (2022). The Roles of Gut Microbiome and Plasma Metabolites in the Associations between ABO Blood Groups and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Metabolites, 12(9), 787. https://doi.org/10.3390/metabo12090787






